Abstract
Acetaldehyde, the first metabolite of ethanol, is active in the central nervous system, where it exerts motivational properties. Acetaldehyde is able to induce drinking behaviour in operant-conflict paradigms that resemble the core features of the addictive phenotype: drug-intake acquisition and maintenance, drug-seeking, relapse and drug use despite negative consequences. Since acetaldehyde directly stimulates dopamine neuronal firing in the mesolimbic system, the aim of this study was the investigation of dopamine D2-receptors' role in the onset of the operant drinking behaviour for acetaldehyde in different functional stages, by the administration of two different D2-receptor agonists, quinpirole and ropinirole. Our results show that acetaldehyde was able to induce and maintain a drug-taking behaviour, displaying an escalation during training, and a reinstatement behaviour after 1-week forced abstinence. Acetaldehyde operant drinking behaviour involved D2-receptor signalling: in particular, quinpirole administration at 0.03 mg/kg, induced a significant decrease in the number of lever presses both in extinction and in relapse. Ropinirole, administered at 0.03 mg/kg during extinction, did not produce any modification but, when administered during abstinence, induced a strong decrease in acetaldehyde intake in the following relapse session. Taken together, our data suggest that acetaldehyde exerts its own motivational properties, involving the dopaminergic transmission: indeed, activation of pre-synaptic D2-receptors by quinpirole, during extinction and relapse, negatively affects operant behaviour for acetaldehyde, likely decreasing acetaldehyde-induced dopamine release. The activation of post-synaptic D2-receptors by ropinirole, during abstinence, decreases the motivation to the consecutive reinstatement of acetaldehyde drinking behaviour, likely counteracting the reduction in the dopaminergic tone typical of withdrawal. These data further strengthen the evidence that acetaldehyde may play a crucial role as mediator of ethanol's central effects.
Introduction
The neurobiological mechanism underlying the pharmacological properties of ethanol is complex and not completely elucidated [1]. In this regard, acetaldehyde (ACD), its first metabolite, has been increasingly recognized as strongly involved in various ethanol neuropharmacological, neurobiological and behavioural effects [2]–[8]. Despite its reputation as an aversive substance for long time [9], ACD possesses motivational and reinforcing properties, highlighted in rodents by different behavioural paradigms, as place conditioning [10], [11] and operant self-administration [12]–[15]; protocols which include reinstatement and conflict procedures have also been reported [16], [17].
Dopamine (DA) plays a prominent role in the different stages of the addiction cycle [18]–[20]. The dopaminergic (DAergic) transmission represents the neurobiological substrate of the acute reinforcing properties of the drugs of abuse [21] and of their enhanced incentive salience [22]. However, the function of the mesolimbic DA system is severely impaired upon cessation of the chronic exposure to several drugs of abuse, including ethanol [23]. Acute withdrawal is associated with an increase in reward thresholds in animals, a finding which mirrors the decreased activity of the mesolimbic dopamine system observed by electrophysiological recordings and in vivo microdialysis [24]–[27]. Furthermore a decrease in the number and function of D2 receptors, observed both in animals [28], [29] and in humans [30]–[33], is consistent with the hypodopaminergic state in ethanol withdrawal, and is functionally correlated to the enhancement in drug craving, drug intake and relapse [34]–[36].
Several reports clearly show that pharmacological properties of ACD involve the DAergic system: ACD is able to increase the neuronal firing of DA neurons in the ventral tegmental area [37], to stimulate DA release from their projections [38]–[40] and to promote, in DA terminal areas, the induction of early-gene protein expression, as c-Fos, considered as a general marker of neural activity [41]. Although this general evidence points to the involvement of mesencephalic DA neurons in ACD neuropharmacological action, the few studies exploring the neurobiological mechanisms underlying the reinforcing and addictive-like properties of oral ACD, have focused on endocannabinoid [17] and opioid [42] systems, while DA's direct contribution to ACD operant drinking behaviour still remains elusive.
We now hypothesize that ACD-induced operant behaviour can be modulated by the direct manipulation of DAergic signalling; we verify our hypothesis by investigating the role of two different D2 receptor agonists, quinpirole and ropinirole, in distinct functional phases of ACD operant-drinking behaviour. D2 receptors have both pre- and post-synaptic localization [43]–[46]: quinpirole, at low doses, has been reported to preferentially bind to D2 autoreceptors [47], [48]. Ropinirole, a post-synaptic D2 agonist, is already used to restore dopaminergic tone in Parkinson's Disease [49], [50], as well as in the normalisation of the behavioural responses to natural rewards in anhedonic states [51]. The effectiveness of these two different D2 receptors agonists in reducing drug seeking and drug taking, during extinction and relapse in ACD-induced operant behaviour, can imply important translational consequences concerning the pharmacological treatments of alcohol addiction.
Materials and Methods
Ethics Statement
The experimental procedures have been carried out in accordance with the Italian legislation D.L. 116/1992 and the EU Directive 2010/63/EU, dealing with research on experimental animals, and approved by the Committee on the Ethics of Animal Experiments of the University of Palermo. All efforts were made to minimize animal suffering.
Animals
Adult male Wistar rats (Harlan, Udine, Italy) (n = 44), weighing 250–300 g, were used in this study. Rats were housed two per cage (standard rat cages, Tecniplast, Italy) with free access to food, (standard rodent diet 4RF18, Mucedola, Italy) and tap water, and kept under controlled environmental conditions (12 h light/dark cycle, temperature 22±2°C, humidity 55±10%). Animals were allowed to habituate to facilities and daily handled before initiation of the experiments, in order to reduce and minimize animal's distress. During operant conditioning experiments, they were water-restricted and allowed to drink 1 hour/day at the end of the experimental sessions. Water intake was recorded. For each experiment, animals were randomly assigned to the different experimental groups.
Drugs
ACD 99,98% (Sigma-Aldrich SRL, Milan Italy) was stocked at −20°C and the solution was prepared with distilled water daily. ACD concentration (3.2%; 1.6 ml in 50 ml of solution) was controlled and measured with UV spectrum analysis, either at the beginning or at the end of the operant session. The procedure confirmed that ACD concentration was preserved along the 20-minute experimental procedure [16].
Quinpirole hydrochloride (Sigma-Aldrich, Milan, Italy), used in Experiment 1, was dissolved in saline solution (0.9% NaCl), and administered intraperitoneally (i.p.) (0.03 mg/kg), 30 minutes prior to start behavioural procedures.
Ropinirole (Sigma-Aldrich, Milan, Italy), used in Experiment 2, was dissolved in saline solution (0.9% NaCl), and administered daily during the 7-day deprivation 2 period (0.03 mg/kg, i.p.).
Operant Apparatus
The experimental sessions were carried out in a custom-built operant-conditioning chamber (30×28×37 cm), placed in a dim-illuminated, ventilated, sound-attenuating cubicle. The chamber was equipped with one active lever and a cup that collected liquid from a corked reservoir, aiming at the preservation of ACD solution from evaporation, with a solenoid-actuated delivery system. It assured the delivery of 0.028 ml of solution for each lever press. Animal performance was recorded on a counter connected to the chamber. The devices were thoroughly cleaned before the introduction of each animal to ensure that the particular rat's behaviour was not affected by the detection of another rat's scent.
Open Field Arena
Locomotor activity was measured in an Open Field with an automatic video-tracking system, Any Maze (Ugo Basile, Italy). The Open Field is a square box, 44 cm long, 44 cm wide, and 20 cm high. The software produces a quali-quantitative mapping of the motor pattern and measures Total Distance Travelled (TDT; cm) along 5 min.
Experimental procedures
Timeline
The operant drinking behaviour paradigm includes consecutive experimental sessions: shaping and training (30 days); deprivation 1 (7 days); relapse 1 (5 days); extinction (1 day); deprivation 2 (7 days); relapse 2 (5 days).
Shaping and Training
Animals (n = 44) were shaped to lever press to obtain water on a continuous reinforcement schedule (fixed ratio 1, FR-1), until they reached the same level of performance. Afterwards training started and rats orally self-administered ACD solution in the operant chamber (FR-1) along 30 days. For each tapping, the system delivered 0.028 ml of 3.2% ACD solution. The number of lever presses was automatically recorded; ACD intake was measured by multiplying the number of lever presses by 0.028 ml, and this value corresponded to the amount of liquid missing from the reservoir at the end of each session. Animals were tested each day at the same time (9:00 to 14:00).
Deprivation 1
During the first 7-day deprivation period, rats were left undisturbed in their home cages and received water and food ad libitum. ACD self-administration was suspended to achieve a forced abstinence.
Relapse 1
After the first deprivation period, rats were exposed again to lever pressing in the operant chamber with a FR-1 response schedule, for 5 days. Responses were then recorded during the 20 min-experimental sessions. ACD solution intake was also recorded.
Experiment 1: administration of quinpirole during extinction and relapse 2
After the first relapse paradigm, the effect of quinpirole on ACD operant drinking behaviour was tested during extinction and relapse 2.
Extinction
Animals underwent an operant responding session during which reward delivery was withheld, in order to test the effect of the pharmacological manipulation on ACD-seeking behaviour. The number of lever presses throughout the 20-min session was tracked. During extinction, rats were administered with 0.03 mg/kg quinpirole (Q-group, n = 8), or vehicle (Vh-group, n = 8), 30 minutes before entering the operant chamber.
Deprivation 2
ACD self-administration was suspended again for 7 days to achieve a forced abstinence. Rats were left undisturbed in their home cages and received water and food ad libitum.
Relapse 2
During this phase, the two experimental groups (n = 8 each) were not treated at relapse day 1, in order to have a baseline value of drinking following abstinence. From day 2 to day 4, they were administered i.p. with 0.03 mg/kg quinpirole, or vehicle, according to their group, 30 minutes prior starting the operant session. Administrations were suspended during session 5.
The dose of Quinpirole was selected because of its capability to reduce ethanol self-administration in an analogous procedure [47]. In order to test the specificity of quinpirole effect on the operant response, a water-drinking group (n = 8) was used and subjected to the same experimental procedures than ACD-drinking rats.
Experiment 2: administration of ropinirole during extinction and deprivation
After the first relapse paradigm, the effect of ropinirole on ACD operant drinking behaviour was tested during the extinction and relapse 2 phases.
Extinction
Animals (n = 20) underwent an operant responding session during which reward delivery was suspended, in order to test the effect of the pharmacological manipulation on ACD-seeking behaviour. The number of lever presses at the end of the 20 min session was recorded. During extinction, rats were administered with 0.03 mg/kg ropinirole (R-group, n = 10) or vehicle (Vh-group, n = 10), 30 minutes before getting in the operant chamber.
Deprivation 2
During this session the distinct groups received ropinirole (0.03 mg/kg, i.p.), or vehicle, once daily at the same time (11:00 a.m.) along 7 days.
Relapse 2
During this phase animals received no administration. The number of lever presses and the amount of ACD consumed was measured after each 20-min session.
Open Field Test
Quinpirole and ropinirole effects on locomotor activity were also tested in the Open Field Test. Rats (n = 36) were administered with quinpirole (0.03 mg/kg, i.p.), ropinirole (0.03 mg/kg, i.p.) or vehicle, 30 minutes before entering the open field arena. Total distance travelled (TDT) at the end of the 5-minute session was measured.
Statistical analysis
A one-way analysis of variance (ANOVA) for repeated measures was conducted in order to evaluate the effect of time on the number of lever presses during training and relapse, while a two-way ANOVA for repeated measures was employed to assess the effect of time and treatment on the number of responses emitted during relapse. Furthermore, in order to compare ACD intake during the different periods of training, and the effect of quinpirole and ropinirole treatments on ACD-seeking behaviour during extinction, and on locomotor activity, a two-tailed Student's t-test for unpaired measures was used. When necessary, simple main effects and post hoc comparisons were calculated with Bonferroni post-test (α = 0.05). Differences were considered statistically significant if p<0.05. Data are reported as mean ± SEM.
Statistical analysis was conducted by using the GraphPad Prism software, v.6.1 (GraphPad Software, San Diego, CA, USA) on data from all experimental animals used.
Results
Operant Self-Administration
Training Period
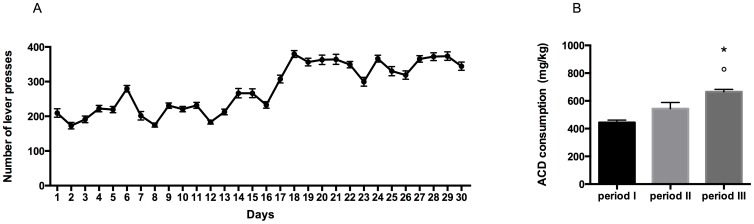
Rats readily acquired 3.2% ACD oral self-administration within the Training period, progressively increasing the number of lever presses along time. The results of a one-way ANOVA for repeated measures, showed a significant effect of time on the number of responses emitted (F(29, 1015) = 68.02, p<0.0001) (Fig.1A).
Figure 1. Operant drinking-behaviour pattern.
Number of lever presses during the 30 days of training in the operant-drinking paradigm (A). ACD consumption (mg/kg) during subsequent periods of training in the operant-drinking paradigm. °p<0.001 Vs period I; *p<0.05 vs period II (B).
In particular, during the first 10 days of exposure to ACD, rats showed a lower number of lever presses, and consequently an average intake of 444,1±124,3 mg/kg. In the second ten days of the paradigm, ACD rats' drinking behaviour increased, displaying a higher number of lever presses and greater liquid intake, and an average intake of 543,2±144,8 mg/kg. During the last 10-day period of training, rats' lever presses for ACD increased significantly with respect to the previous days, reaching a mean value of 665,3±118,8 mg/kg.
When ACD intake within the three subsequent training periods was analyzed by a two-tailed Student's t-test for paired measures, a significant difference in the amount of ACD consumed in period III with respect to period I and period II (t = 7.705, df = 9, p<0.001; t = 2.470, df = 9, p<0.0356) was observed (Fig.1B).
No differences were observed in body weight and water intake during the free-drinking hour at the end of the experimental sessions, along the 30-day training period between the two groups.
Relapse 1
Following the first 7 days of abstinence from ACD self-administration, rats were tested in the operant chamber to assess whether deprivation could influence their drinking behaviour. A one-way ANOVA for repeated measures showed a statistically significant effect of days on the number of responses emitted (F(4, 140) = 6.188, p = 0.0001). Bonferroni post-hoc analysis evidenced a significant increase in number of lever presses in day 2 with respect to day 3 (t = 4.789, df = 140, p<0.001) and day 5 (t = 3.483, df = 140, p<0.01).
Experiment 1: administration of quinpirole during extinction and relapse 2.
Extinction
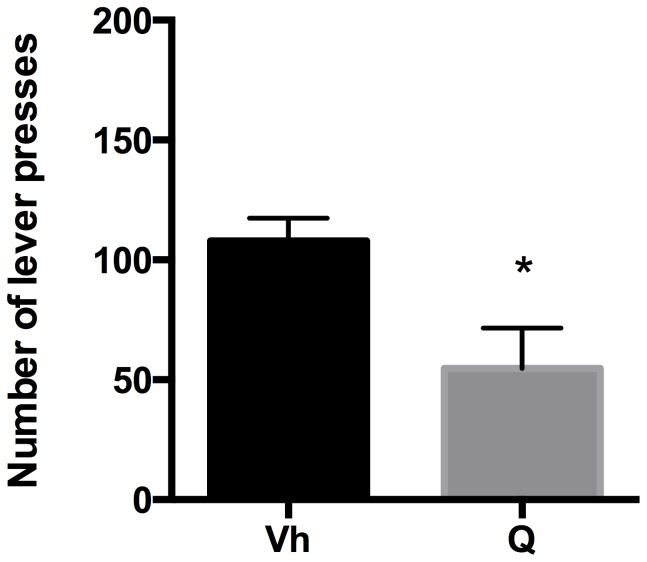
Rats were tested in the operant condition paradigm to assess quinpirole effect on drug seeking when reward delivery was suspended. The effect of quinpirole on the number of non-rewarded lever presses was analyzed by a two-tailed Student's t-test for unpaired measures. Our data indicated that quinpirole administration induced a significant reduction in the number of lever presses (t = 2.740, df = 14, p = 0.0160) with respect to vehicle along the 20 min experimental session (Fig. 2).
Figure 2. Quinpirole and Extinction.
Number of lever presses during the 20-min Extinction session, in vehicle (Vh) - and quinpirole (Q) treated rats. *p<0.05 Vs Vh.
Relapse 2
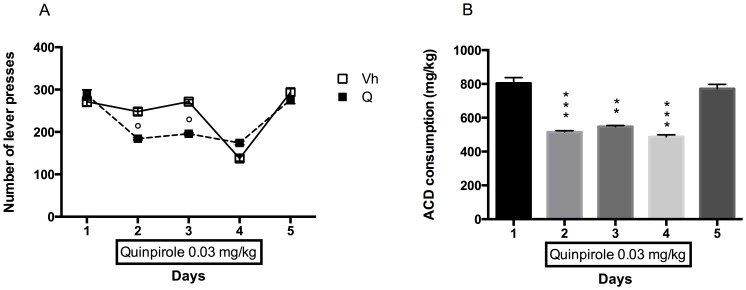
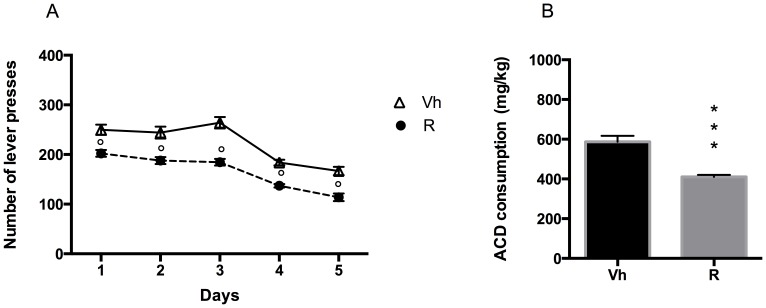
The results of a 2-way ANOVA for repeated measures including "Quinpirole Treatment" as the between-subjects factor and “Days” as within-subjects factor showed a significant effect of time, treatment, and their interaction on the number of responses emitted, F(4, 56) = 88.05, p<0.0001; F(1, 14) = 26.10, p = 0.0002; F(4, 56) = 18.95, p<0.0001. Bonferroni post hoc analysis showed that quinpirole was able to induce a reduction in the number of lever presses with respect to vehicle in 2 out of 3 days of administration: day 2 (t = 5.883, df = 70, p<0.0001), day 3 (t = 6.991, df = 70, p<0.0001); the two ACD-drinking groups showed no statistically significant differences in days 1 and 5, when quinpirole was not administered (Fig. 3A).
Figure 3. Quinpirole and Relapse.
Number of lever presses in Vh and Q groups during Relapse following 1 week abstinence. °p<0.001 Vs Vh (A). ACD consumption (mg/kg) during Relapse following 1 week abstinence. **p<0.01; ***p<0.001 Vs Vh (B).
Quinpirole effect was also analyzed within the treated group day by day. The results of a one-way ANOVA for repeated measure, including "quinpirole treatment" as the between columns factor, showed a statistically significant effect of the drug on the amount of ACD consumed (F(1.490, 10.43) = 60.60, p<0.0001). Bonferroni post hoc analysis showed that quinpirole administration decreased the amount of ACD ingested in day 2 (t = 7.933, df = 7, p<0.001); 3 (t = 6.736, df = 7, p<0.01) and 4 (t = 9.242, df = 7, p<0.001) with respect to day 1, and also when compared to day 5, when quinpirole administration was suspended (day 2: t = 8.311, df = 7, p<0.001; day 3: t = 7.504, df = 7, p<0.01; day 4: t = 9.994, df = 7, p<0.001). No significant difference in mg/kg of ACD consumed was observed between day 5 and day 1 (Fig. 3B).
When quinpirole was administered in controls, i.e. water-drinking rats, the results of a 2-way ANOVA for repeated measures showed no significant effect on the number of lever presses emitted along the experimental days (F(4,24) = 1.405, p = 0.2623).
Experiment 2: administration of ropinirole during extinction and deprivation 2.
Extinction
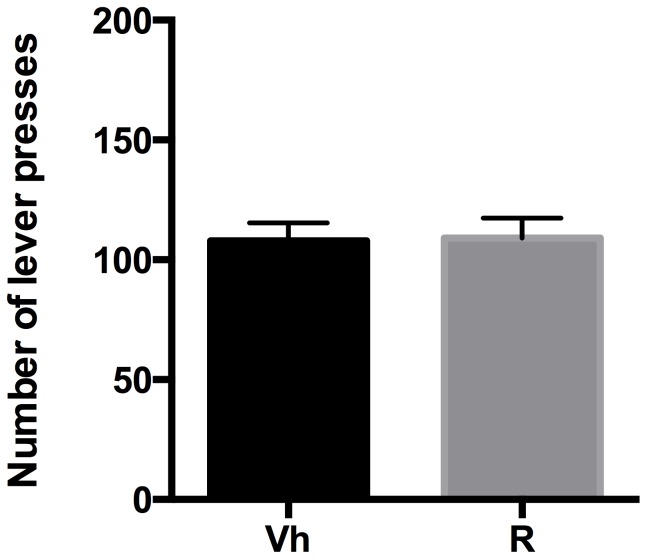
Rats were tested on the operant condition paradigm to assess ropinirole effect on drug seeking when reward delivery was suspended. The effect of ropinirole treatment on non-rewarded lever presses was analyzed by a two-tailed Student's t-test for unpaired measures. Our data indicated that ropinirole treatment, 30 minutes before the extinction session, was ineffective and no significant difference in the number of lever presses was observed (t = 0.09720, df = 18, p = 0.9236), compared to vehicle (Fig.4).
Figure 4. Ropinirole and Extinction.
Number of lever presses during the 20-min Extinction session, in vehicle (Vh) - and ropinirole (R) treated rats.
Relapse 2
Following 7 days of abstinence from ACD self-administration in deprivation 2, rats were administered daily with 0.03 mg/kg ropinirole, and were tested again in the operant chamber to assess whether the treatment could influence their drinking behaviour in relapse 2. The results of a 2-way ANOVA for repeated measures including "Ropinirole treatment" as the between-subjects factor and “Days” as within-subjects factor, showed a significant effect of time, treatment, and their interaction on the number of responses emitted (F(4, 72) = 105.2, p<0.0001; F(1, 18) = 36.11, p<0.0001; F(4, 72) = 2.926, p = 0.0267). Bonferroni post hoc analysis showed that ropinirole-treated group displayed a decrease in the number of lever presses in all days of relapse, on day 1, 2, 3, 4 and 5 (t = 4.047, df = 90, p = 0.0005; t = 4.800, df = 90, p<0.0001; t = 6.774, df = 90, p<0.0001; t = 3.991, df = 90, p = 0.0007; t = 4.516, df = 90, p<0.0001) when compared to vehicle (Fig. 5A).
Figure 5. Ropinirole and Relapse.
Number of lever presses in Vh and R groups during Relapse following 1 week abstinence. °p<0.001 Vs Vh (A). ACD consumption (mg/kg) during Relapse following 1 week abstinence. ***p<0.001 Vs Vh (B).
Accordingly, a two-tailed Student's t-test for unpaired measures showed a significant reduction in the amount of ACD in ropinirole -treated group, with respect to vehicle (t = 5.617, df = 18, p<0.001) (Fig. 5B).
Open Field Test
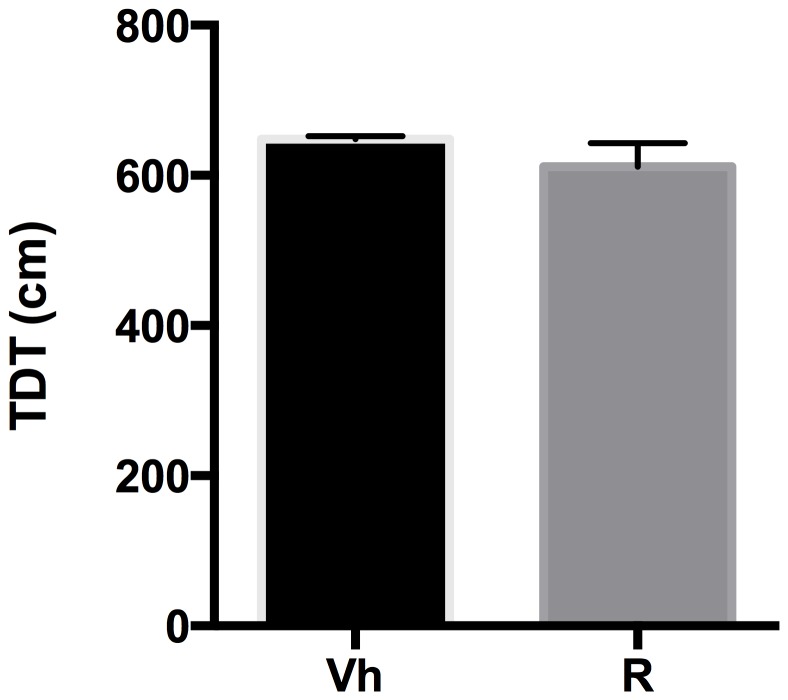
The effects of quinpirole and ropinirole treatment on locomotion in terms of TDT in the Open Field Test were analyzed by a two-tailed Student's t-test for unpaired measures. Our data indicate that quinpirole administration induced a significant reduction in TDT (t = 10.93, df = 14, p<0.0001) with respect to vehicle (Fig. 6). Ropinirole administration induced no variation in TDT (t = 1.147, df = 18, p = 0.2665) with respect to vehicle (Fig. 7).
Figure 6. Quinpirole and OF.
Effect of quinpirole on the total distance travelled (TDT) in the open field arena. ***p<0.001 Vs Vh.
Figure 7. Ropinirole and OF.
Effect of ropinirole on the total distance travelled (TDT) in the open field arena.
Discussion
The aim of the present study was to investigate the neuropharmacological basis underpinning discrete aspects of operant drinking behaviour for ACD in male rats.
Previous self-administration studies demonstrated that ACD possesses its own reinforcing and motivational properties [10], [15], [52], [53] since it is able to induce and maintain an operant behaviour in rats and promotes different drug-related behaviours, such as resilience to extinction, induction to relapse and to compulsive-like behaviour [12], [16], [17]. Recent works by Karahanian and colleagues elegantly demonstrated that ACD has a crucial role in mediating ethanol reinforcement in the VTA. Indeed, reducing ACD generation, or increasing its metabolism in the VTA, can lead to a marked reduction of ethanol intake in naive rats [5], [54], but the increase in ACD metabolism in VTA failed to affect ethanol intake in animals that consumed ethanol chronically for 2–3 months [54]. In this regard, a role for high ACD peripheral levels, able to cross the blood-brain barrier, cannot be ruled out. Since chronic ethanol exposure leads to CYP2E1 induction and decreased activity of aldehyde dehydrogenase [55]–[58], it is worth exploring the pharmacological potential properties of peripheral ACD, which may also account for its positive reinforcing effects. Remarkably, some of the behavioural features of orally self-administered ACD are sensitive to the pharmacological modulation of the cannabinoid CB1 receptor [17], as well as of the opioid neurotransmission [42]. These systems are largely involved in the induction of alcohol drinking behaviour and relapse [59], [60], [35] and can likely influence ACD drinking behaviour through the modulation of the DAergic reward pathway, thus causing DA release in the nucleus accumbens [61].
In the current experiments, the induction of ACD drinking behaviour was acquired along 30 days. Our data show that in the last period of training, rats' ACD intake was significantly higher than in the previous weeks suggesting that the incentive motivation for the substance had also been increasing along time [62]. Operant conditioning is a behavioural paradigm specifically tailored to reflect the measure of the reinforcing properties of self-administered drugs [63]–[68]. Chronic exposure to relative high ACD concentrations, as those used in the present study (3.2%), aims at creating conditions for ACD central accumulation, since this small molecule can be found in the central nervous system when administered i.p. at doses of 100 mg/kg [69], [70]. According to our data, ACD intake reached mean values of 665,3 mg/kg, within the 20 min-operant session, and it is conceivable that such chronic and increasing intake of ACD can overwhelm the metabolic barrier constituted by epithelial aldehyde dehydrogenase (ALDH1), a low Km ACD-oxidizing enzyme expressed in gastrointestinal tract [71]. Systemic absorption after ACD oral ingestion has been already demonstrated by previous studies on ACD self-administration by Peana and colleagues [12]. Therefore, increasing blood ACD concentrations could saturate the moderate aldehyde dehydrogenase activity of BBB capillaries [72]–[74], [8], enter the brain, and exert central activity.
Our results are in agreement with previous data from this laboratory [16], and can be ascribed to the rewarding and motivational properties of ACD. Animals easily self-administer ACD [13]–[16], likely as a consequence of its reinforcing properties, reported to be 1000-fold stronger than ethanol's [15], [53]. Although apparently in opposition with reports on primarily ACD aversive effects following acute peripheral administration [75], our results must be interpreted in the context of the particular experimental protocol. ACD operant-drinking behaviour is induced and maintained along a relatively long period, which is likely required for exerting pharmacologically significant central effects. On the other hand, our findings are in line with early studies reporting positive euphoric effects following moderate consumption of ethanol in subjects treated with aldehyde dehydrogenase inhibitors, such as disulfiram [76].
DA involvement in the operant behaviour for ACD was assessed by using quinpirole and ropinirole, DA D2 receptor-selective ligands with a different pharmacodynamic profile, whose administration aimed at selectively modulate the DAergic synapse in different functional states. Indeed, the rationale of the experiment was that quinpirole, administered during extinction and relapse, could phasically inhibit DAergic signalling, as a consequence of its activity as agonist at D2 receptors in the presynaptic terminal, and reduce thus drug-seeking behaviour; ropinirole, on the other hand, as a D2-D3 receptor agonist, was administered daily during the deprivation period in order to stimulate the DAergic post-synaptic terminal, thus reducing the craving for the substance during reinstatement.
Our data clearly show that, when tested during extinction, quinpirole acute administration was able to decrease the number of lever presses, when compared with vehicle. Lever pressing is thought to reflect learned processes related to motivation to seek the substance [77] and it is to be considered as a measure of appetitive “ACD seeking-like” behaviour, which is in turn related to the induction of mesolimbic DA release [61].
Our evidence shows that quinpirole, at the doses used in this study, is responsible for the decrease in the number of lever presses through the reduction in DA neural firing, obtained by the activation of D2 pre-synaptic receptors [45], [46], indicating that ACD-seeking behaviour directly depends on DA neurotransmission.
In order to assess the role of dopamine neurotransmission on the reinstatement of ACD consumption following a period of forced abstinence, quinpirole was administered during relapse. In particular, along the relapse experiment, animals were administered with quinpirole during day 2, 3 and 4, in order to consider day 1 as a reference of the baseline drinking behaviour, and to evaluate drinking behaviour restoration, once the administration ceased at day 5.
Quinpirole was able to decrease the number of responses emitted and, induced a significant reduction in ACD intake, within the quinpirole treated group, when compared with the first and the last relapse days. Accordingly, the dose of quinpirole used in these experiments has previously been shown to decrease ethanol-reinforced responding [47], likely by disrupting dopamine transmission, via pre-synaptic receptors stimulation [48], [78], [79].
It has long been known that activation of D2-like receptors hyperpolarizes DA neurons and inhibits their firing activity [80]–[82]. Consistent with these data, anatomical studies revealed that D2-like receptors are predominantly expressed on the dendrites of DA neurons, where their inhibitory activity plays a predominant role [83]. Quinpirole, activating D2 pre-synapting autoreceptors, appears to dissociate the process of primary reinforcement from processes regulating instrumental response initiation, maintenance, and selection [84], hence leading to a decrease in ACD-attributed salience, and consequently in the motivation to work for drug self-administration.
In agreement with previous studies [85]–[87] and consistently with the pre-synaptic action exerted, quinpirole reduced locomotor activity in terms of total distance travelled, when tested in the Open Field. Nevertheless, no differences in rats' behavioural reactivity in the operant chamber were observed during the operant self-administration sessions, ruling out a non-specific effect of the drug in the reduction of the operant behaviour. Indeed, when tested on the operant-drinking behaviour for water, quinpirole did not exert any effect, with respect to vehicle, highlighting a specific activity on motivation for ACD.
A different activity on the DAergic synapse, i.e. a post-synaptic receptor modulation, was achieved by ropinirole administration. Ropinirole is a well-tolerated, selective D2-D3 agonist used in improving the motor symptoms of Parkinson's Disease, hence able to increase DA neurotransmission [49], [86], [88]. Due to its post-junctional activity, ropinirole was administered during extinction in order to verify whether it was able to affect drug-seeking behaviour. Our results show that ropinirole failed to induce any modification in lever pressing. This result is consistent with ropinirole activity in promoting dopamine signalling, but since ropinirole is devoid of abuse liability, it was not able, in our experimental conditions, to modify motivation when lever pressing was not associated to reinforcement.
The acute administration of low dose-ropinirole did not induce significant variations in total distance travelled in the Open Field, when compared to vehicle, confirming a mild D2-D3 receptor stimulation. On the contrary when high dose-dopamine agonists are employed, long-lasting alterations in DAergic functions appear. These are associated with increased locomotor activity and enhanced DA overflow in brain limbic areas, defined as behavioural sensitization [89], [90].
Abrupt withdrawal from alcohol and other substances of abuse is associated with a decrease in DAergic neuronal activity [24]–[27], [91], [92]. This effect has been indicated as contributing to the dysphoric state of abstinence [36], and to the enhancement in drug intake during relapse [23], [34]. Moreover, decreased levels of D2-D3 receptors in the striatum can be visualized by brain imaging in abstinent alcoholics: this feature has become a common marker of addiction in human patients [30], [32], [93].
On the other hand, manipulation of the D2 receptors seems effective in reducing drug-intake and relapse: when rodents receive viral-mediated gene transfer of D2 receptors to the nucleus accumbens, they display attenuated ethanol consumption [94].
The extent to which a mild D2-D3 stimulation, during withdrawal, can influence the reinstatement behaviour for ACD, was thus investigated.
Once acquired a long habit to ACD self-administration, and in order to be tested for reinstatement, rats underwent forced abstinence, during which they were daily administered with a low dose of ropinirole. The subsequent testing for relapse shows that ropinirole was able to significantly decrease the number of lever presses for ACD, with respect to vehicle, along the whole relapse phase. Ropinirole administration during abstinence could provide a mild stimulation of post-synaptic D2-D3 receptors, overcoming the dysfunctional reduction in DA neurotransmission associated with craving and vulnerability to relapse. Our data are in line with the hypothesis that long lasting ACD self-administration, and related behaviours, can induce a hypo-DAergic tone during withdrawal, as already demonstrated for alcohol and other addictive substances [23], likely induced by a dysfunctional inhibition of DA VTA neurons [95].
Recent reports demonstrate that ropinirole is able to reduce the subjective reinforcing effect of cocaine in humans [96], [97]; moreover Hoefer et al. [51] showed that ropinirole was able to counteract the reward deficit in methamphetamine withdrawal in rats, supporting the hypothesis that activating, rather than blocking, the DAergic system could help attenuating drug seeking behaviour [98]. Additional studies need to be undertaken to evaluate ropinirole efficacy in alcohol relapse in order to suggest its administration as a pharmacological aid in controlling the chronic relapsing nature of alcohol abuse.
Conclusions
Taken together, these findings show that the pharmacological modulation of DA synapse has a direct impact on ACD-related behaviour. Animals do self-administer ACD, and its oral consumption, or the associated conditioning context and cues, exerts a DA-releasing effect, as suggested by the different sensitivity to distinctly acting D2 agonists. Manipulation of the D2 signalling represents a promising strategy to finely tune DA control over alcohol and ACD actions in the brain. Post-synaptic D2 receptors are involved in the modulation of ACD-induced phenotypes, since their mild and subchronic activation during withdrawal is able to affect the subsequent drug seeking and intake during relapse, Moreover, ropinirole treatment during abstinence has face validity within the clinical setting, and this enhances the translational relevance of preclinical animal testing for medications in treating substance use disorders.
Funding Statement
The research was supported by a grant from the Department of Drug Sciences and Products for Health - University of Messina, Italy. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Söderpalm B, Ericson M (2013) Neurocircuitry involved in the development of alcohol addiction: the dopamine system and its access points. Curr Top Behav Neurosci 13: 127–61. [DOI] [PubMed] [Google Scholar]
- 2. Cannizzaro C, La Barbera M, Plescia F, Cacace S, Tringali G (2010) Ethanol modulates corticotropin releasing hormone release from the rat hypothalamus: does acetaldehyde play a role? Alcoholism, clinical and experimental research 34: 588–93. [DOI] [PubMed] [Google Scholar]
- 3. Correa M, Salamone JD, Segovia KN, Pardo M, Longoni R, et al. (2012) Piecing together the puzzle of acetaldehyde as a neuroactive agent. Neuroscience and biobehavioral reviews 36: 404–30. [DOI] [PubMed] [Google Scholar]
- 4. Deehan GA, Brodie MS, Rodd ZA (2013) What is in that drink: the biological actions of ethanol, acetaldehyde, and salsolinol. Current topics in behavioral neurosciences 13: 163–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karahanian E, Quintanilla ME, Tampier L, Rivera-Meza M, Bustamante D, et al. (2011) Ethanol as a prodrug: brain metabolism of ethanol mediates its reinforcing effects. Alcoholism, clinical and experimental research 35: 606–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McBride WJ, Li TK, Deitrich RA, Zimatkin S, Smith BR, et al. (2002) Involvement of acetaldehyde in alcohol addiction. Alcohol Clin Exp Res 26(1): 114–9. [PubMed] [Google Scholar]
- 7. Muggironi G, Fois GR, Diana M (2013) Ethanol-derived acetaldehyde: pleasure and pain of alcohol mechanism of action. Frontiers in behavioral neuroscience 7: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quertemont E, Grant KA, Correa M, Arizzi MN, Salamone JD, et al. (2005) The Role of Acetaldehyde in the Central Effects of Ethanol. Alcoholism: Clinical & Experimental Research 29: 221–234. [DOI] [PubMed] [Google Scholar]
- 9. Hunt WA (1996) Role of acetaldehyde in the actions of ethanol on the brain- a review. Alcohol 13(2): 147–5110. [DOI] [PubMed] [Google Scholar]
- 10. Quertemont E, De Witte P (2001) Conditioned stimulus preference after acetaldehyde but not ethanol injections. Pharmacol Biochem Behav 68(3): 449–54. [DOI] [PubMed] [Google Scholar]
- 11. Smith BR, Amit Z, Splawinsky J (1984) Conditioned place preference induced by intraventricular infusions of acetaldehyde. Alcohol 1(3): 193–5. [DOI] [PubMed] [Google Scholar]
- 12. Peana AT, Muggironi G, Diana M (2010) Acetaldehyde-reinforcing effects: a study on oral self-administration behavior. Frontiers in psychiatry/Frontiers Research Foundation 1: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown ZW, Amit Z, Rockman GE (1979) Intraventricular self-administration of acetaldehyde, but not ethanol, in naive laboratory rats. Psychopharmacology (Berl) 64(3): 271–6. [DOI] [PubMed] [Google Scholar]
- 14. Myers WD, Ng KT, Singer G (1982) Intravenous self-administration of acetaldehyde in the rat as a function of schedule, food deprivation and photoperiod. Pharmacol Biochem Behav 17(4): 807–11. [DOI] [PubMed] [Google Scholar]
- 15. Rodd-Henricks ZA, Melendez RI, Zaffaroni A, Goldstein A, McBride WJ, et al. (2002) The reinforcing effects of acetaldehyde in the posterior ventral tegmental area of alcohol-preferring rats. Pharmacology, biochemistry, and behavior 72: 55–64. [DOI] [PubMed] [Google Scholar]
- 16. Cacace S, Plescia F, Barberi I, Cannizzaro C (2012) Acetaldehyde oral self-administration: evidence from the operant-conflict paradigm. Alcoholism, clinical and experimental research 36: 1278–87. [DOI] [PubMed] [Google Scholar]
- 17. Plescia F, Brancato A, Marino RAM, Cannizzaro C (2013) Acetaldehyde as a drug of abuse: insight into AM281 administration on operant-conflict paradigm in rats. Frontiers in behavioral neuroscience 7: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, et al. (2004) Dopamine and drug addiction: the nucleus accumbens shell connection. Neuropharmacology 47(1): 227–41. [DOI] [PubMed] [Google Scholar]
- 19. Koob GF (2013) Addiction is a Reward Deficit and Stress Surfeit Disorder. Front Psychiatry 4: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koob GF (2013) Negative reinforcement in drug addiction: the darkness within. Curr Opin Neurobiol 23(4): 559–63. [DOI] [PubMed] [Google Scholar]
- 21. Wise RA, Bozarth MA (1987) A psychomotor stimulant theory of addiction. Psychol Rev 94(4): 469–92. [PubMed] [Google Scholar]
- 22. Berridge KC (2007) The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 191(3): 391–431. [DOI] [PubMed] [Google Scholar]
- 23. Diana M (2011) The dopamine hypothesis of drug addiction and its potential therapeutic value. Front Psychiatry 2: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Diana M, Pistis M, Carboni S, Gessa GL, Rossetti ZL (1993) Profound decrement of mesolimbic dopaminergic neuronal activity during ethanol withdrawal syndrome in rats: electrophysiological and biochemical evidence. Proc Natl Acad Sci U S A 90(17): 7966–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Diana M, Pistis M, Muntoni A, Gessa GL (1995) Profound decrease of mesolimbic dopaminergic neuronal activity in morphine withdrawn rats. The Journal of pharmacology and experimental therapeutics 272: 781–785. [PubMed] [Google Scholar]
- 26. Diana M, Melis M, Muntoni AL, Gessa GL (1998) Mesolimbic dopaminergic decline after cannabinoid withdrawal. Proc Natl Acad Sci U S A 95: 10269–10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Epping-Jordan MP, Watkins SS, Koob GF, Markou A (1998) Dramatic decreases in brain reward function during nicotine withdrawal. Nature 393(6680): 76–9. [DOI] [PubMed] [Google Scholar]
- 28. Franklin KM, Engleman EA, Ingraham CM, McClaren JA, Keith CM, et al. (2009) A single, moderate ethanol exposure alters extracellular dopamine levels and dopamine d receptor function in the nucleus accumbens of wistar rats. Alcohol Clin Exp Res 33(10): 1721–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thielen RJ, Engleman EA, Rodd ZA, Murphy JM, Lumeng L, et al. (2004) Ethanol drinking and deprivation alter dopaminergic and serotonergic function in the nucleus accumbens of alcohol-preferring rats. J Pharmacol Exp Ther 309(1): 216–25. [DOI] [PubMed] [Google Scholar]
- 30. Heinz A, Siessmeier T, Wrase J, Buchholz HG, Gründer G, et al. (2005) Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: a combined [18F]DOPA and [18F]DMFP PET study in detoxified alcoholic patients. Am J Psychiatry 162(8): 1515–20. [DOI] [PubMed] [Google Scholar]
- 31. Hietala J, West C, Syvälahti E, Någren K, Lehikoinen P, et al. (1994) Striatal D2 dopamine receptor binding characteristics in vivo in patients with alcohol dependence. Psychopharmacology (Berl) 116(3): 285–90. [DOI] [PubMed] [Google Scholar]
- 32. Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, et al. (2005) Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry 58(10): 779–86. [DOI] [PubMed] [Google Scholar]
- 33. Volkow ND, Wang GJ, Maynard L, Fowler JS, Jayne B, et al. (2002) Effects of alcohol detoxification on dopamine D2 receptors in alcoholics: a preliminary study. Psychiatry Res 116 (3): 163–72. [DOI] [PubMed] [Google Scholar]
- 34. Ahmed SH, Koob GF (2005) Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology 180: 473–490. [DOI] [PubMed] [Google Scholar]
- 35. Koob GF (2013) Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci 13: 3–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Melis M, Spiga S, Diana M (2005) The dopamine hypothesis of drug addiction: hypodopaminergic state. Int Rev Neurobiol 63: 101–54. [DOI] [PubMed] [Google Scholar]
- 37. Foddai M, Dosia G, Spiga S, Diana M (2004) Acetaldehyde increases dopaminergic neuronal activity in the VTA. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 29: 530–6. [DOI] [PubMed] [Google Scholar]
- 38. Deehan GA Jr, Engleman EA, Ding ZM, McBride WJ, Rodd ZA (2013) Microinjections of acetaldehyde or salsolinol into the posterior ventral tegmental area increase dopamine release in the nucleus accumbens shell. Alcohol Clin Exp Res 37(5): 722–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Enrico P, Sirca D, Mereu M, Peana AT, Lintas A, et al. (2009) Acetaldehyde sequestering prevents ethanol-induced stimulation of mesolimbic dopamine transmission. Drug Alcohol Depend 100(3): 265–71. [DOI] [PubMed] [Google Scholar]
- 40. Melis M, Enrico P, Peana AT, Diana M (2007) Acetaldehyde mediates alcohol activation of the mesolimbic dopamine system. The European journal of neuroscience 26: 2824–33. [DOI] [PubMed] [Google Scholar]
- 41. Segovia KN, Vontell R, López-Cruz L, Salamone JD, Correa M (2013) c-Fos immunoreactivity in prefrontal, basal ganglia and limbic areas of the rat brain after central and peripheral administration of ethanol and its metabolite acetaldehyde. Front Behav Neurosci 7: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peana AT, Muggironi G, Fois GR, Zinellu M, Vinci S, et al. (2011) Effect of opioid receptor blockade on acetaldehyde self-administration and ERK phosphorylation in the rat nucleus accumbens. Alcohol 45: 773–83. [DOI] [PubMed] [Google Scholar]
- 43. Gingrich JA, Caron MG (1993) Recent advances in the molecular biology of dopamine receptors. Annu Rev Neurosci 16: 299–321. [DOI] [PubMed] [Google Scholar]
- 44. De Mei C, Ramos M, Iitaka C, Borrelli E (2009) Getting specialized: presynaptic and postsynaptic dopamine D2 receptors. Current Opinion in Pharmacology 9: 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Picetti R, Saiardi A, Abdel Samad T, Bozzi Y, Baik JH, et al. (1997) Dopamine D2 receptors in signal transduction and behavior. Critical Reviews in Neurobiology 11: 121–142. [DOI] [PubMed] [Google Scholar]
- 46. Usiello A, Baik JH, Rougé-Pont F, Picetti R, Dierich A, et al. (2000) Distinct functions of the two isoforms of dopamine D2 receptors. Nature 408: 199–203. [DOI] [PubMed] [Google Scholar]
- 47. Cohen C, Perrault G, Sanger DJ (1998) Preferential involvement of D3 versus D2 dopamine receptors in the effects of dopamine receptor ligands on oral ethanol self-administration in rats. Psychopharmacology 140: 478–85. [DOI] [PubMed] [Google Scholar]
- 48. Panin F, Cathala A, Piazza PV, Spampinato U (2012) Coupled intracerebral microdialysis and electrophysiology for the assessment of dopamine neuron function in vivo. Journal of pharmacological and toxicological methods 65: 83–92. [DOI] [PubMed] [Google Scholar]
- 49. Brooks DJ, Abbott RJ, Lees AJ, Martignoni E, Philcox DV, et al. (1998) A placebo-controlled evaluation of ropinirole, a novel D2 agonist, as sole dopaminergic therapy in Parkinson's disease. Clin Neuropharmacol 21(2): 101–7. [PubMed] [Google Scholar]
- 50. Vidailhet MJ, Bonnet AM, Belal S, Dubois B, Marle C, et al. (1990) Ropinirole without levodopa in Parkinson's disease. Lancet 336(8710): 316–7. [DOI] [PubMed] [Google Scholar]
- 51. Hoefer ME, Voskanian SJ, Koob GF, Pulvirenti L (2006) Effects of terguride, ropinirole, and acetyl-L-carnitine on methamphetamine withdrawal in the rat. Pharmacology, biochemistry, and behavior 83: 403–9. [DOI] [PubMed] [Google Scholar]
- 52. Cannizzaro C, Plescia F, Cacace S (2011) Role of acetaldehyde in alcohol addiction: current evidence and future perspectives. Malta Med. J 23: 27. [Google Scholar]
- 53. Rodd ZA, Bell RL, Zhang Y, Murphy JM, Goldstein A, et al. (2005) Regional heterogeneity for the intracranial self-administration of ethanol and acetaldehyde within the ventral tegmental area of alcohol-preferring (P) rats: involvement of dopamine and serotonin. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 30: 330–8. [DOI] [PubMed] [Google Scholar]
- 54. Karahanian E, Rivera-Meza M, Tampier L, Quintanilla ME, Herrera-Marschitz M, et al. (2014) Long-term inhibition of ethanol intake by the administration of an aldehyde dehydrogenase-2 (ALDH2)-coding lentiviral vector into the ventral tegmental area of rats. Addict Biol. 26 doi: 10.1111/adb.12130. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 55. Lieber CS, De Carli LM (1968) Ethanol oxidation by hepatic microsomes: adaptive increase after ethanol feeding. Science 162: 917–918. [DOI] [PubMed] [Google Scholar]
- 56. Lieber CS (1999) Microsomal ethanol-oxidizing system: the first 30 years (1968–1998)—a review. Alcohol Clin Exp Res 23: 991–1007. [PubMed] [Google Scholar]
- 57. Takahashi T, Lasker JM, Rosman AS, Lieber CS (1993) Induction of cytochrome P-4502E1 in the human liver by ethanol is caused by a corresponding increase in encoding messenger RNA. Hepatology 17: 236–245. [PubMed] [Google Scholar]
- 58. Nuutinen H, Lindros KO, Salaspuro M (1983) Determinants of blood acetaldehyde level during ethanol oxidation in chronic alcoholics. Alcohol Clin Exp Res 7: 163–168. [DOI] [PubMed] [Google Scholar]
- 59. Alen F, Moreno-Sanz G, Isabel de Tena, A, Brooks RD, López-Jimenez A, et al. (2008) Pharmacological activation of CB1 and D2 receptors in rats: predominant role of CB1 in the increase of alcohol relapse. The European journal of neuroscience 27: 3292–8. [DOI] [PubMed] [Google Scholar]
- 60. Colombo G, Agabio R, Fà M, Guano L, Lobina C, et al. (1998) Reduction of voluntary ethanol intake in ethanol-preferring sP rats by the cannabinoid antagonist SR-141716. Alcohol and alcoholism 33: 126–30. [DOI] [PubMed] [Google Scholar]
- 61. Vengeliene V, Bilbao A, Molander A, Spanagel R (2008) Neuropharmacology of alcohol addiction. British journal of pharmacology 154: 299–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Edwards S, Koob GF (2013) Escalation of drug self-administration as a hallmark of persistent addiction liability. Behav Pharmacol 24(5–6): 356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.June HL, Gilpin NW (2010) Operant self-administration models for testing the neuropharmacological basis of ethanol consumption in rats. Current protocols in neuroscience; Chapter 9, Unit 9.12: .1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Katz JL, Higgins ST (2003) The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology 168: 21–30. [DOI] [PubMed] [Google Scholar]
- 65. Reichel CM, Bevins RA (2009) Forced abstinence model of relapse to study pharmacological treatments of substance use disorder. Current drug abuse reviews 2: 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sanchis-Segura C, Spanagel R (2006) Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol 11(1): 2–38. [DOI] [PubMed] [Google Scholar]
- 67. Shaham Y, Miczek KA (2003) Reinstatement? Toward a model of relapse. Psychopharmacology 168: 1–2. [DOI] [PubMed] [Google Scholar]
- 68. Spanagel R (2000) Recent animal models of alcoholism. Alcohol research health the journal of the National Institute on Alcohol Abuse and Alcoholism 24: 124–131. [PMC free article] [PubMed] [Google Scholar]
- 69. Quertemont E (2004) Genetic polymorphism in ethanol metabolism: acetaldehyde contribution to alcohol abuse and alcoholism. Mol Psychiatry 9(6): 570–81. [DOI] [PubMed] [Google Scholar]
- 70. Ward RJ, Colantuoni C, Dahchour A, Quertemont E, De Witte P (1997) Acetaldehyde-induced changes in monoamine and amino acid extracellular microdialysate content of the nucleus accumbens. Neuropharmacology 36(2): 225–32. [DOI] [PubMed] [Google Scholar]
- 71. Westerlund M1, Belin AC, Felder MR, Olson L, Galter D (2007) High and complementary expression patterns of alcohol and aldehyde dehydrogenases in the gastrointestinal tract: implications for Parkinson's disease. FEBS J 274(5): 1212–23. [DOI] [PubMed] [Google Scholar]
- 72. Zimatkin SM Histochemical study of aldehyde dehydrogenase in the rat CNS. J Neurochem 56(1): 1–11. [DOI] [PubMed] [Google Scholar]
- 73. Hoover DJ, Brien JF (1981) Acetaldehyde concentration in rat blood and brain during the calcium carbimide—ethanol interaction. Can J Physiol Pharmacol 59(1): 65–70. [DOI] [PubMed] [Google Scholar]
- 74. Westcott JY, Weiner H, Shultz J, Myers RD (1980) In vivo acetaldehyde in the brain of the rat treated with ethanol. Biochem Pharmacol 29(3): 411–7. [DOI] [PubMed] [Google Scholar]
- 75. Escrig MA, Pardo M, Aragon CM, Correa M (2012) Anxiogenic and stress-inducing effects of peripherally administered acetaldehyde in mice: similarities with the disulfiram-ethanol reaction. Pharmacol Biochem Behav 100(3): 404–12. [DOI] [PubMed] [Google Scholar]
- 76. Brown ZW, Amit Z, Smith BR, Sutherland EA, Selvaggi N (1983) Alcohol-induced euphoria enhanced by disulfiram and calcium carbimide. Alcohol Clin Exp Res 7(3): 276–8. [DOI] [PubMed] [Google Scholar]
- 77. Samson HH, Slawecki CJ, Sharpe AL, Chappell A (1998) Appetitive and consummatory behaviors in the control of ethanol consumption: a measure of ethanol seeking behavior. Alcohol Clin Exp Res 22(8): 1783–7. [PubMed] [Google Scholar]
- 78. Widzowski DV, Cory-Slechta DA (1993) Apparent mediation of the stimulus properties of a low dose of quinpirole by dopaminergic autoreceptors. The Journal of pharmacology and experimental therapeutics 266: 526–534. [PubMed] [Google Scholar]
- 79. Furmidge L, Tong ZY, Petry N, Clark D (1991) Effects of low, autoreceptor selective doses of dopamine agonists on the discriminative cue and locomotor hyperactivity produced by d-amphetamine. Journal Of Neural Transmission General Section 86: 61–70. [DOI] [PubMed] [Google Scholar]
- 80. Grace AA, Bunney BS (1985) Low doses of apomorphine elicit two opposing influences on dopamine cell electrophysiology. Brain Research 333: 285–298. [DOI] [PubMed] [Google Scholar]
- 81. Johnson S, North R (1992) Two types of neurone in the rat ventral tegmental area and their synaptic inputs. The Journal of physiology 450: 455–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lacey MG, Mercuri NB, North RA (1987) Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. The Journal of Physiology 392: 397–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sesack SR, Aoki C, Pickel VM (1994) Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. Journal of Neuroscience 14: 88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Salamone JD, Cousins MS, Snyder BJ (1997) Behavioral functions of nucleus accumbens dopamine: empirical and conceptual problems with the anhedonia hypothesis. Neuroscience & Biobehavioral Reviews 21: 341–359. [DOI] [PubMed] [Google Scholar]
- 85. Abrahao KP, Quadros IMH, Andrade ALM, Souza-Formigoni MLO (2012) Accumbal dopamine D2 receptor function is associated with individual variability in ethanol behavioral sensitization. Neuropharmacology 62: 882–9. [DOI] [PubMed] [Google Scholar]
- 86. Beaulieu JM, Gainetdinov RR (2011) The Physiology, Signaling, and Pharmacology of Dopamine Receptors. Pharmacol Rev 63(1): 182–217. [DOI] [PubMed] [Google Scholar]
- 87. Broadbent J, Kampmueller KM, Koonse S (2005) Role of dopamine in behavioral sensitization to ethanol in DBA/2J mice. Alcohol (Fayetteville, N.Y.) 35: 137–48. [DOI] [PubMed] [Google Scholar]
- 88. Emilien G, Maloteaux JM, Geurts M, Hoogenberg K, Cragg S (1999) Dopamine receptors - physiological understanding to therapeutic intervention potential. Pharmacology therapeutics 84: 133–156. [DOI] [PubMed] [Google Scholar]
- 89. Richtand NM, Goldsmith RJ, Nolan JE, Berger SP (2001) The D3 dopamine receptor and substance dependence. Journal of Addictive Diseases 20: 19–32. [DOI] [PubMed] [Google Scholar]
- 90. Vezina P (2004) Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neuroscience & Biobehavioral Reviews 27: 827–839. [DOI] [PubMed] [Google Scholar]
- 91. Rossetti ZL, Melis F, Carboni S, Diana M, Gessa GL (1992) Alcohol withdrawal in rats is associated with a marked fall in extraneuronal dopamine. Alcoholism: Clinical and Experimental Research 16: 529–532. [DOI] [PubMed] [Google Scholar]
- 92. Weiss F, Parsons LH, Schulteis G, Hyytiä P, Lorang MT, et al. (1996) Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J Neurosci 16(10): 3474–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F (2009) Imaging dopamine's role in drug abuse and addiction. Neuropharmacology 56(1): 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Thanos PK, Michaelides M, Umegaki H, Volkow ND (2008) D2R DNA transfer into the nucleus accumbens attenuates cocaine self-administration in rats. Synapse 62: 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Perra S, Clements MA, Bernier BE, Morikawa H (2011) In vivo ethanol experience increases D(2) autoinhibition in the ventral tegmental area. Neuropsychopharmacology 36: 993–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Maremmani AGI, Pacini M, Rovai L, Rugani F, Dell'Osso L, et al. (2011) Can ropinirole modulate reinforcing subjective effects of cocaine in humans? Front Psychiatry 2: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Meini M, Moncini M, Cecconi D, Cellesi V, Biasci L, et al. (2011) Aripiprazole and ropinirole treatment for cocaine dependence: evidence from a pilot study. Current Pharmaceutical Design 17: 1376–1383. [DOI] [PubMed] [Google Scholar]
- 98. Blum K, Chen AL, Chen TJ, Braverman ER, Reinking J, et al. (2008) Activation instead of blocking mesolimbic dopaminergic reward circuitry is a preferred modality in the long term treatment of reward deficiency syndrome (RDS): a commentary. Theor Biol Med Model 12: 5–24. [DOI] [PMC free article] [PubMed] [Google Scholar]