Abstract
Introduction
Abnormal body temperatures (Tb) are frequently seen in patients with severe sepsis. However, the relationship between Tb abnormalities and the severity of disease is not clear. This study investigated the impact of Tb on disease severity and outcomes in patients with severe sepsis.
Methods
We enrolled 624 patients with severe sepsis and grouped them into 6 categories according to their Tb at the time of enrollment. The temperature categories (≤35.5°C, 35.6–36.5°C, 36.6–37.5°C, 37.6–38.5°C, 38.6–39.5°C, ≥39.6°C) were based on the temperature data of the Acute Physiology and Chronic Health Evaluation II (APACHE II) scoring. We compared patient characteristics, physiological data, and mortality between groups.
Results
Patients with Tb of ≤36.5°C had significantly worse sequential organ failure assessment (SOFA) scores when compared with patients with Tb >37.5°C on the day of enrollment. Scores for APACHE II were also higher in patients with Tb ≤35.5°C when compared with patients with Tb >36.5°C. The 28-day and hospital mortality was significantly higher in patients with Tb ≤36.5°C. The difference in mortality rate was especially noticeable when patients with Tb ≤35.5°C were compared with patients who had Tb of >36.5°C. Although mortality did not relate to Tb ranges of ≥37.6°C as compared to reference range of 36.6–37.5°C, relative risk for 28-day mortality was significantly greater in patients with 35.6–36.5°C and ≤35.5°C (odds ratio; 2.032, 3.096, respectively). When patients were divided into groups based on the presence (≤36.5°C, n = 160) or absence (>36.5°C, n = 464) of hypothermia, disseminated intravascular coagulation (DIC) as well as SOFA and APACHE II scores were significantly higher in patients with hypothermia. Patients with hypothermia had significantly higher 28-day and hospital mortality rates than those without hypothermia (38.1% vs. 17.9% and 49.4% vs. 22.6%, respectively). The presence of hypothermia was an independent predictor of 28-day mortality, and the differences between patients with and without hypothermia were observed irrespective of the presence of septic shock.
Conclusions
In patients with severe sepsis, hypothermia (Tb ≤36.5°C) was associated with increased mortality and organ failure, irrespective of the presence of septic shock.
Trial registration
UMIN-CTR ID UMIN000008195
Introduction
Body temperature (Tb) abnormalities are amongst the most commonly noted symptoms of critically ill patients. Fever occurs in approximately half of patients admitted to the ICU and has been associated with adverse outcomes [1]. Fever is one of the most prominent symptoms of infection [2] and it is part of the host acute-phase response to infectious as well as non-infectious inflammatory stimuli [3]. Fever is also believed to be harmful, especially in patients with life-threatening illness, because febrile responses are known to increase the metabolic rate and minutes ventilation and oxygen consumption, and it can have adverse effects on neurological outcomes [4-6]. Fever could also be beneficial because it is believed to reduce bacterial growth, and a higher Tb is believed to promote the synthesis of antibodies and cytokines, thereby activating immune cells and improving survival [7-9]. Several studies have suggested that suppression of the febrile response with antipyretic drugs could worsen patient outcomes [10,11].
A large epidemiological study that included patients with and without infection reported that the presence of fever per se could not be associated with increased ICU mortality. Nevertheless, fever with Tb ≥39.5°C was associated with a significant increase in mortality (20.3% versus 12.0% (P <0.001) for patients with Tb ≥39.5°C and <39.5°C, respectively). These very high fevers could be complicated with cardiac arrhythmias, tachycardia, increased oxygen demand, convulsions, and brain damage [1]. A recent study that used data from Australia, New Zealand, and the United Kingdom investigated the association between peak Tb in the first 24 h after admission to ICU and in-hospital mortality [12]. This study showed that elevated peak Tb in the first 24 h in the ICU could be associated with decreased in-hospital mortality in patients with infection. The lowest mortality risk was among patients with Tb between 39.0°C and 39.4°C. However, mortality risk was increased among patients who did not have infection. Patients with fever in response to non-infective causes may well experience the harmful effects of fever without any fever-related benefits, such as protection against viruses or bacteria.
Hypothermia can be caused by a variety of factors including cold exposure, severe infection, endocrine abnormalities, and drug overdoses, and hypothermic patients require immediate medical intervention [13-15]. Although hypothermia may be an unintended consequence of critical illness and may be associated with an increased risk of mortality in patients with sepsis and non-infectious conditions, the influence of hypothermia on the physiological severity and outcome of critically ill patients, particularly patients with severe sepsis, is not well understood [12,16-22].
Although there are many reports of Tb abnormalities in patients with sepsis, there is a relative paucity of information on the influences of hyper- or hypothermia on disease severity and outcomes in patients with severe sepsis. The aim of present study was to investigate the association between Tb and disease severity and patient outcomes in patients with a definitive diagnosis of severe sepsis.
Materials and methods
This was a prospective study conducted as a part of a multicenter prospective evaluation of severe sepsis in Japan, undertaken by the Japanese Association for Acute Medicine Sepsis Registry (JAAMSR) Study Group [23]. Both the Japanese Association for Acute Medicine and the Ethics Committees of the hospitals that participated in this study approved the study protocol. Data collection was performed as part of the routine clinical examinations without any medical intervention. Data management and statistical analyses were processed anonymously. Based on these reasons, written informed consent was waived by both the Japanese Association for Acute Medicine and the Ethics Committees of the participating hospitals. The study was registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR ID: UMIN000008195).
Patients
Between June 1, 2010, and May 31, 2011, we enrolled 624 patients in this study. All of the patients were diagnosed with severe sepsis and admitted to one of 15 critical care centers in the tertiary care hospitals in Japan. We did not have any exclusion criteria.
Definitions
Sepsis, severe sepsis, septic shock, and systemic inflammatory response syndrome (SIRS) were defined according to the American College of Chest Physicians/Society of Critical Care Medicine consensus conference and its revised version of 2003 [24,25]. The severity of illness was evaluated according to the acute physiology and chronic health evaluation (APACHE) II score at the time of enrollment [26]. Organ dysfunction was assessed according to the sequential organ failure assessment (SOFA) score [27]. Multiple organ dysfunction syndrome (MODS) was defined as a SOFA score ≥12 [27]. A diagnosis of disseminated intravascular coagulation (DIC) was made on the basis of the scoring system of the International Society on Thrombosis and Haemostasis (ISTH) [28]. The change in fibrin/fibrinogen degradation product (FDP) was used as the fibrin-related marker for the ISTH criteria. FDP values of <10, ≥10 but <25, and ≥25 mg/L, were defined as no increase, moderate increase, and strong increase, respectively. The outcome measure was the 28-day and hospital all-cause mortality.
Body temperature
Tb recorded within 24 h of a diagnosis of severe sepsis was used for the APACHE II score and the recorded temperature was used in this analysis. We recorded the value measured by the method most preferred by the American College of Critical Care Medicine and the Infectious Diseases Society of America [2]. Although the method used to measure core Tb was not standardized and the specific methods used for each individual measurement of core Tb was not recorded in this survey, all the institutions that participated in this study used standard methods for determining core Tb. The sites used for Tb measurement at the institutions were as follows: urinary bladder, ten institutions; urinary bladder or rectal, three institutions; rectal, one institution; and intravascular, one institution. To ascertain the effect of Tb aberrance on disease severity and outcome, patients were grouped into one of six categories based on their core Tb as recorded for the APACHE II scoring. The categories were ≤35.5°C, 35.6 to 36.5°C, 36.6 to 37.5°C, 37.6 to 38.5°C, 38.6 to 39.5°C, and ≥39.6°C, as previously reported. A core Tb of 35.5°C was taken as the lowest Tb value because previous studies have reported this temperature as the threshold of hypothermia with high mortality [16,29-34]. Although previous studies considered 35.5°C as the threshold for hypothermia [16,29-32], we opted to use 36.5°C as the threshold because we found significant differences in the mortality of patients with Tb ≤36.5°C compared with those with Tb >36.5°C based on the results of a temperature categorical analysis, as shown in Tables 1 and 2. We also compared the mortality of patients divided into two groups using 36.5°C as a cutoff value. This analysis demonstrated significant differences between groups in both the 28-day and hospital mortality (P <0.001). Based on these findings, we evaluated the effect of hypothermia defined as Tb ≤36.5°C on not only mortality but also disease severity, which may affect mortality. For analysis, patients were divided into two groups, namely, hypothermia (Tb ≤36.5°C, n = 160) and no-hypothermia (Tb >36.5°C, n = 464).
Table 1.
Baseline characteristics and outcome of the enrolled patients (n = 624)
| Characteristic | Value |
|---|---|
| Age, years |
72 (61 – 81) |
| Gender, male/female |
391/233 |
| APACHE II score |
23 (17 – 29) |
| SOFA score |
8 (6 – 11) |
| MODS, n (%) |
144 (23.1) |
| Septic shock, n (%) |
282 (45.2) |
| Admission category |
|
| Medical |
519 (83.2%) |
| Trauma |
27 (4.3%) |
| Surgery |
19 (3.0%) |
| Burns |
19 (3.0%) |
| Other |
40 (6.4%) |
| Site of Infection |
|
| Pulmonary |
261 (41.8%) |
| Intra-abdominal |
133 (21.3%) |
| Urinary |
78 (12.5%) |
| Skin/soft tissue |
78 (12.5%) |
| Meningitis |
15 (2.4%) |
| Catheter-related |
11 (1.8%) |
| Bone/joint |
10 (1.6%) |
| Infective endocarditis |
3 (0.5%) |
| Other |
25 (4.0%) |
| 28-day mortality, n (%) |
144 (23.1%) |
| Hospital mortality, n (%) | 184 (29.5%) |
Values are presented as median (IQR) or number (%). APACHE, acute physiology and chronic health evaluation; SOFA, sequential organ failure assessment; MODS, multiple organ dysfunction syndrome.
Table 2.
Body temperature and severity of coagulation abnormality/organ failure scores
| |
Body temperature, °C |
|||||
|---|---|---|---|---|---|---|
| ≤35.5 (n = 99) | 35.6 to 36.5 (n = 61) | 36.6 to 37.5 (n = 112) | 37.6 to 38.5 (n = 155) | 38.6 to 39.5 (n = 133) | ≥39.6 (n = 64) | |
| Age, years |
76 (65 to 82)de |
78 (62.5 to 83.5)de |
75 (66 to 84)de |
72 (61 to 82)de |
67 (58 to 76) |
65 (45.25 to 78.75) |
| Septic shock, n (%) |
62 (62.6%)bcde |
33 (54.1%) |
46 (41.1%) |
63 (40.6%) |
54 (40.6%) |
24 (37.5%) |
| SIRS criteria |
4 (3 to 4)abc |
3 (2 to 4)bde |
3 (2 to 3)cde |
3 (3 to 4)de |
4 (3 to 4) |
4 (3 to 4) |
| DIC score |
5 (2 to 6)c |
4 (2 to 6) |
4 (2 to 5) |
3 (2 to 5) |
3 (2 to 5) |
3 (2 to 5) |
| DIC ≥5, n (%) |
28 (28.3%)cde |
15 (24.6%)c |
22 (19.6%) |
20 (12.9%) |
21 (15.8%) |
7 (10.9%) |
| SOFA score |
10 (7 to 12)cde |
10 (7 to 13)cde |
8 (5 to 11) |
8 (5 to 11) |
7 (5 to 10.75) |
7 (6 to 10) |
| MODS, n (%) |
35 (35.4%)cde |
23 (37.8%)cde |
27 (24.1%) |
32 (20.6%) |
23 (17.3%) |
10 (15.6%) |
| APACHE II |
28 (23 to 33)bcde |
24 (20 to 29) |
21 (16.25 to 27) |
21 (16 to 27) |
22 (17 to 26) |
22 (17.25 to 30) |
| Outcome |
|
|
|
|
|
|
| 28-day mortality, n (%) |
40 (40.4%)bcde |
21 (34.4%)cde |
23 (20.5%) |
28 (18.1%) |
21 (15.8%) |
11 (17.2%) |
| Hospital mortality, n (%) | 52 (52.5%)bcde | 27 (44.3%)bcde | 27 (24.1%) | 39 (25.2%) | 26 (19.5%) | 13 (20.3%) |
SIRS, systemic inflammatory response syndrome; DIC, disseminated intravascular coagulation; SOFA, sequential organ failure assessment; MODS, multiple organ dysfunction syndrome; APACHE, acute physiology and chronic health evaluation. aP <0.0033 (after Bonferroni correction) versus 35.6 to 36.5°C; bversus 36.6 to 37.5°C; cversus 37.6 to 38.5°C; dversus 38.6 to 39.5°C; eversus ≥39.6°C.
Assessments
Blood was collected at the time of admission to ICU and then daily thereafter as part of the routine clinical and laboratory tests using established standard laboratory techniques. Platelet counts and coagulation variables necessary to diagnose DIC were collected and APACHE II, SOFA, and DIC scores were assessed.
Statistical analysis
Data are expressed as medians and interquartile ranges. All statistical analyses were performed using SPSS 19.0 for Windows (SPSS, Chicago, IL, USA). Comparisons between the 2 groups were performed using the Mann-Whitney’s U test, and categorical variables were summarized using proportions and compared between groups using either the Pearson’s chi-square or Fisher’s exact test, where appropriate. Kruskal-Wallis one-way analysis of variance and multiple chi-square tests were used for comparisons between multiple groups, and P-values were adjusted with the Bonferroni correction for multiple testing.
Odds ratios (OR) are reported relative to a reference range of Tb, as previously reported [33]. We defined the reference range here as the Tb category of 36.6 to 37.5°C. Additionally, survival curves were derived by the Kaplan-Meier method and compared by the log-rank test for each range. We used a multivariate logistic model to assess the relationships between 28-day mortality and independent variables in patients with severe sepsis. Outcome (dead, 1; survived, 0) was used as the criterion variable, and age, gender (male or female), admission category of underlying medical condition (medical or other cause), SOFA score, APACHE II score, positive blood culture (yes or no), the presence of comorbidity (yes or no), and hypothermia (Tb ≤36.5°C or >36.5°C) were used as explanatory variables. Results are reported as OR, P-values, and 95% CI. Differences with a P-value <0.05 were considered to be statistically significant. Furthermore, P <0.0033 (after Bonferroni correction) was used for comparisons between groups in multiple testing (Table 2).
Results
Baseline characteristics and patient outcome
During the 1-year study period, a total of 14,417 patients were admitted to the 15 critical care centers, and 624 (4.3%) of these patients were diagnosed with severe sepsis and enrolled in this study. The characteristics at enrollment and outcomes of patients are shown in Table 1. The mean age was 69 years, and the mean initial APACHE II score and SOFA scores were 23.4 and 8.6, respectively. The major sites of infection were pulmonary, intra-abdominal, urinary, and skin/soft tissue. More than half of the patients had dysfunction of three or more organ systems. The 28-day mortality was 23.1% and the overall hospital mortality was 29.5%. Sepsis-related hospital mortality was 25.6% (160/624 patients).
Relationships between body temperature and severity scores
Patients with Tb >38.5°C were significantly younger than patients with Tb ≤38.5°C. The prevalence of septic shock was significantly higher among patients with Tb ≤35.5°C when compared with the incidence of septic shock among patients in the other Tb categories (Table 2). MODS and SOFA on the day of enrollment were significantly higher in patients with Tb 35.6 to 36.5°C and ≤35.5°C when compared with patients who had Tb >37.5°C. The APACHEII scores in patients with Tb ≤35.5°C were significantly higher when compared with patients who had Tb of >36.5°C.
For mortality rates, patients who had Tb ≤36.5°C had significantly higher 28-day and hospital mortality rates when compared with patients who had Tb >36.5°C. The mortality rate among patients who had Tb ≤35.5°C was especially high at 40.4% and 52.5% for 28-day and hospital mortality rates, respectively. The lowest 28-day and hospital mortality were noted in patients with Tb between 38.6 and 39.5°C (15.8% and 19.5%, for 28-day and hospital mortality, respectively) (Table 2).
Body temperature and mortality
Table 3 shows 28-day mortality and OR for each Tb (taken on day 1) relative to the reference range of 36.6 to 37.5°C. We found no relationships between mortality and Tb in patients in the following categories: 37.6 to 38.5°C, 38.6 to 39.5°C, and ≥39.6°C. The relationship between mortality and Tb was significant in patients in the Tb categories of 35.6 to 36.5°C (OR 2.032, P = 0.047) and ≤35.5°C (OR 3.096, P = 0.001). Kaplan-Meier estimates for the probability of survival at 28 days were lower in patients with Tb between 35.6°C and 36.5°C and ≤35.5°C compared to patients who had Tb ≥36.6°C (Figure 1).
Table 3.
Day-1 body temperature and 28-day mortality
| Range of body temperature (°C) | 28-day mortality | Unadjusted odds ratio | 95% CI | P -value |
|---|---|---|---|---|
| ≤35.5 |
40.4% |
3.096 |
1.611, 5.947 |
0.001 |
| 35.6 to 36.5 |
34.4% |
2.032 |
1.009, 4.088 |
0.047 |
| 36.6 to 37.5 |
20.5% |
1.000 |
(reference) |
|
| 37.6 to 8.5 |
18.1% |
0.853 |
0.461, 1.577 |
0.621 |
| 38.6 to 39.5 |
15.8% |
0.726 |
0.377, 1.395 |
0.404 |
| ≥39.6 | 17.2% | 0.803 | 0.363, 1.778 | 0.693 |
Figure 1.
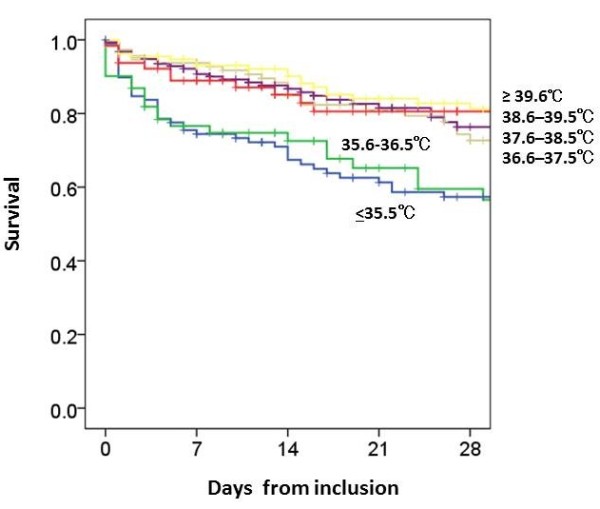
Body temperature within 24 h of ICU admission and survival of patients with severe sepsis. This figure depicts the Kaplan-Meier estimates for the probability of survival, which at 28 days was lower in patients with body temperature of ≤35.5°C and 35.6 to 36.5°C, as compared to patients with body temperatures of 36.6 to 37.5°C, 37.6 to 38.5°C, 38.6 to 39.5°C, and ≥39.6°C (P <0.001). Body temperature was recorded as the highest score on the acute physiology and chronic health evaluation (APACHE) II scoring system and as the farthest value from 36.5 to 37.0°C within 24 h from the time of enrollment, which was divided into categorical variables with 1°C increments. Thus, body temperature was analyzed in six range categories: ≤35.5°C, 35.6 to 36.5°C, 36.6 to 37.5°C, 37.6 to 38.5°C, 38.6 to 39.5°C, and ≥39.6°C.
Severity scores and outcome in hypothermic and non-hypothermic patients
After analyzing the data for the different Tb categories, we defined 36.5°C as the threshold temperature for hypothermia and compared variables and outcomes between patients with hypothermia (≤36.5°C, n = 160) and those without hypothermia (>36.5°C, n = 464).
The incidence of septic shock was significantly higher in patients with hypothermia compared to patients without hypothermia. DIC, SOFA, and APACHE II scores and the incidence of MODS were significantly increased among hypothermic patients (Table 4). In hypothermic patients, 28-day and hospital mortality were higher (more than double) than the mortality rates of patients without hypothermia (38.1% versus 17.9%, 49.4% versus 22.6%, for 28-day and for hospital mortality, respectively) (Table 4).
Table 4.
Characteristics, physiology on day 1 and outcome in hypothermic (body temperature ≤36.5°C) and non-hypothermic severe sepsis patients
| Hypothermia (n = 160) | Non-hypothermia (n = 464) | P -value | |
|---|---|---|---|
| Age, years |
76 (64.25 to 83) |
71 (60 to 80) |
0.001 |
| Septic shock |
59.4% (n = 95) |
40.3% (n = 187) |
<0.001 |
| DIC score |
4 (2 to 6) |
3 (2 to 5) |
0.009 |
| SOFA score |
10 (7 to 13) |
8 (5 to 11) |
<0.001 |
| APACHE II score |
26 (21 to 32) |
21 (16.25 to 27) |
<0.001 |
| Outcome |
|
|
|
| 28-day mortality |
38.1% (n = 61) |
17.9% (n = 83) |
<0.001 |
| Hospital mortality | 49.4% (n = 79) | 22.6% (n = 105) | <0.001 |
Results are presented as median (IQR) or % (number). DIC, disseminated intravascular coagulation; SOFA, sequential organ failure assessment; APACHE, acute physiology and chronic health evaluation.
Comparisons of severity scores and outcome between hypothermic and non-hypothermic patients with and without septic shock
The incidence of septic shock was significantly higher in patients with hypothermia. We separately evaluated the influence of hypothermia on variables and outcomes in patients with and without septic shock, because mortality and severity scores in patients with septic shock were significantly higher when compared with other patients [23]. Patients with septic shock had higher DIC, SOFA, and APACHE II scores if they were hypothermic at the time of diagnosis. In these hypothermic patients, both 28-day and hospital mortality were nearly twice those of patients with septic shock and no hypothermia (Table 5).
Table 5.
Characteristics, physiology on day 1, and outcome in hypothermic (body temperature ≤36.5°C) and non-hypothermic patients with septic shock
| Hypothermia (n = 95) | Non-hypothermia (n = 187) | P -value | |
|---|---|---|---|
| Age, years |
75 (62 to 83) |
72 (61 to 79) |
0.069 |
| DIC score |
4.0 (2.0 to 5.0) |
3.0 (2.0 to 5.0) |
0.047 |
| SOFA score |
11.0 (9.0 to 13.0) |
10.0 (8.0 to 13.0) |
0.039 |
| APACHE II score |
29.0 (23.0 to 35.0) |
25.0 (19.0 to 31.0) |
0.001 |
| Outcome |
|
|
|
| 28-day mortality |
49.5% (n = 47) |
24.6% (n = 46) |
< 0.001 |
| Hospital mortality | 62.1% (n = 59) | 31.0% (n = 58) | < 0.001 |
Results are presented as median (IQR) or % (number). DIC, disseminated intravascular coagulation; SOFA, sequential organ failure assessment; APACHE, acute physiology and chronic health evaluation.
In patients without septic shock, hypothermic patients had a significantly higher incidence of MODS and they also had significantly higher SOFA and APACHE II scores when compared with patients who did not have hypothermia. Although the 28-day mortality was not significantly different between hypothermic and non-hypothermic patients, hospital mortality in hypothermic patients was nearly twice that of non-hypothermic patients (30.8% versus 17.0%) (Table 6). The 28-day and hospital mortality rates in hypothermic patients without septic shock and non-hypothermic patients with septic shock were both double those in patients who were non-hypothermic and did not have septic shock. In addition, the 28-day and hospital mortality rates in hypothermic patients with septic shock were almost four times higher than those in non-hypothermic patients without septic shock.
Table 6.
Characteristics, physiology on day 1, and outcome in hypothermic (body temperature ≤36.5°C) and non-hypothermic patients without septic shock
| Hypothermia (n = 65) | Non-hypothermia (n = 277) | P -value | |
|---|---|---|---|
| Age, years |
78 (70 to 82.5) |
71 (58 to 81) |
0.004 |
| DIC score |
3 (2 to 5) |
3 (2 to 5) |
0.133 |
| SOFA score |
7.5 (5 to 11) |
6 (4 to 8) |
0.004 |
| APACHEII score |
24 (18 to 27) |
19 (15 to 24) |
<0.001 |
| Outcome |
|
|
|
| 28-day mortality |
21.5% (n = 14) |
13.4% (n = 37) |
0.096 |
| Hospital mortality | 30.8% (n = 20) | 17.0% (n = 47) | 0.012 |
DIC, disseminated intravascular coagulation; SOFA, sequential organ failure assessment; APACHE, acute physiology and chronic health evaluation. Results are presented as median (IQR) or % (number).
Table 7 shows that hypothermia, defined as a core Tb of ≤36.5°C, was an independent predictor of 28-day mortality in patients with severe sepsis, especially in the presence of septic shock.
Table 7.
Results of multivariate logistic regression analysis for the prediction of 28-day mortality
| Factors | Odds ratio | P value | 95% CI |
|---|---|---|---|
| Severe sepsis (n = 602) | |||
| Age |
1.026 |
0.001 |
1.010–1.042 |
| Gender (male) |
1.476 |
0.091 |
0.940–2.317 |
| Admission category (medical conditions) |
1.098 |
0.778 |
0.572–2.109 |
| SOFA score |
1.111 |
0.002 |
1.041–1.186 |
| APACHE II score |
1.062 |
0.000 |
1.029–1.095 |
| Positive blood culture |
1.471 |
0.073 |
0.965–2.242 |
| Presence of comorbidity |
0.880 |
0.569 |
0.566–1.367 |
| Hypothermia (body temperature <36.5°C) |
1.952 |
0.003 |
1.253–3.040 |
| Severe sepsis with septic shock (n = 273) | |||
| Age |
1.036 |
0.001 |
1.014–1.059 |
| Gender (male) |
1.676 |
0.095 |
0.914–3.074 |
| Admission category (medical conditions) |
1.110 |
0.829 |
0.431–2.860 |
| SOFA score |
1.078 |
0.119 |
0.981–1.186 |
| APACHE II score |
1.050 |
0.019 |
1.008–1.094 |
| Positive blood culture |
1.761 |
0.052 |
0.996–3.114 |
| Presence of comorbidity |
0.722 |
0.290 |
0.395–1.319 |
| Hypothermia (body temperature <36.5°C) | 2.778 | 0.001 | 1.555–4.965 |
CI, confidence interval; SOFA, Sequential Organ Failure Assessment; APACHE, Acute Physiology and Chronic Health Evaluation; Comorbidity, at least one comorbidity.
Discussion
The results of this study clearly indicate that the mortality rate amongst patients with severe sepsis is significantly higher among those who have a Tb of ≤36.5°C compared to those who have a Tb of >36.5°C measured within 24 h of diagnosis. In this study, the mortality rate was more than two times higher among patients with severe sepsis who were hypothermic compared to patients with severe sepsis who had no hypothermia. Furthermore, the higher mortality rate was associated with a deterioration of organ function and DIC. The effect of hypothermia on mortality rate was consistently observed in patients with and without septic shock.
In our study, elevated Tb was not associated with an increase in disease severity or risk of mortality. Moreover, elevated Tb was not associated with a progressive increase in disease severity or mortality when compared with the reference Tb range of 36.6 to 37.5°C. Our results suggest that higher Tb is not harmful in patients with severe sepsis. Studies investigating the effect of fever control by means of antipyretic treatment or external cooling and the risk of mortality have reported contrasting results, and it is clear that the role of fever and its control in patients with severe sepsis still needs to be elucidated [34,35].
It has been suggested that hypothermia is associated with an increased risk of mortality in critically ill patients [12,33]. Moreover, the effect of hypothermia on increased mortality has been shown in patients with and without infection [12,17,18]. In the Methylprednisolone Severe Sepsis Study database, the Veterans Administration Systemic Sepsis Cooperative Study of Glucocorticoid Therapy, and the Ibuprofen Sepsis Study, the threshold Tb for hypothermia was set at 35.5°C and patients with severe sepsis were included. The incidence of hypothermia (<35.5°C), 28- or 30-day mortality in patients with hypothermia versus patients without hypothermia in these studies were 9%, 62% versus 26%; 10%, 57% versus 28%; and 9.6%, 70% versus 35%, respectively. The NORASEPT II study included only patients with septic shock and the incidence of hypothermia among these patients was 21%. The mortality in patients with hypothermia and in those without hypothermia was 59% and 34%, respectively. In the present study, the incidence of mortality among patients with Tb ≤35.5°C was 15.9% (99/624 patients), and the 28-day and hospital mortality rates were also significantly higher when compared with other patients (Tb >35.5°C; 40.4% versus 19.8%, 52.5% versus 25.1%, for 28-day and hospital mortality, respectively). Although the underlying mechanism of sepsis-related hypothermia is still unclear, our results are consistent with previous studies [16,29-32].
We defined 36.5°C as the threshold of hypothermia based on the results of our evaluation of the outcomes for the different Tb categories. We also generated the receiver operating characteristic curves using Tb on the day of enrollment for the 28-day and hospital mortality evaluation. The analysis revealed that the cutoff values for predicting the 28-day and hospital mortality were 36.9°C and 36.3°C, respectively, for maximizing both sensitivity and specificity (data not shown), and these suggest a Tb of 36.5°C as an acceptable cutoff value to define hypothermia in this study.
Although the impact of hypothermia, defined as a threshold temperature of 35.5°C, on mortality has been demonstrated in previous studies [16,29-32], the effect of hypothermia on disease severity has not been fully evaluated. Therefore, we evaluated the effects of Tb ≤36.5°C on both mortality and disease severity by comparing patients with and without hypothermia. The incidence of organ failure, DIC and outcomes were significantly different in patients with Tb of ≤36.5°C compared to those with Tb of >36.5°C, and there was no significant difference between patients who had Tb of ≤35.5°C compared to those with a Tb of >35.5°C. Therefore, 36.5°C was considered the threshold for hypothermia in patients with severe sepsis, irrespective of the presence of septic shock.
It is important to note that the inclusion of Tb abnormalities as a measure of the severity of illness varies between different scoring systems. APACHE II assigns points for patients with either high or low Tb, SAPS II only assigns points for high Tb, and SAPS III only assigns points for low Tb[26,36,37]. Although it is widely accepted that fever has an adverse effect on patients with neurologic injury [38], little is known about the impact of temperature abnormalities on the outcome of other ICU patients, especially patients with sepsis [1,18]. The results of this study add valuable knowledge with regard to the influence of Tb abnormalities on the outcome of patients with severe sepsis. From our results, it is clear that hypothermia has a greater impact on organ dysfunction and outcomes. Thus far, the mechanism underlying the harmful effects of hypothermia is not yet known, but it is evident that hypothermia is more important than elevated temperature for the severity of illness scores in patients with severe sepsis.
Limitations
There are some limitations to our study. Tb was recorded within 24 h of a diagnosis of severe sepsis as the highest core Tb value of the APACHE II score. However, we did not standardize the method by which core Tb was measured, and we did not attempt to differentiate between patients who had an elevated Tb as a result of hyperthermia syndrome or because of fever. Although some patients might have been categorized differently if we employed a systematic protocol for measuring Tb, our recorded core Tb data were not merely arbitrary values obtained within the 24-h period after a diagnosis of severe sepsis, but these measurements were assessed objectively. In addition, the outcome and influence of treatment may vary significantly on that basis.
We did not specifically control for therapeutic modalities that may have influenced Tb, such as antipyretic drugs or active external temperature control strategies. Although it is widely accepted that temperature control improves outcome in patients with neurologic injury, the effect of acetaminophen, ibuprofen, or external control on the outcome of other critically ill patients is not well understood.
Conclusions
Tb of patients with severe sepsis, as measured at the time of diagnosis, significantly affected patient outcome. In our study, hypothermia (≤36.5°C) was associated with a significantly higher risk of mortality. The risk of mortality was almost double among hypothermic patients compared to patients without hypothermia. Hypothermia was also associated with a significant physiological decline in these patients, irrespective of whether they experienced septic shock or not. Elevated Tb was not associated with an increased disease severity and risk of mortality.
Key messages
•In patients with severe sepsis, the impact of elevated body temperature and hypothermia on mortality and severity of physiologic decline is different.
•Hypothermia, defined as body temperature of ≤36.5°C, is significantly associated with an increased mortality risk of more than double that of non-hypothermic patients; moreover, it is associated with a physiological decline in severe sepsis, irrespective of the presence of septic shock.
•Elevated body temperature was not associated with increased disease severity or risk of mortality.
Abbreviations
APACHE: Acute physiology and chronic health evaluation; DIC: Disseminated intravascular coagulation; FDP: Fibrin/fibrinogen degradation product; ISTH: International Society on Thrombosis and Haemostasis; JAAM: Japanese Association for Acute Medicine; JAAMSR: Japanese Association for Acute Medicine Sepsis Registry; MODS: Multiple organ dysfunction syndrome; OR: Odds ratio; SAPS: Simplified acute physiology score; SIRS: Systemic inflammatory response syndrome; SOFA: Sequential organ failure assessment; Tb: Body temperature.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SK participated in study design and data collection and interpretation, performed the statistical analysis, and drafted the manuscript. SG, DS, HO, NT, SF, TM, TA, HI, JK, YM, SS, KS, YS, KT, RT, YY, NY, and NA participated in study design and data collection and interpretation, performed the statistical analysis, and helped to draft the manuscript. All authors read and approved the final version of the manuscript.
Contributor Information
Shigeki Kushimoto, Email: kussie@emergency-medicine.med.tohoku.ac.jp.
Satoshi Gando, Email: gando@med.hokudai.ac.jp.
Daizoh Saitoh, Email: ds0711@ndmc.ac.jp.
Toshihiko Mayumi, Email: mtoshi@med.nagoya-u.ac.jp.
Hiroshi Ogura, Email: ogura@hp-emerg.med.osaka-u.ac.jp.
Seitaro Fujishima, Email: fujishim@z6.keio.jp.
Tsunetoshi Araki, Email: t9240araki@matsunami-hsp.or.jp.
Hiroto Ikeda, Email: ikeda@med.teikyo-u.ac.jp.
Joji Kotani, Email: kotanijo@hyo-med.ac.jp.
Yasuo Miki, Email: yasuo@aichi-med-u.ac.jp.
Shin-ichiro Shiraishi, Email: shinshi@nms.ac.jp.
Koichiro Suzuki, Email: ksuzuki@med.kawasaki-m.ac.jp.
Yasushi Suzuki, Email: ysuzuki@iwate-med.ac.jp.
Naoshi Takeyama, Email: takeyama@fujita-hu.ac.jp.
Kiyotsugu Takuma, Email: takuma@fa2.so-net.ne.jp.
Ryosuke Tsuruta, Email: ryosan-ygc@umin.ac.jp.
Yoshihiro Yamaguchi, Email: gaius@kyorin-u.ac.jp.
Norio Yamashita, Email: norio22@med.kurume-u.ac.jp.
Naoki Aikawa, Email: aikawa7@rc4.so-net.ne.jp.
Acknowledgements
This study was funded and supported by the Japanese Association for Acute Medicine. This study was approved by the Japanese Association for Acute Medicine and by the following institutional review boards or ethics committees of each hospital: Institutional Review Board of Hokkaido University Hospital for Clinical Research; Ethics Committee of Nagoya University of Graduate School of Medicine; Ethics Committee of Keio University School of Medicine; Fujita Health University ethical review board for epidemiological and clinical studies; Teikyo University Review Board; Ethics Committee of Nippon Medical School Hospital; Ethics Committee of Nippon Medical School Hospital; Center for Clinical Research, Yamaguchi University Hospital; The Ethics Review Board of Hyogo College of Medicine; Ethics Committee of Osaka University Hospital; Ethics Committee of Kyorin University; Ethics Committee of Tohoku University Hospital; Ethics Committee of Kawasaki Municipal Hospital; The Ethical Committee of Kurume University; and Research Ethics Committee of Kawasaki Medical School and Hospital.
References
- Laupland KB, Shahpori R, Kirkpatrick AW, Ross T, Gregson DB, Stelfox HT. Occurrence and outcome of fever in critically ill adults. Crit Care Med. 2008;17:1531–1535. doi: 10.1097/CCM.0b013e318170efd3. [DOI] [PubMed] [Google Scholar]
- O’Grady NP, Barie PS, Bartlett JG, Bleck T, Carroll K, Kalil AC, Linden P, Maki DG, Nierman D, Pasculle W, Masur H. American college of critical care medicine, infectious diseases society of America: guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American college of critical care medicine and the infectious diseases society of America. Crit Care Med. 2008;17:1330–1349. doi: 10.1097/CCM.0b013e318169eda9. [DOI] [PubMed] [Google Scholar]
- Hawksworth JS, Leeser D, Jindal RM, Falta E, Tadaki D, Elster EA. New directions for induction immunosuppression strategy in solid organ transplantation. Am J Surg. 2009;17:515–524. doi: 10.1016/j.amjsurg.2008.04.025. [DOI] [PubMed] [Google Scholar]
- Manthous CA, Hall JB, Olson D, Singh M, Chatila W, Pohlman A, Kushner R, Schmidt GA, Wood LD. Effect of cooling on oxygen consumption in febrile critically ill patients. Am J Respir Crit Care Med. 1995;17:10–14. doi: 10.1164/ajrccm.151.1.7812538. [DOI] [PubMed] [Google Scholar]
- The Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;17:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;17:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- Villar J, Ribeiro SP, Mullen JB, Kuliszewski M, Post M, Slutsky AS. Induction of the heat shock response reduces mortality rate and organ damage in a sepsis-induced acute lung injury model. Crit Care Med. 1994;17:914–921. [PubMed] [Google Scholar]
- Kluger MJ, Kozak W, Conn CA, Leon LR, Soszynski D. The adaptive value of fever. Infect Dis Clin North Am. 1996;17:1–20. doi: 10.1016/S0891-5520(05)70282-8. [DOI] [PubMed] [Google Scholar]
- Mackowiak PA. Fever: blessing or curse? A unifying hypothesis. Ann Intern Med. 1994;17:1037–1040. doi: 10.7326/0003-4819-120-12-199406150-00010. [DOI] [PubMed] [Google Scholar]
- Eyers S, Weatherall M, Shirtcliffe P, Perrin K, Beasley R. The effect on mortality of antipyretics in the treatment of influenza infection: systematic review and meta-analysis. J R Soc Med. 2010;17:403–411. doi: 10.1258/jrsm.2010.090441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandts CH, Ndjavé M, Graninger W, Kremsner PG. Effect of paracetamol on parasite clearance time in Plasmodium falciparum malaria. Lancet. 1997;17:704–709. doi: 10.1016/S0140-6736(97)02255-1. [DOI] [PubMed] [Google Scholar]
- Young PJ, Saxena M, Beasley R, Bellomo R, Bailey M, Pilcher D, Finfer S, Harrison D, Myburgh J, Rowan K. Early peak temperature and mortality in critically ill patients with or without infection. Intensive Care Med. 2012;17:437–444. doi: 10.1007/s00134-012-2478-3. [DOI] [PubMed] [Google Scholar]
- Mégarbane B, Axler O, Chary I, Pompier R, Brivet FG. Hypothermia with indoor occurrence is associated with a worse outcome. Intensive Care Med. 2000;17:1843–1849. doi: 10.1007/s001340000702. [DOI] [PubMed] [Google Scholar]
- Brivet F, Carras PM, Dormont J, Guidet B, Offenstadt G, Gachot B, Wolf M, Timsit JF, Misset B. Hypothermia, a pertinent clinical prognostic factor in severe systemic inflammatory response syndrome. Crit Care Med. 1994;17:533–534. doi: 10.1097/00003246-199403000-00029. [DOI] [PubMed] [Google Scholar]
- Clemmer TP, Fisher CJ Jr, Bone RC, Slotman GJ, Metz CA, Thomas FO. Hypothermia in the sepsis syndrome and clinical outcome. The Methylprednisolone Severe Sepsis Study Group. Crit Care Med. 1992;17:1395–1401. doi: 10.1097/00003246-199210000-00006. [DOI] [PubMed] [Google Scholar]
- Arons MM, Wheeler AP, Bernard GR, Christman BW, Russell JA, Schein R, Summer WR, Steinberg KP, Fulkerson W, Wright P, Dupont WD, Swindell BB. Effects of ibuprofen on the physiology and survival of hypothermic sepsis. Crit Care Med. 1999;17:699–707. doi: 10.1097/00003246-199904000-00020. [DOI] [PubMed] [Google Scholar]
- Tiruvoipati R, Ong K, Gangopadhyay H, Arora S, Carney I, Botha J. Hypothermia predicts mortality in critically ill elderly patients with sepsis. BMC Geriatr. 2010;17:70. doi: 10.1186/1471-2318-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres Bota D, Lopes Ferreira F, Melot C, Vincent JL. Body temperature alterations in the critically ill. Intensive Care Med. 2004;17:811–816. doi: 10.1007/s00134-004-2166-z. [DOI] [PubMed] [Google Scholar]
- Karalapillai D, Story DA, Calzavacca P, Licari E, Liu YL, Hart GK. Inadvertent hypothermia and mortality in postoperative intensive care patients: retrospective audit of 5050 patients. Anaesthesia. 2009;17:968–972. doi: 10.1111/j.1365-2044.2009.05989.x. [DOI] [PubMed] [Google Scholar]
- Laupland KB, Davies HD, Church DL, Louie TJ, Dool JS, Zygun DA, Doig CJ. Bloodstream infection-associated sepsis and septic shock in critically ill adults: A population-based study. Infection. 2004;17:59–64. doi: 10.1007/s15010-004-3064-6. [DOI] [PubMed] [Google Scholar]
- den Hartog AW, de Pont AC, Robillard LB, Binnekade JM, Schultz MJ, Horn J. Spontaneous hypothermia on intensive care unit admission is a predictor of unfavorable neurological outcome in patients after resuscitation: An observational cohort study. Crit Care. 2010;17:R121. doi: 10.1186/cc9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuzzo M, Moreno RP, Jordan B, Bauer P, Alvisi R, Metnitz PG. Predictors of early recovery of health status after intensive care. Intensive Care Med. 2006;17:1832–1838. doi: 10.1007/s00134-006-0307-2. [DOI] [PubMed] [Google Scholar]
- Ogura H, Gando S, Saitoh D, Takeyama N, Kushimoto S, Fujishima S, Mayumi T, Araki H, Ikeda H, Kotani J, Miki Y, Shiraishi S, Suzuki K, Suzuki Y, Takuma K, Tsuruta R, Yamaguchi Y, Yamashita N, Aikawa N. Epidemiology of severe sepsis in Japan: Results of a multicenter, prospective survey. J Infect Chemother. 2013;17: . doi: 10.1016/j.jiac.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Members of the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference committee: American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference. Definition for sepsis and organ failure and guidelines for the use innovative therapies in sepsis. Crit Care Med. 1992;17:864–874. doi: 10.1097/00003246-199206000-00025. [DOI] [PubMed] [Google Scholar]
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. SCCM/ESICM/ACCP/ATS/SIS: 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;17:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- Knaus WA, Draper EA, Wanger DP, Zimmerman JE. APACHE II: A severity classification system. Crit Care Med. 1985;17:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;17:1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- Taylor FBJ, Toh CH, Hoots WK, Wada H, Levi M. Toward definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;17:1327–1330. [PubMed] [Google Scholar]
- Marik PE, Zaloga GP. Norasept II study investigators. Hypothermia and cytokines in septic shock. Intensive Care Med. 2000;17:716–721. doi: 10.1007/s001340051237. [DOI] [PubMed] [Google Scholar]
- Clemmer TP, Fisher CJ Jr, Bone RC, Slotman GJ, Metz CA, Thomas FO. Hypothermia in the sepsis syndrome and clinical outcome. Crit Care Med. 1992;17:1395–1401. doi: 10.1097/00003246-199210000-00006. [DOI] [PubMed] [Google Scholar]
- Anonymous. Effect of high dose glucocorticoid therapy on mortality in patients with clinical signs of systemic sepsis. The veterans administration systemic sepsis cooperative study group. N Engl J Med. 1987;17:659–665. doi: 10.1056/NEJM198709103171102. [DOI] [PubMed] [Google Scholar]
- Sprung CL, Peduzzi PN, Shatney CH, Schein RM, Wilson MF, Sheagren JN, Hinshaw LB. Impact of encephalopathy on mortality in the sepsis syndrome. Crit Care Med. 1990;17:801–806. doi: 10.1097/00003246-199008000-00001. [DOI] [PubMed] [Google Scholar]
- Laupland KB, Zahar JR, Adrie C, Schwebel C, Goldgran-Toledano D, Azoulay E, Garrouste-Orgeas M, Cohen Y, Jamali S, Souweine B, Darmon M, Timsit JF. Determinants of temperature abnormalities and influence on outcome of critical illness. Crit Care Med. 2012;17:145–151. doi: 10.1097/CCM.0b013e31822f061d. [DOI] [PubMed] [Google Scholar]
- Lee BH, Inui D, Suh GY, Kim JY, Kwon JY, Park J, Tada K, Tanaka K, Ietsugu K, Uehara K, Dote K, Tajimi K, Morita K, Matsuo K, Hoshino K, Hosokawa K, Lee KH, Lee KM, Takatori M, Nishimura M, Sanui M, Ito M, Egi M, Honda N, Okayama N, Shime N, Tsuruta R, Nogami S, Yoon SH, Fujitani S, Koh SO, Takeda S, Saito S, Hong SJ, Yamamoto T, Yokoyama T, Yamaguchi T, Nishiyama T, Igarashi T, Kakihana Y, Koh Y. Fever and Antipyretic in Critically ill patients Evaluation (FACE) Study Group: Association of body temperature and antipyretic treatments with mortality of critically ill patients with and without sepsis: multi-centered prospective observational study. Crit Care. 2012;17:R33. doi: 10.1186/cc11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schortgen F, Clabault K, Katsahian S, Devaquet J, Mercat A, Deye N, Dellamonica J, Bouadma L, Cook F, Beji O, Brun-Buisson C, Lemaire F, Brochard L. Fever control using external cooling in septic shock: a randomized controlled trial. Am J Respir Crit Care Med. 2012;17:1088–1095. doi: 10.1164/rccm.201110-1820OC. [DOI] [PubMed] [Google Scholar]
- Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/north American multicenter study. JAMA. 1993;17:2957–2963. doi: 10.1001/jama.1993.03510240069035. [DOI] [PubMed] [Google Scholar]
- Moreno RP, Metnitz PG, Almeida E, Jordan B, Bauer P, Campos RA, Iapichino G, Edbrooke D, Capuzzo M, Le Gall JR. SAPS 3 Investigators. SAPS 3–From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;17:1345–1355. doi: 10.1007/s00134-005-2763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diringer MN, Reaven NL, Funk SE, Uman GC. Elevated body temperature independently contributes to increased length of stay in neurologic intensive care unit patients. Crit Care Med. 2004;17:1489–1495. doi: 10.1097/01.CCM.0000129484.61912.84. [DOI] [PubMed] [Google Scholar]


