Abstract
Objective
We conducted a prospective cohort study to estimate the incidence of mild cognitive impairment (MCI) by baseline neuropsychiatric status, in the setting of the Mayo Clinic Study of Aging.
Method
A classification of normal cognitive aging, MCI, and dementia was adjudicated by an expert consensus panel based on published criteria. Hazard ratios (HR) and 95% confidence intervals (95% CI) were computed using Cox proportional hazards model, with age as a time scale. Baseline Neuropsychiatric Inventory Questionnaire data were available on 1,587 cognitively normal persons who underwent at least one follow-up visit.
Results
We followed the cohort (N=1,587) to incident MCI (N=365) or censoring variables (N=179) for a median of 5 years. The following baseline neuropsychiatric symptoms significantly predicted incident MCI, after adjusting for age, sex, education and medical comorbidity: agitation (HR=3.06; 95% CI=1.89–4.93), apathy (HR=2.26; 95% CI=1.49–3.41), anxiety (HR=1.87; 95% CI=1.28–2.73), irritability (HR=1.84; 95% CI=1.31–2.58), and depression (HR=1.63; 95% CI=1.23–2.16). Delusion (HR=0.55; 95% CI=0.08–3.95) and hallucination (HR=1.48; 95% CI=0.37–5.99) did not predict incident MCI. A secondary analysis showed that euphoria (HR=11.3; 95% CI=3.44–37.2), disinhibition (HR=5.18; 95% CI=2.24–12.0) and nighttime behavior (HR=2.04; 95% CI=1.11–3.76) were significant predictors of non-amnestic MCI but not of amnestic MCI. By contrast, depression predicted amnestic MCI (HR=1.74; 95% CI=1.22–2.47) but not non-amnestic MCI (HR=1.18; 95% CI=0.64–2.16).
Conclusions
Non-psychotic symptoms predicted incident MCI. However, the associations between baseline euphoria, disinhibition, delusions, hallucinations, and the outcome of incident MCI should be considered preliminary since the observations were based on small number of events.
Mild cognitive impairment (MCI) is the intermediate stage between normal cognitive aging and dementia (1–3). Subjects with MCI constitute a high-risk group because they develop dementia at a rate of 10%–15% per year as compared to 1%–2% per year in the general population (4). Therefore, it is critical to understand the risk factors for MCI in order to intervene where possible.
Investigators have examined the outcome of incident dementia as determined by baseline neuropsychiatric symptoms among subjects with prevalent MCI (5–9). However, few studies examined the risk of incident MCI in a cognitively normal cohort by neuropsychiatric status at baseline (10–12). Therefore, we conducted a population-based study to estimate the risk of incident MCI among cognitively normal subjects with or without baseline neuropsychiatric symptoms.
Methods
Study Design
This is a prospective cohort study.
Setting
The Mayo Clinic Study of Aging is a population-based study (13) designed to estimate the prevalence (14) and incidence (15) of MCI in Olmsted County, Minnesota. Briefly, October 1, 2004, was selected as the prevalence date and elderly individuals were recruited by using a stratified random sampling from the target population of nearly 10,000 elderly individuals residing in Olmsted County (16). After complete description of the study to the subjects, written informed consent was obtained. The study was conducted with the approval of the Institutional Review Boards of the Mayo Clinic and Olmsted Medical Center in Rochester, Minnesota.
Cognitive Evaluation
Each participant underwent the following three face-to-face evaluations: 1) neurological evaluation by a physician; 2) risk factor assessment by a nurse or study coordinator; and 3) neuropsychological testing that was interpreted by a neuropsychologist. The interview by the nurse or study coordinator included administration of the Clinical Dementia Rating Scale (17) to the participant and to an informant. The neurological evaluation was performed by a physician and included administration of the Short Test of Mental Status (18), medical history review, and a complete neurological examination.
Neuropsychological testing was performed to assess four cognitive domains: 1) memory (Logical Memory-II [delayed recall] and Visual Reproduction-II [delayed recall] from Wechsler Memory Scale-Revised, and delayed recall from the Auditory Verbal Learning Test) (19–22); 2) executive function (Trail Making Test B (23), and Digit Symbol Substitution from Wechsler Adult Intelligent Scale-Revised); 3) language (Boston Naming Test (24), and category fluency) (25); and 4) visuospatial skills (Picture Completion and Block Design from WAIS-R). The raw neuropsychological test scores were transformed to age-adjusted scores, and were scaled to have a mean of 10 and a SD of 3 in reference to a normative data of Mayo’s Older American Normative Studies (26). Cognitive domain scores were obtained for each subject; additionally we calculated z-scores in order to make comparisons across the four cognitive domains. Each person’s domain score was compared to the mean (SD) from Mayo’s Older American Normative Studies. Thus, a z score of ≥1.0 below the mean in a specific domain, e.g., memory domain, indicated memory impairment. However, the final decision about impairment in any cognitive domain was made during the weekly consensus panel of the research team that includes physicians, neuropsychologists and research nurses.
MCI Criteria
We used the revised Mayo Clinic criteria for MCI: 1) cognitive concern expressed by a physician, informant, participant, or nurse; 2) cognitive impairment in one or more domains (executive function, memory, language, or visuospatial); 3) normal functional activities; and 4) not demented (27, 28). Subjects with MCI could have a Clinical Dementia Rating Scale score of 0 or 0.5; however, the final diagnosis of MCI was not based exclusively on the clinical dementia rating, but rather on all available data. The diagnosis of normal cognition, MCI, dementia, or Alzheimer’s disease was made by an expert consensus panel of physicians, psychologists, and nurses based on published criteria (1, 13, 28–30). The panel meets once per week and reviews three independent sources of data, i.e., the clinical data collected by behavioral neurologists and physicians of other specialties with expertise in dementia and MCI, neuropsychological data collected by psychometrists who are supervised by neuropsychologists, and nursing data gathered by research nurses (13).
MCI Subtypes
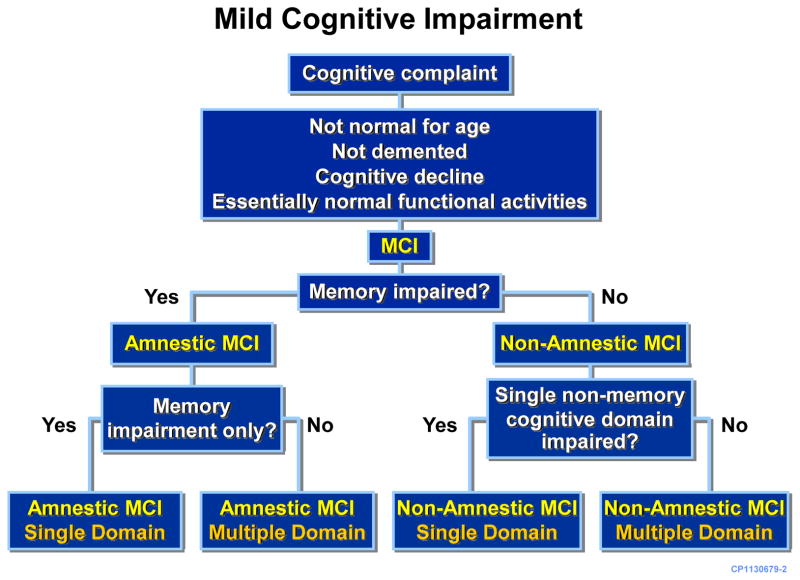
Subjects that met the criteria for MCI were further classified as having amnestic or non-amnestic MCI, based on whether memory domain was impaired or not. Additionally, subjects were further classified as having single or multiple domain MCI according to the number of domains that were impaired (27), e.g., a subject with impairment of memory domain only as defined by z score of ≥1.0 below the mean would be classified to have amnestic MCI, single-domain type whereas a subject with impairments of both memory and attention domains would be classified as having amnestic MCI, multiple-domain type. Furthermore, a subject with impairment in attention domain only would be classified as having non-amnestic MCI, single-domain type whereas if both attention and language domains were impaired then the subject would be classified as having non-amnestic MCI, multiple-domain type (Figure 1).
Figure 1.
Neuropsychiatric Assessment
We assembled a cohort of cognitively normal persons on whom Neuropsychiatric Inventory Questionnaire (NPI-Q) data were available. The exposed cohort consisted of cognitively normal persons with one or more neuropsychiatric symptoms at baseline. The outcome of interest was incident MCI as measured by modified Mayo Clinic criteria (27). The baseline administration of the NPI-Q took place between October 1, 2004, and September 1, 2007. We have previously reported the population-based prevalence of baseline neuropsychiatric symptoms in MCI and normal cognitive aging (31). At baseline, MCI subjects were excluded for the current incidence study. There were 1,640 cognitively normal persons; however, NPI-Q data were not available for 53 participants. Thus, baseline NPI-Q data were available for 1,587 cognitively normal persons. Because 35 subjects died and 144 were lost to follow-up before the first follow-up visit, our analyses included a total of 1,408 subjects.
The NPI-Q was administered as a structured interview to a spouse or an informant of each study participant (32). The NPI-Q is a shorter version of Neuropsychiatric Inventory (NPI) which is a structured interview with established reliability and validity (33). Both NPI and NPI-Q measure 12 emotional behavioral domains. We used the NPI-Q because it was selected by the Uniform Data Set initiative of the National Institute on Aging (34).
The structured interview addressed 12 neuropsychiatric domains, i.e., agitation, delusion, hallucination, depression, anxiety, euphoria, apathy, disinhibition, irritability, aberrant motor behavior, sleep, and eating/appetite. The categorical outcome of the presence or absence of a neuropsychiatric symptom was documented and served as the exposure of interest of the study. Our primary goal was to determine the risk of incident MCI based on the presence or absence of baseline neuropsychiatric symptoms but not determining the severity of neuropsychiatric symptoms. This goal was generated from our previous study derived from a clinical sample (10); wherein we examined whether the presence or absence of baseline depression predicted the risk of incident MCI. Therefore, we sought to estimate a population-based risk of incident MCI by baseline presence or absence of neuropsychiatric symptoms, and we did not investigate the severity of neuropsychiatric symptoms.
Statistical Analyses
We conducted cohort analyses to determine the risk of incident MCI among cognitively normal subjects with or without a specific neuropsychiatric symptom at baseline. We computed hazards ratios (HR) and 95% confidence intervals (95% CI) using Cox Proportional Hazards model. The HR (95% CI) for each neuropsychiatric symptom quantified the risk of developing incident MCI associated with a specific symptom at baseline after adjusting for age, sex, education, and medical comorbidity (35). The Charlson Comorbidity Index was calculated by using Deyo’s method wherein numeric values were assigned to comorbid medical conditions, e.g., a score of 1 was assigned for congestive heart failure and a score of 6 assigned for malignant tumor. A composite index was then calculated by using Deyo’s method of Charlson index (35, 36). Adjusting for age, sex, education and medical comorbidity ensured that baseline neuropsychiatric symptoms predicted incident MCI over and above that can be explained by these potential confounders. We also conducted secondary analyses for MCI subtypes by separating amnestic versus non-amnestic MCI.
Statistical testing was done at the conventional two-tailed alpha level of 0.05. All analyses were performed using SAS® (Cary, NC).
Results
Demographic description of the sample is displayed in Table 1. We followed the cohort of cognitively normal persons with NPI-Q data (N=1, 587), to the outcomes of incident MCI (N=365) or censoring events (death [N=35]; loss to longitudinal follow-up [n=144]) for a median (interquartile range [IQR]) of 5.0 [3.8, 5.3] years. At baseline, there were differences in the frequency of neuropsychiatric symptoms by sex. There were more men than women in the agitation, apathy, irritability, and disinhibition groups whereas there were more women than men in the depression, anxiety, and euphoria groups. The median (IQR) age of the cohort was 79.3 (75.0, 83.4) years. The median (IQR) years of education was 13 (12, 16) years. The median (IQR) number of comorbid medical conditions was 3 (1, 5) as measured by Charlson index.
TABLE 1.
Demographics Characteristics of Study Participants by Baseline Non-Psychotic Neuropsychiatric Symptoms
| Total (N=1,408) |
Depression Cohort (N=153) |
Apathy Cohort (N=57) |
Anxiety Cohort (N=66) |
Agitation Cohort (N=33) |
Irritability Cohort (N=96) |
Appetite/Eating Cohort (N=67) |
Motor Disturbance Cohort (N=7) |
Nighttime Behaviors Cohorta (N=122) |
|
|---|---|---|---|---|---|---|---|---|---|
| Male Gender
| |||||||||
| N | 704 | 72 | 33 | 28 | 20 | 62 | 3 | 4 | 68 |
| % | 50.0 | 47.1 | 57.9 | 42.4 | 60.6 | 64.6 | 64.2 | 57.1 | 55.7 |
|
| |||||||||
| Age (years)
| |||||||||
| Median | 79.3 | 79.8 | 79.1 | 81.3 | 79.1 | 79.3 | 81.5 | 79.4 | 80.2 |
| IQR | 75.0, 83.4 | 75.2, 83.6 | 76.2, 82.7 | 75.9, 83.9 | 75.3, 82.7 | 75.1, 83.3 | 76.9, 84.3 | 72.8, 83.1 | 75.2, 82.6 |
| 70–79 | |||||||||
| N | 741 | 79 | 31 | 30 | 20 | 52 | 27 | 4 | 61 |
| % | 52.6 | 51.6 | 54.4 | 45.5 | 60.6 | 54.2 | 40.3 | 57.1 | 50.0 |
| 80–91 | |||||||||
| N | 667 | 74 | 26 | 36 | 13 | 44 | 40 | 3 | 61 |
| % | 47.4 | 48.4 | 45.6 | 54.5 | 39.4 | 45.8 | 59.7 | 42.9 | 50.0 |
|
| |||||||||
| Education (years)
| |||||||||
| Median | 13 | 12 | 13 | 13 | 13 | 13 | 13 | 12 | 13 |
| IQR | 12, 16 | 12, 15 | 12, 16 | 12, 16 | 12, 16 | 12, 16 | 12, 16 | 12, 17 | 12, 16 |
| >12 Years | |||||||||
| N | 801 | 74 | 29 | 36 | 20 | 50 | 38 | 3 | 70 |
| % | 56.9 | 48.4 | 50.9 | 54.5 | 60.6 | 52.1 | 56.7 | 42.9 | 57.4 |
|
| |||||||||
| Charlson Index
| |||||||||
| Median | 3 | 3 | 4 | 3 | 4 | 3 | 4 | 2 | 3 |
| IQR | 1,5 | 2,5 | 2, 8 | 2, 5 | 2, 6 | 1, 5 | 2, 7 | 2, 6 | 2, 6 |
|
| |||||||||
| Time in Study (years)
| |||||||||
| Median | 5.03 | 4.5 | 4.1 | 4.5 | 4.3 | 4.6 | 4.5 | 4.1 | 5.1 |
| IQR | 5.3, 8.0 | 2.9, 5.2 | 2.7, 5.2 | 3.2, 5.2 | 3.1, 5.2 | 2.9, 5.2 | 3.6, 5.3 | 3.9, 5.3 | 3.1, 5.3 |
|
| |||||||||
| Incident MCI
| |||||||||
| N | 364 | 59 | 25 | 30 | 18 | 38 | 25 | 3 | 38 |
| % | 25.9 | 38.6 | 43.9 | 45.5 | 54.5 | 39.6 | 37.3 | 42.9 | 31.1 |
| Rateb | 68 | 109 | 142 | 138 | 186 | 119 | 103 | 116 | 86 |
| 95% CI | 61, 76 | 83, 141 | 92, 210 | 93, 197 | 110, 295 | 85, 164 | 67, 152 | 24, 338 | 61, 118 |
Abbreviations: CI, confidence interval; IQR, interquartile range.
N (%), unless otherwise indicated.
Each p value is for the neuropsychiatric cohort versus its referent cohort (referent cohort columns not shown).
271 subjects did not have nighttime behaviors assessment available (informant unable to assess).
Age- and sex-standardized incidence rate of MCI (per 1,000 person-years).
We used person-years and survival analyses to calculate the incidence of MCI as predicted by baseline neuropsychiatric status. Thus, the age-sex standardized incidence rate of MCI was 68 per 1,000 person-years. After adjusting for age, sex, education, and medical comorbidity, we observed that the following baseline neuropsychiatric symptoms significantly predicted incident MCI: agitation (HR=3.06; 95% CI=1.89–4.93; p<0.001), apathy (HR=2.26; 95% CI=1.49–3.41; p<0.001), anxiety (HR=1.87; 95% CI=1.28–2.73; p<0.001), irritability (HR=1.84; 95% CI=1.31–2.58; p<0.001), and depression (HR=1.63; 95% CI=1.23–2.16; p<0.001). Baseline delusion and hallucination did not predict incident MCI. There were substantial missing data for nighttime behavior (missing data for 271 subjects); thus, the HR of nighttime behavior (HR=1.46; 95% CI=1.03–2.06; p=0.033) should be interpreted with caution. Even though euphoria (HR=5.10; 95% CI=2.24–11.6); p<0.001) and disinhibition (HR=2.59; 95% CI=1.42–4.73); p=0.002) were significant predictors of incident MCI, these analyses were based on relatively small events. For example, there were only seven cognitively normal persons with baseline euphoria, out of whom six developed incident MCI during subsequent follow-up. Similarly, there were only 22 cognitively normal persons with baseline disinhibition, out of who 11 developed incident MCI. Details of these findings are displayed in Table 2. The four most frequent neuropsychiatric symptoms at baseline were agitation, apathy, depression, and anxiety. At baseline, no one had all four symptoms simultaneously. Only one person had apathy, agitation, and anxiety at the same time at baseline. This person developed incident MCI during follow-up. Twenty-eight persons had comorbid depression and apathy; 10 of them developed incident MCI during subsequent follow-up.
TABLE 2.
Demographics Characteristics of Study Participants by Baseline Psychotic Symptoms and Other Emotional Behaviors
| Disinhibition Cohort (N=22) | Euphoria Cohort (N=7) | Delusions Cohort (N=5) | Hallucinations Cohort (N=5) | |
|---|---|---|---|---|
| Male Gender
| ||||
| N | 12 | 3 | 2 | 3 |
| % | 54.5 | 42.9 | 40.0 | 60.0 |
|
| ||||
| Age (years)
| ||||
| Median | 80.3 | 81.3 | 80.9 | 86.2 |
| IQR | 76.2, 84.3 | 78.0, 82.0 | 78.4, 83.5 | 82.7, 86. |
| 70–79 | ||||
| N | 9 | 3 | 2 | 0 |
| % | 40.9 | 42.9 | 40.0 | 0.0 |
| 80–91 | ||||
| N | 13 | 4 | 3 | 5 |
| % | 59.1 | 57.1 | 60.0 | 100.0 |
|
| ||||
| Education (years)
| ||||
| Median | 12 | 16 | 13 | 13 |
| IQR | 12, 14 | 13, 16 | 13, 1 | 13, 14 |
| >12 Years | ||||
| N | 10 | 6 | 4 | 4 |
| % | 45.5 | 85.7 | 80.0 | 80.0 |
|
| ||||
| Charlson Index
| ||||
| Median | 3.5 | 4 | 3 | 4 |
| IQR | 2, 5 | 3, 4 | 1, 5 | 4, 5 |
|
| ||||
| Time in Study (years)
| ||||
| Median | 3.0 | 5.4 | 2.7 | 2.9 |
| IQR | 2.6, 5.2 | 3.1, 5.4 | 2.7, 5.2 | 2.7, 4.2 |
|
| ||||
| Incident MCI
| ||||
| N | 11 | 6 | 1 | 2 |
| % | 50.0 | 85.7 | 20.0 | 40.0 |
| Rateb | 177 | 265 | 55 | 162 |
| 95% CI | 89, 317 | 97, 576 | 1, 308 | 20, 583 |
Abbreviations: CI, confidence interval; IQR, interquartile range.
N (%), unless otherwise indicated.
Each p value is for the neuropsychiatric cohort versus its referent cohort (referent cohort columns not shown).
271 subjects did not have nighttime behaviors assessment available (informant unable to assess).
Age- and sex-standardized incidence rate of MCI (per 1,000 person-years).
Secondary Analyses
The primary outcome of interest was incident MCI. We conducted secondary analyses to examine whether neuropsychiatric symptoms differentially predicted amnestic versus non-amnestic MCI (Tables 3 and 4). Euphoria (HR=11.3; 95% CI=3.44–37.2; p<0.001) and disinhibition (HR=5.18, 95% CI=2.24–12.0; p<0.001) were significant predictors of non-amnestic MCI. However, neither disinhibition (HR=1.48; 95% CI=0.55–4.00; p=0.44) nor euphoria (HR=2.41; 95% CI=0.59–9.83; p=0.22) significantly predicted amnestic MCI. Nighttime behavior was a significant predictor for non-amnestic MCI (HR=2.04; 95% CI=1.11–3.76; p=0.021) but not for amnestic MCI (HR=1.44; 95% CI=0.93–2.25; p=0.10). Depression predicted amnestic MCI (HR=1.74; 95% CI=1.22–2.47; p=0.002) but not non-amnestic MCI (HR=1.18; 95% CI=0.64–2.16; p=0.60). Apathy predicted both amnestic (HR=1.93; 95% CI=1.09–3.41; p=0.023); and non-amnestic MCI (HR=3.19; 95% CI=1.62–6.26; p<0.001). Additional findings are displayed in Tables 3 and 4.
TABLE 3.
Risk of Incident MCI by Baseline Non-Psychotic Neuropsychiatric Symptoms
| Psychiatric Symptom | HR (95% CI)a | pa | HR (95% CI)b | pb |
|---|---|---|---|---|
| Total MCI
| ||||
| Depression | 1.68 (1.27–2.22) | <0.001 | 1.63 (1.23–2.16) | <0.001 |
| Apathy | 2.46 (1.63–3.70) | <0.001 | 2.26 (1.49–3.41) | <0.001 |
| Anxiety | 1.91 (1.31–2.78) | <0.001 | 1.87 (1.28–2.73) | 0.001 |
| Agitation | 3.13 (1.94–5.05) | <0.001 | 3.06 (1.89–4.93) | <0.001 |
| Irritability | 1.85 (1.32–2.60) | <0.001 | 1.84 (1.31–2.58) | <0.001 |
| Appetite/Eating | 1.44 (0.96–2.17) | 0.08 | 1.34 (0.89–2.02) | 0.16 |
| Motor disturbance | 1.63 (0.52–5.11) | 0.40 | 1.60 (0.51–5.00) | 0.42 |
| Nighttime behaviors | 1.48 (1.05–2.08) | 0.027 | 1.46 (1.03–2.06) | 0.033 |
|
| ||||
| Amnestic MCI
| ||||
| Depression | 1.75 (1.23–2.48) | 0.002 | 1.74 (1.22–2.47) | 0.002 |
| Apathy | 1.98 (1.13–3.47) | 0.018 | 1.93 (1.09–3.41) | 0.023 |
| Anxiety | 1.65 (0.99–2.76) | 0.05 | 1.64 (0.98–2.74) | 0.06 |
| Agitation | 2.18 (1.07–4.44) | 0.032 | 2.16 (1.06–4.41) | 0.033 |
| Irritability | 1.69 (1.09–2.64) | 0.020 | 1.69 (1.08–2.63) | 0.021 |
| Appetite/Eating | 1.09 (0.61–1.95) | 0.78 | 1.06 (0.59–1.91) | 0.85 |
| Motor disturbance | 0.84 (0.12–6.01) | 0.86 | 0.84 (0.12–5.97) | 0.86 |
| Nighttime behaviors | 1.44 (0.93–2.24) | 0.11 | 1.44 (0.93–2.25) | 0.10 |
|
| ||||
| Non-amnestic MCI
| ||||
| Depression | 1.26 (0.68–2.31) | 0.46 | 1.18 (0.64–2.16) | 0.60 |
| Apathy | 3.81 (1.97–7.38) | <0.001 | 3.19 (1.62–6.26) | <0.001 |
| Anxiety | 2.84 (1.50–5.35) | 0.001 | 2.74 (1.45–5.16) | 0.002 |
| Agitation | 5.14 (2.46–10.7) | <0.001 | 4.92 (2.36–10.3) | <0.001 |
| Irritability | 2.18 (1.18–4.02) | 0.013 | 2.18 (1.18–4.03) | 0.012 |
| Appetite/Eating | 1.52 (0.70–3.30) | 0.29 | 1.31 (0.60–2.85) | 0.50 |
| Motor disturbance | 4.12 (1.00–16.9) | 0.049 | 3.89 (0.94–16.0) | 0.06 |
| Nighttime behaviors | 2.11 (1.15–3.88) | 0.016 | 2.04 (1.11–3.76) | 0.021 |
Adjusted for age (scale), sex, education.
Additionally adjusted for medical comorbidity.
TABLE 4.
Risk of Incident MCI by Baseline Psychotic Symptoms and Other Emotional Behaviors
| Psychiatric Symptoms | HR (95% CI)a | pa | HR (95% CI)b | pb |
|---|---|---|---|---|
| Total MCI
| ||||
| Disinhibition | 2.60 (1.42–4.75) | 0.002 | 2.59 (1.42–4.73) | 0.002 |
| Euphoria | 5.07 (2.23–11.5) | <0.001 | 5.10 (2.24–11.6) | <0.001 |
| Delusions | 0.60 (0.08–4.27) | 0.61 | 0.55 (0.08–3.95) | 0.55 |
| Hallucinations | 1.57 (0.39–6.37) | 0.52 | 1.48 (0.37–5.99) | 0.58 |
|
| ||||
| Amnestic MCI
| ||||
| Disinhibition | 1.49 (0.55–4.01) | 0.43 | 1.48 (0.55–4.00) | 0.44 |
| Euphoria | 2.42 (0.59–9.84) | 0.22 | 2.41 (0.59–9.83) | 0.22 |
| Delusions | 1.02 (0.14–7.34) | 0.98 | 1.00 (0.14–7.15) | 1.00 |
| Hallucinations | 1.32 (0.18–9.52) | 0.78 | 1.30 (0.18–9.34) | 0.80 |
|
| ||||
| Non-amnestic MCI
| ||||
| Disinhibition | 5.22 (2.26–12.0) | <0.001 | 5.18 (2.24–12.0) | <0.001 |
| Euphoria | 10.7 (3.27–35.1) | <0.001 | 11.3 (3.44–37.2) | <0.001 |
| Delusions | NA | 0.99 | NA | 0.99 |
| Hallucinations | 3.10 (0.42–22.7) | 0.27 | 2.76 (0.38–20.3) | 0.32 |
Adjusted for age (scale), sex, education.
Additionally adjusted for medical comorbidity.
Discussion
Here we report the population-based risk of incident MCI as predicted by baseline neuropsychiatric symptoms among cognitively normal persons. At baseline there were sex differences in the frequency of neuropsychiatric symptoms, i.e., more men than women were observed to have agitation, apathy, irritability and disinhibition whereas more women than men were observed to have depression, anxiety, and euphoria. These findings were by and large consistent with previously reported observations, e.g., a study in Helsinki reported a slightly higher rate of apathy in men than women (37), a Japanese study reported that physical agitation but not verbal agitation was higher in men than women (38), several studies including the Cache County study (39), and large scale epidemiological studies (40, 41) reported that depression is higher in women than women. Furthermore, factoring in neuropsychiatric symptoms has not substantially altered the age-sex standardized incidence rate of MCI that was previously reported by our research group (15). The reader is referred to our previous publication (15) for a detailed discussion of the incidence of MCI wherein we indicated that few studies reported age-sex standardized incidence rates (42, 43).
We observed that non-psychotic symptoms strongly predicted incident MCI. How do these neuropsychiatric symptoms compare with genetic, biomarker, and demographic predictors of incident MCI? Such comparisons are best done with studies that utilized similar if not identical methods with that of our study. Therefore, here we compare our findings with the biomarker predictors of incident MCI reported by our colleagues that specialize in the imaging work of the Mayo Clinic Study of Aging. Our imaging team reported that the HR (95% CI) for hippocampal volume (as measured by brain MRI) in predicting incident MCI was HR=1.8 (95% CI=1.4–2.20) (44) whereas here we report that the HR (95% CI) for apathy in predicting incident MCI is HR=2.26 (95% CI=1.49–3.41), and it is even higher for agitation (HR=3.06; 95% CI=1.89–4.93). This is an informative comparison because the difference in the strength of predicting incident MCI by a biomarker versus a neuropsychiatric symptom cannot simply be attributed to methodological difference because both the imaging and neuropsychiatric research took place in the context of the Mayo Clinic Study of Aging. Similarly, the risk of incident MCI given exposure to baseline neuropsychiatric symptoms was as strong as or even stronger than APOE ε4 (10), comorbid medical conditions (45) or demographic variables such as lower education (15).
Delusions and hallucinations did not predict incident MCI. Even though euphoria and disinhibition were significant predictors of incident MCI, their risk estimates were based on few subjects. There were only seven cognitively normal persons with baseline euphoria, out of whom six developed incident MCI. Similarly, there were 22 cognitively normal persons with baseline disinhibition, out of whom 11 developed incident MCI. In view of these reported small events, the observed associations between these rarely reported neuropsychiatric symptoms and MCI should be considered preliminary until confirmed by future studies.
A secondary analysis showed that euphoria and disinhibition were significant predictors of non-amnestic MCI but not of amnestic MCI. Given the small number of participants that reported these symptoms, at best we can only hypothesize that disinhibition and euphoria at baseline in a cognitively normal elderly person may increase the risk of non-amnestic MCI that may progress to fronto-temporal dementia. Similarly, nighttime behavior was a significant predictor of non-amnestic MCI but not of amnestic MCI, and these subjects may progress to dementia with Lewy bodies (46).
Few studies have investigated the prediction of incident MCI by baseline neuropsychiatric symptoms (10–12). Most studies examined the prediction of incident dementia by baseline neuropsychiatric symptoms (7, 47). The Sydney Memory and Ageing Study recently reported the prediction of cognitive impairment by baseline neuropsychiatric symptoms in 879 subjects aged 70–90 years. Consistent with our study, they measured baseline neuropsychiatric symptoms by using the neuropsychiatric inventory (34). The Australian investigators defined cognitive impairment by diagnostic category (prevalent MCI or incident dementia) or by neuropsychological performance. They followed the cohort of cognitively normal persons and subjects with prevalent MCI over a period of 2 years to the outcomes of cognitive decline defined as worse neuropsychological performance or incident dementia. They observed that agitation and anxiety predicted cognitive decline (12). The Sydney investigators also observed that agitation, apathy, irritability, and anxiety were associated with prevalent MCI. A study that examined the outcome of incident MCI by baseline neuropsychiatric symptoms would be the ideal one to compare with our study. The Chicago Health and Aging Study examined the outcome of incident MCI as predicted by baseline status of proneness to chronic psychological distress as measured by the NEO Personality Inventory (48). They observed that a “distress prone” elderly person at baseline was 40% more likely to develop incident MCI than a person who reported to be less distress prone (11). The construct of chronic proneness to psychological distress is not identical with the neuropsychiatric construct as measured by the Neuropsychiatric Inventory Questionnaire; however, both instruments measured emotional behavior among a cohort of elderly persons that were recruited for cognitive research. Thus, we can suggest that emotional behavior at baseline in a cognitively person may be associated with increased risk of MCI.
We did not investigate the possible mechanisms linking baseline neuropsychiatric symptoms with incident MCI. In the past, we have proposed possible explanations for the link between baseline depression and the outcome of incident MCI (10). It is possible that baseline neuropsychiatric symptoms could be the non-cognitive manifestation of the underlying neurodegenerative disorder (reverse causality). Alternatively, an underlying neuropathology may be causing both cognitive and emotional behavior manifestations (shared etiology model). The third possibility is that a synergistic interaction between neuropsychiatric symptoms and a biological factor (e.g., APOE ε4 genotype) may lead to clinical outcomes such as MCI.
Our findings should be interpreted in light of the strengths and weaknesses of the study. There are several strengths. First, we conducted our study in a population-based setting, involving a large cohort that was followed for several years; thus, our findings are less prone to referral bias (49–51). Second, we were able to examine a spectrum of emotional behavior by investigating several neuropsychiatric symptoms as predictors of incident MCI. Third, we measured MCI using a face-to-face evaluation adjudicated by an expert consensus panel at a center that has a well established reputation for measuring MCI. On the other hand, our study also has limitations. The NPI/NPI-Q gathers information from an informant who is knowledgeable about the participant. In our sample, 90% of the informants were spouses. Even though such data have the advantage of being observed behaviors, the informant may not be able to recognize subtle signs. However, other studies, e.g., the Sydney Aging and Memory Study, that used NPI also reported similar results, e.g., agitation and anxiety predicted cognitive decline both in the Sydney study and our study. Even though our study’s goal of examining the presence or absence of a baseline neuropsychiatric symptom in predicting incident MCI addresses a clinically relevant important question, it is possible that factoring in severity of symptoms might have added more depth to our findings.
In summary, in this population-based study, we assembled a cohort of cognitively normal persons on whom we acquired baseline neuropsychiatric symptoms data. We then followed the cognitively normal cohort forward in time to the outcomes of incident MCI or censoring events. Non-psychotic neuropsychiatric symptoms at baseline were significant predictors of incident MCI. Euphoria, disinhibition, and nighttime behavior predicted incident non-amnestic MCI but not amnestic MCI. Psychotic symptoms (delusions and hallucinations) predicted neither amnestic nor non-amnestic MCI.
Acknowledgments
Funding/Support: This research was supported by National Institutes of Health (K01 MH068351, U01 AG006786 and K01 AG028573), RR024150 (Mayo Clinic CTSA [Career Transition Award]), the RWJ Foundation (Harold Amos Scholar), Robert H. and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program; and in part supported by the European Regional Development Fund - Project FNUSA-ICRC (No. CZ.1.05/1.1.00/02.0123), the Czech Ministry of Health (NT13434–4/20012), and the Mayo Clinic Center for Individualized Medicine.
Footnotes
Financial Disclosures: No potential conflicts of interest relevant to this article were reported.
Data Access and Responsibility: Dr. Geda (the Principal Investigator who is a full-time employee of the College of Medicine, Mayo Clinic) had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 2.Grundman M, Petersen RC, Ferris SH, Thomas RG, Aisen PS, Bennett DA, Foster NL, Jack CR, Jr, Galasko DR, Doody R, Kaye J, Sano M, Mohs R, Gauthier S, Kim HT, Jin S, Schultz AN, Schafer K, Mulnard R, van Dyck CH, Mintzer J, Zamrini EY, Cahn-Weiner D, Thal LJ. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey A, Pfeiffer E, Sano M, van Dyck CH, Thal LJ. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 5.Richard E, Schmand B, Eikelenboom P, Yang SC, Ligthart SA, Moll van Charante EP, van Gool WA. Symptoms of apathy are associated with progression from mild cognitive impairment to Alzheimer’s disease in non-depressed subjects. Dement Geriatr Cogn Disord. 2012;33:204–209. doi: 10.1159/000338239. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell RA, Herrmann N, Lanctot KL. The role of dopamine in symptoms and treatment of apathy in Alzheimer’s disease. CNS Neurosci Ther. 2011;17:411–427. doi: 10.1111/j.1755-5949.2010.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmer K, Di Iulio F, Varsi AE, Gianni W, Sancesario G, Caltagirone C, Spalletta G. Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer’s disease: the role of depression and apathy. J Alzheimers Dis. 2010;20:175–183. doi: 10.3233/JAD-2010-1352. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg PB, Mielke MM, Appleby B, Oh E, Leoutsakos JM, Lyketsos CG. Neuropsychiatric symptoms in MCI subtypes: the importance of executive dysfunction. Int J Geriatr Psychiatry. 2011;26:364–372. doi: 10.1002/gps.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramakers IH, Visser PJ, Aalten P, Kester A, Jolles J, Verhey FR. Affective symptoms as predictors of Alzheimer’s disease in subjects with mild cognitive impairment: a 10-year follow-up study. Psychol Med. 2010;40:1193–1201. doi: 10.1017/S0033291709991577. [DOI] [PubMed] [Google Scholar]
- 10.Geda YE, Knopman DS, Mrazek DA, Jicha GA, Smith GE, Negash S, Boeve BF, Ivnik RJ, Petersen RC, Pankratz VS, Rocca WA. Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment: a prospective cohort study. Arch Neurol. 2006;63:435–440. doi: 10.1001/archneur.63.3.435. [DOI] [PubMed] [Google Scholar]
- 11.Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68:2085–2092. doi: 10.1212/01.wnl.0000264930.97061.82. [DOI] [PubMed] [Google Scholar]
- 12.Brodaty H, Heffernan M, Draper B, Reppermund S, Kochan NA, Slavin MJ, Trollor JN, Sachdev PS. Neuropsychiatric symptoms in older people with and without cognitive impairment. J Alzheimers Dis. 2012;31:411–420. doi: 10.3233/JAD-2012-120169. [DOI] [PubMed] [Google Scholar]
- 13.Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, Ivnik RJ, Tangalos EG, Petersen RC, Rocca WA. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, Boeve BF, Tangalos EG, Ivnik RJ, Rocca WA. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology. 2010;75:889–897. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, Tangalos EG, Ivnik RJ, Rocca WA, Petersen RC. The incidence of MCI differs by subtype and is higher in men: the Mayo Clinic Study of Aging. Neurology. 2012;78:342–351. doi: 10.1212/WNL.0b013e3182452862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. Am J Epidemiol. 2011;173:1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 18.Kokmen E, Smith GE, Petersen RC, Tangalos E, Ivnik RC. The Short Test of Mental Status: Correlations With Standardized Psychometric Testing. Arch Neurol. 1991;48:725–728. doi: 10.1001/archneur.1991.00530190071018. [DOI] [PubMed] [Google Scholar]
- 19.Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC, Kokmen E, Kurland LT. Mayo’s Older Americans Normative Studies: WAIS-R norms for ages 56 to 97. Clin Neuropsychol. 1992;6:1–30. [Google Scholar]
- 20.Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC, Kokmen E, Kurland LT. Mayo’s Older Americans Normative Studies: WMS-R norms for ages 56 to 94. Clin Neuropsychol. 1992;6:49–82. [Google Scholar]
- 21.Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC, Kokmen E, Kurland LT. Mayo’s Older Americans Normative Studies: Updated AVLT norms for ages 56 to 97. Clin Neuropsychol. 1992;6:83–104. [Google Scholar]
- 22.Malec JF, Ivnik RJ, Smith GE, Tangalos EG, Petersen RC, Kokmen E, Kurland LT. Mayo’s Older Americans Normative Studies: Utility of corrections for age and education for the WAIS-R. Clin Neuropsychol. 1992;6:31–47. [Google Scholar]
- 23.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 24.Kaplan E, Goodglass H, Brand S. Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 25.Lucas JA, Ivnik RJ, Smith GE, Bohac DL, Tangalos EG, Graff-Radford NR, Petersen RC. Mayo’s Older Americans Normative Studies: category fluency norms. J Clin Exp Neuropsychol. 1998;20:194–200. doi: 10.1076/jcen.20.2.194.1173. [DOI] [PubMed] [Google Scholar]
- 26.Ivnik R, Malec J, Smith G, Tangalos E, Petersen R, Kokmen E, Kurland L. Mayo’s Older Americans Normative Studies: WAIS-R, WMS-R, and AVLT norms for ages 56 through 97. Clin Neuropsychol. 1992;6:1–104. [Google Scholar]
- 27.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 28.Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, van Duijn C, Visser P, Petersen RC. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 29.American Psychiatric Association, American Psychiatric Association. Task Force on DSM-IV. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 30.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA work group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 31.Geda YE, Roberts RO, Knopman DS, Petersen RC, Christianson TJ, Pankratz VS, Smith GE, Boeve BF, Ivnik RJ, Tangalos EG, Rocca WA. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry. 2008;65:1193–1198. doi: 10.1001/archpsyc.65.10.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, Lopez OL, DeKosky ST. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 33.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 34.Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, Beekly D, Ramos EM, Kukull WA. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 35.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 36.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 37.Hölttä EH, Laakkonen M-L, Laurila JV, Strandberg TE, Tilvis RS, Pitkälä KH. Apathy: Prevalence, Associated Factors, and Prognostic Value Among Frail, Older Inpatients. Journal of the American Medical Directors Association. 2012;13:541–545. doi: 10.1016/j.jamda.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Schreiner AS. Aggressive behaviors among demented nursing home residents in Japan. Int J Geriatr Psychiatry. 2001;16:209–215. doi: 10.1002/1099-1166(200102)16:2<209::aid-gps314>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 39.Steffens DC, Norton MC, Hart AD, Skoog I, Corcoran C, Breitner JC. Apolipoprotein E genotype and major depression in a community of older adults. The Cache County Study. Psychol Med. 2003;33:541–547. doi: 10.1017/s0033291702007201. [DOI] [PubMed] [Google Scholar]
- 40.Kessler RC, McGonagle KA, Nelson CB, Hughes M, Swartz M, Blazer DG. Sex and depression in the National Comorbidity Survey. II: Cohort effects. J Affect Disord. 1994;30:15–26. doi: 10.1016/0165-0327(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 41.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caracciolo B, Palmer K, Monastero R, Winblad B, Backman L, Fratiglioni L. Occurrence of cognitive impairment and dementia in the community: a 9-year-long prospective study. Neurology. 2008;70:1778–1785. doi: 10.1212/01.wnl.0000288180.21984.cb. [DOI] [PubMed] [Google Scholar]
- 43.Luck T, Luppa M, Briel S, Matschinger H, Konig HH, Bleich S, Villringer A, Angermeyer MC, Riedel-Heller SG. Mild cognitive impairment: incidence and risk factors: results of the leipzig longitudinal study of the aged. J Am Geriatr Soc. 2010;58:1903–1910. doi: 10.1111/j.1532-5415.2010.03066.x. [DOI] [PubMed] [Google Scholar]
- 44.Kantarci K, Weigand SD, Przybelski SA, Preboske GM, Pankratz VS, Vemuri P, Senjem ML, Murphy MC, Gunter JL, Machulda MM, Ivnik RJ, Roberts RO, Boeve BF, Rocca WA, Knopman DS, Petersen RC, Jack CR., Jr MRI and MRS predictors of mild cognitive impairment in a population-based sample. Neurology. 2013;81:126–133. doi: 10.1212/WNL.0b013e31829a3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luck T, Riedel-Heller SG, Luppa M, Wiese B, Wollny A, Wagner M, Bickel H, Weyerer S, Pentzek M, Haller F, Moesch E, Werle J, Eisele M, Maier W, van den Bussche H, Kaduszkiewicz H. Risk factors for incident mild cognitive impairment--results from the German Study on Ageing, Cognition and Dementia in Primary Care Patients (AgeCoDe) Acta Psychiatr Scand. 2010;121:260–272. doi: 10.1111/j.1600-0447.2009.01481.x. [DOI] [PubMed] [Google Scholar]
- 46.Molano J, Boeve B, Ferman T, Smith G, Parisi J, Dickson D, Knopman D, Graff-Radford N, Geda Y, Lucas J, Kantarci K, Shiung M, Jack C, Silber M, Pankratz VS, Petersen R. Mild cognitive impairment associated with limbic and neocortical Lewy body disease: a clinicopathological study. Brain. 2010;133:540–556. doi: 10.1093/brain/awp280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenberg PB, Mielke MM, Appleby BS, Oh ES, Geda YE, Lyketsos CG. The association of neuropsychiatric symptoms in MCI with incident dementia and Alzheimer disease. Am J Geriatr Psychiatry. 2013 doi: 10.1016/j.jagp.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Costa PT, Jr, McCrae RR. Personality in adulthood: a six-year longitudinal study of self-reports and spouse ratings on the NEO Personality Inventory. J Pers Soc Psychol. 1988;54:853–863. doi: 10.1037//0022-3514.54.5.853. [DOI] [PubMed] [Google Scholar]
- 49.Kokmen E, Ozsarfati Y, Beard CM, O’Brien PC, Rocca WA. Impact of referral bias on clinical and epidemiological studies of Alzheimer’s disease. J Clin Epidemiol. 1996;49:79–83. doi: 10.1016/0895-4356(95)00031-3. [DOI] [PubMed] [Google Scholar]
- 50.Tsuang D, Kukull W, Sheppard L, Barnhart RL, Peskind E, Edland SD, Schellenberg G, Raskind M, Larson EB. Impact of sample selection on APOE epsilon 4 allele frequency: a comparison of two Alzheimer’s disease samples. J Am Geriatr Soc. 1996;44:704–707. doi: 10.1111/j.1532-5415.1996.tb01836.x. [DOI] [PubMed] [Google Scholar]
- 51.Szklo M, Nieto FJ. Epidemiology: Beyond the Basics. 2. Sudbury, Mass: Jones and Bartlett Publishers; 2007. [Google Scholar]