Abstract
Background
In the last 10 years, multiple new targeted agents have been developed for patients with human epidermal growth factor receptor 2-positive (HER2+) breast cancer. Up to 55% of patients with HER2+ breast cancer will develop brain metastases requiring some form of radiation therapy. The interaction between radiation and these targeted agents is unknown and previously unreported.
Methods
In this series, we describe 4 patients who developed clinically significant brain edema at sites of treated brain metastases. These patients were treated with stereotactic radiosurgery and trastuzumab emtansine, the newest FDA-approved agent for metastatic HER2+ breast cancer. Additionally, we present rates of clinically significant radiation necrosis among all breast cancer patients treated during this same time period.
Results
Using previously published clinical and preclinical data, we then hypothesize possible mechanisms for this striking interaction.
Conclusion
Increased awareness of potential interactions between targeted agents and radiation to the brain is crucial.
Keywords: brain metastases, HER2 positive breast cancer, stereotactic radiosurgery, targeted therapy
Approximately 20%–25% of breast cancers overexpress human epidermal growth factor receptor 2 (HER2).1,2 In the last 15 years, multiple targeted agents directed at HER2 have emerged, leading to improved progression-free and overall survival.3,4 Recently, trastuzumab emtansine (T-DM1) was approved by the FDA for metastatic HER2-overexpressing (HER2+) breast cancers previously treated with trastuzumab and a taxane. This approval came after a phase III trial showed a 5-month improvement in median overall survival and an objective response rate of 43.6% with T-DM1 compared with 30.8% on lapatinib/capecitabine.5 Toxicity, including grade 3, was lower with T-DM1 and included fatigue, nausea, elevated liver enzymes, gastrointestinal symptoms, and cytopenias.
Among patients with HER2+ breast cancer, up to 55% will develop brain metastases (BM),6 with studies suggesting up to a 4 times greater incidence relative to patients with non-HER2–expressing cancers.7 This increased incidence has been attributed to an “unmasking” effect of excellent systemic control prolonging survival, with poor drug CNS penetration. Preclinical data also suggest that the quantitative HER2 protein expression may be directly related to the development of BM, suggesting a possible causative mechanism for increased BM in the setting of HER2 overexpression.8
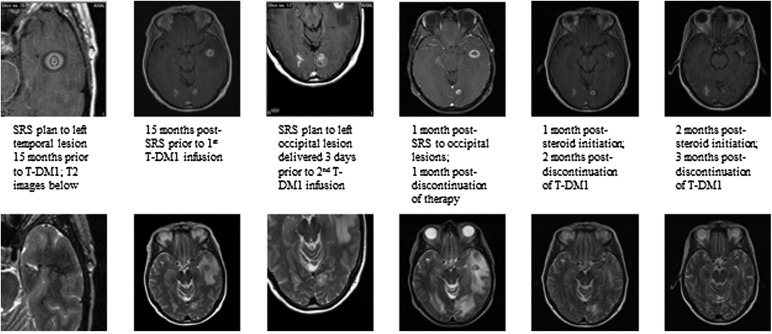
Many patients with metastatic HER2+ breast cancer undergo CNS radiation, including stereotactic radiosurgery (SRS), at some point in their treatment course. The interaction between SRS and these newer targeted agents is unknown and previously unreported. Since the FDA approval of T-DM1, we have observed an unexpected reaction among patients treated with T-DM1 who received SRS for BM within 15 months of undergoing T-DM1 therapy. These 4 patients, all with HER2+ metastatic breast cancer, presented shortly after a T-DM1 infusion with clinically significant increased edema as evidenced by neurologic changes and MRI findings of increased T2 signal in the area of previously treated BM (Fig. 1).
Fig. 1.
Image series from one patient who received a total of 2 infusions of T-DMI.
The 4 patients, of median age 56.5 years (range, 37y–57y), had received SRS to one or more lesions at a median of 8.5 days (range: 3d–449d) prior to a T-DM1 infusion. Two patients experienced symptoms immediately after the first infusion; one patient received 5 infusions on trial (NCT01276041) and then became symptomatic after the second infusion off trial; one patient developed symptoms on her fifth infusion. Symptoms included headaches, nausea/vomiting, speech impairment, short-term memory deficits, imbalance, gait disturbance, and visual deficits. For all 4 patients, fusion of MRI with the SRS treatment plan revealed that the area of T1-contrast enhancement was entirely encompassed by the radiation prescription isodose line, suggesting no evidence of tumor progression.
All 4 patients initiated steroids with eventual clinical and radiographical improvement over the weeks to months following steroid initiation. Three patients stopped T-DM1 due to neurologic symptoms; the patient who continued developed worsening symptoms and required steroid resumption. Due to severity of symptoms, this latter patient was taken to the operating room for resection of a single posterior fossa metastasis. Pathology revealed severe radionecrosis with no viable tumor cells identified.
For comparison, we then evaluated all patients with metastatic breast cancer and brain metastases who were treated with SRS at our institution during the 2-year time period when these 4 patients had been treated. Overall, a total of 13 breast cancer patients received SRS. Of these, only the 4 patients previously reported developed clinically significant radiation necrosis. Three additional HER2+ breast cancer patients were treated with T-DM1 and SRS and did not develop radiation necrosis, for an overall rate of clinically significant radiation necrosis among this treatment group of 57% (Table 1).
Table 1.
Clinical and treatment characteristics for 7 patients with HER2+ breast cancer treated with SRS and T-DM1 over a 2-year period
| Patient | Age (years) | CSRN | Prior Systemic Therapy | Total no. Cycles T-DM1 | T-DM1 On-Trial | Total no. Treated BM | SRS Dose (Gy) | Maximum Size of Treated Lesion (cm3) | Interval to CSRN From T-DM1 (days) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 37 | Yes | T, S | 1 | No | 4 | 24 | 1.1 | 10 |
| 2 | 56 | Yes | AC, ET | 7 | Yes | 1 | 18 | 1.6 | 7 |
| 3 | 57 | Yes | APx, XT, TV, GTCaL | 5 | No | 5 | 16-20 | 0.9 | 35 |
| 4 | 57 | Yes | ACPx, T, S | 2 | No | 5 | 24 | 4.5 | 3 |
| 5 | 49 | No | TPx, AC, DPT | 4 | No | 3 | 20 | 0.5 | N/A |
| 6 | 46 | No | TaPT, CaL | 2 | Yes | 2 | 20 | 0.9 | N/A |
| 7 | 57 | No | AC,TPx, PTPx, V, L | 31 | Yes | 2 | 18 | 5.0 | N/A |
Abbreviations: A, Doxorubicin; C, cyclophosphamide; Ca, capecitabine; CSRN, clinically significant radiation necrosis; D, docetaxel; E, eribulin; G, gemcitabine; L, lapatinib; no., number; P, carboplatin; S, study; Px, Paclitaxel; T, trastuzumab; V, vinorelbine; X. abraxane
Discussion
These patients represent a heterogeneous group of HER2+ metastatic breast cancer patients with multiple prior therapies and varying timing of prior SRS treatment, T-DM1 initiation, and symptom development; however, the unexpected correlation between T-DM1 and significantly increased cerebral swelling at sites of previous SRS was striking. Previously reported rates of symptomatic radiation necrosis among breast cancer patients who received SRS for BM suggest an incidence of 6%–11%,9,10 which correlates with a 2-year actuarial rate of radiation necrosis of 11%, as seen in the initial Radiation Therapy Oncology Group (RTOG) SRS dose-escalation study (RTOG 90-05).11 This incidence is far lower than the 57% incidence found among patients treated with both SRS and T-DM1 in this series. With the high frequency of BM in this patient population, this finding is potentially applicable to a large number of patients.
To our knowledge, there is no currently published data on CNS penetration of T-DM1. We draw upon both preclinical and clinical data with trastuzumab, data on the effects of radiation on the permeability of the blood-brain barrier (BBB), the mechanism of action of T-DM1, and HER2 expression in normal glial cells, to hypothesize a possible mechanism for these findings.
Multiple series suggest increased survival and time to progression among HER2+ breast cancer patients with BM treated with trastuzumab, as well as a delay in BM among patients on trastuzumab.12,13 These findings have been explained by increased systemic control with trastuzumab rather than by direct BBB penetration. Initial pharmacokinetic trials with trastuzumab reported an apparent lack of penetration of trastuzumab into the cerebrospinal fluid (CSF), with CSF levels 300-fold lower than serum levels.14 However, a more recent study reported increased levels of trastuzumab in the CSF after radiation, with ratio of trastuzumab in serum to trastuzumab in CSF going from 420:1 to 76:1 after whole brain radiation or SRS.15 Changes in BBB permeability after radiation to the brain have been well documented; preclinical and clinical data suggest increased permeability after both fractionated and high-dose single fraction radiation with changes lasting between days to months after radiation delivery.16
T-DM1 has several clinically unique features that distinguish it from trastuzumab. Trastuzumab is thought to inhibit intracellular proliferative signals, activate antibody-dependent cellular cytotoxicity, and inhibit tumor-induced angiogenesis.17 T-DM1 is an antibody drug conjugate that uses the trastuzumab antibody to deliver a cytotoxic agent to antigen-expressing tumors. The agent, mysantine (DM1), induces apoptosis in a fashion similar to Vinca alkaloids.18 In vitro cell viability studies have shown that DM1 is 25–4,000-fold more potent than current clinically used chemotherapy agents.19 Lewis Phillips et al. determined that T-DM1 specifically targets HER2-overexpressing cells, with persistent activity after HER2-overexpressing cells no longer responded to trastuzumab. Mechanisms of cell death determined by cell-proliferation assays included both cellular lysis and apoptosis.18 More recent work suggests that T-DM1 also uniquely promotes mitotic catastrophe within cells.20 These preclinical data provide evidence that subsequent cell death from T-DM1 may be more proinflammatory than the cytostatic mechanism of trastuzumab.
Limited data exist on new molecular targeted agents and concurrent SRS treatment. A large review of the use of targeted agents combined with radiation found unexpected systemic toxicity, with little increased CNS toxicity and no reports of CNS toxicity with trastuzumab.21 In 2009, Schwer et al. reported on 15 patients with high-grade gliomas enrolled in a clinical trial of SRS and gefitinib, an epidermal growth factor receptor inhibitor.22 At 5 to 7 months post SRS, a 37.8% median increase in T2 signal was seen. Two patients underwent resection and were found to have extensive radiation necrosis, similar to the patient in our series who went to surgery; imaging changes were attributed to a vigorous treatment response. Among our patients, increase in T2 signal was seen uniformly within 1 to 2 weeks after a T-DM1 infusion in the setting of prior SRS. Unlike the aforementioned study suggesting tumor radiosensitization, this timing is more consistent with a radiation-recall effect whereby administration of T-DM1 precipitates an inflammatory reaction within SRS-treated lesions and leads to T2 changes and clinical symptoms.
One hypothesis of the mechanism comes from preclinical data showing that radiation upregulates HER2/neu gene expression in human breast cancer cell lines, which leads to a heightened response to trastuzumab.23 An alternative theory proposes that T-DM1 may be targeting normal glial cells. ErbB2, the proto-oncogene that encodes HER2, is known to play a role in glial cell formation, and preclinical data show upregulation of erbB2 in response to neuronal injury.24,25 T-DM1 targeting of upregulated HER2 in glial cells could lead to T-DM1-mediated death and dysfunction of glial cells, with a subsequent inflammatory response characterized by increased levels of glutamate and release of cytokines including tumor necrosis factor and interleukins, as seen in brain injury models.26 This latter theory is supported by the extensive radiation necrosis seen in the resection specimen of one patient in our series, with no viable tumor detected. This patient had received SRS to this lesion prior to any T-DM1 therapy, with no evidence of progression in the posttreatment interval. While it is impossible to know for certain, if no residual tumor was present at the time of T-DM1 therapy, injured glial cells would represent an alternative target for T-DM1 in the context of prior radiation-induced increased permeability of the drug.
Seven clinical studies have been published, reviewing 1 455 patients treated with T-DM1, with limited data on BM and no mention of increased CNS toxicity.5,27 However, there are two possible explanations. First, the closest clinical surrogate for CNS toxicity may be headaches. Grades 1 and 2 headaches were twice as likely with T-DM1 compared with trastuzumab plus docetaxel (40.6% vs 18.2%).27 Further evaluation of significant headaches was not reported. Also, untreated or symptomatic BM were exclusion criteria in both recent trials, with a 60-day interval required between treatment of BM and enrollment. However, our series does include one patient treated with SRS more than 2 months prior to T-DM1, suggesting that this toxicity could have been captured in similar clinical scenarios on trial.
While this series is limited, with varied timing of SRS and T-DM1 infusion, the correlation was quite salient. Numerous new targeted agents are developed every month, providing significant clinical benefit for patients. SRS is also being used with increased frequency. The intersection of these treatment modalities will continue; thorough reporting and evaluation of adverse effects with concurrent SRS and targeted agents are essential. The use of T-DM1 and SRS for HER2+ metastatic breast cancer is especially significant, given the high incidence of BM among this patient population.
Funding
No funding support of this work.
Conflict of interest statement. Dr. Anthony Elias reports a compensated consultant relationship to Genentech, as well as research funding from Genentech.
References
- 1.Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clinical Breast Cancer. 2004;5(1):63–69. doi: 10.3816/cbc.2004.n.011. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Godophin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244(4905):707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 4.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 5.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olson EM, Najita JS, Sohl J, et al. Clinical outcomes and treatment practice patterns of patients with HER2-positive metastatic breast cancer in the post-trastuzumab era. Breast. 2013;22(4):525–531. doi: 10.1016/j.breast.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musolino A, Ciccolallo L, Panebianco M, et al. Multifactorial central nervous system recurrence susceptibility in patients with HER2-positive breast cancer: epidemiological and clinical data from a population-based cancer registry study. Cancer. 2011;117(9):1837–1846. doi: 10.1002/cncr.25771. [DOI] [PubMed] [Google Scholar]
- 8.Duchnowska R, Biernat W, Szostakiewicz B, et al. Correlation between quantitative HER-2 protein expression and risk for brain metastases in HER-2+ advanced breast cancer patients receiving trastuzumab-containing therapy. Oncologist. 2012;17(1):26–35. doi: 10.1634/theoncologist.2011-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muacevic A, Kreth FW, Tonn JC, et al. Stereotactic radiosurgery for multiple brain metastases from breast carcinoma. Cancer. 2004;100(8):1705–1711. doi: 10.1002/cncr.20167. [DOI] [PubMed] [Google Scholar]
- 10.Kondziolka D, Kano H, Harrison GL, et al. Stereotactic radiosurgery as primary and salvage treatment for brain metastases from breast cancer. Clinical article. J Neurosurg. 2011;114(3):792–800. doi: 10.3171/2010.8.JNS10461. [DOI] [PubMed] [Google Scholar]
- 11.Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90–05. Int J Radiat Oncol Biol Phys. 2000;47(2):291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 12.Kirsch DG, Ledezma CJ, Mathews CS, et al. Survival after brain metastases from breast cancer in the trastuzumab era. J Clin Oncol. 2005;23(9):2114–2116. doi: 10.1200/JCO.2005.05.249. author reply 2116–2117. [DOI] [PubMed] [Google Scholar]
- 13.Metro G, Sperduti I, Russillo M, et al. Clinical utility of continuing trastuzumab beyond brain progression in HER-2 positive metastatic breast cancer. Oncologist. 2007;12(12):1467–1469. doi: 10.1634/theoncologist.12-12-1467. author reply 1469–1471. [DOI] [PubMed] [Google Scholar]
- 14.Pestalozzi BC, Brignoli S. Trastuzumab in CSF. J Clin Oncol. 2000;18(11):2349–2351. doi: 10.1200/JCO.2000.18.11.2349. [DOI] [PubMed] [Google Scholar]
- 15.Stemmler HJ, Schmitt M, Willems A, et al. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs. 2007;18(1):23–28. doi: 10.1097/01.cad.0000236313.50833.ee. [DOI] [PubMed] [Google Scholar]
- 16.van Vulpen M, Kal HB, Taphoorn MJ, et al. Changes in blood-brain barrier permeability induced by radiotherapy: implications for timing of chemotherapy?. (Review) Oncol Rep. 2002;9(4):683–688. [PubMed] [Google Scholar]
- 17.Mehta AI, Brufsky AM, Sampson JH. Therapeutic approaches for HER2-positive brain metastases: circumventing the blood-brain barrier. Cancer Treat Rev. 2013;39(3):261–269. doi: 10.1016/j.ctrv.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis Phillips GD, Li G, Dugger DL, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68(22):9280–9290. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 19.Kovtun YV, Audette CA, Mayo MF, et al. Antibody-maytansinoid conjugates designed to bypass multidrug resistance. Cancer Res. 2010;70(6):2528–2537. doi: 10.1158/0008-5472.CAN-09-3546. [DOI] [PubMed] [Google Scholar]
- 20.Barok M, Tanner M, Koninki K, Isola J. Trastuzumab-DM1 causes tumour growth inhibition by mitotic catastrophe in trastuzumab-resistant breast cancer cells in vivo. Breast Cancer Res. 2011;13(2):R46. doi: 10.1186/bcr2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niyazi M, Maihoefer C, Krause M, et al. Radiotherapy and “new” drugs-new side effects? Radiat Oncol. 2011;6:177. doi: 10.1186/1748-717X-6-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwer AL, Kavanagh BD, McCammon R, et al. Radiographic and histopathologic observations after combined EGFR inhibition and hypofractionated stereotactic radiosurgery in patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2009;73(5):1352–1357. doi: 10.1016/j.ijrobp.2008.06.1919. [DOI] [PubMed] [Google Scholar]
- 23.Voutsas IF, Mahaira LG, Fotopoulou K, et al. Gamma-irradiation induces HER-2/neu overexpression in breast cancer cell lines and sensitivity to treatment with trastuzumab. Int J Radiat Biol. 2013;89(5):319–325. doi: 10.3109/09553002.2013.765617. [DOI] [PubMed] [Google Scholar]
- 24.Liang C, Tao Y, Shen C, et al. Erbin is required for myelination in regenerated axons after injury. J Neurosci. 2012;32(43):15169–15180. doi: 10.1523/JNEUROSCI.2466-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang H, Ling W, Vitale A, et al. ErbB2 activation contributes to de-differentiation of astrocytes into radial glial cells following induction of scratch-insulted astrocyte conditioned medium. Neurochem Int. 2011;59(7):1010–1018. doi: 10.1016/j.neuint.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Swanson RA. Astrocytes and brain injury. J Cereb Blood Flow Metab. 2003;23(2):137–149. doi: 10.1097/01.WCB.0000044631.80210.3C. [DOI] [PubMed] [Google Scholar]
- 27.Hurvitz SA, Dirix L, Kocsis J, et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2013;31(9):1157–1163. doi: 10.1200/JCO.2012.44.9694. [DOI] [PubMed] [Google Scholar]