Abstract
Sensory cortices can not only detect and analyze incoming sensory information but can also undergo plastic changes while learning behaviorally important sensory cues. This experience-dependent cortical plasticity is essential for shaping and modifying neuronal circuits to perform computations of multiple, previously unknown sensations, the adaptive process that is believed to underlie perceptual learning. Intensive efforts to identify the mechanisms of cortical plasticity have provided several important clues; however, the exact cellular sites and mechanisms within the intricate neuronal networks that underlie cortical plasticity have yet to be elucidated. In this review, we present several parallels between cortical plasticity in the auditory cortex and recently discovered mechanisms of synaptic plasticity gating at thalamocortical projections that provide the main input to sensory cortices. Striking similarities between the features and mechanisms of thalamocortical synaptic plasticity and those of experience-dependent cortical plasticity in the auditory cortex, especially in terms of regulation of an early critical period, point to thalamocortical projections as an important locus of plasticity in sensory cortices.
Keywords: Synaptic plasticity, auditory cortex, perceptual memory, thalamocortical synapses, presynaptic, adenosine
Introduction
The thalamus and its afferent projections provide the main pathway of sensory input to the primary sensory cortices of the brain. Thalamocortical (TC) projections send streams of information to sensory cortices, which constantly receive incoming signals, analyze their attributes, and sort them into distinct categories such as intensity, location, movement, and other characteristics of sound, light, and touch. With such an overwhelming amount of input, sensory cortices require a sorting mechanism that selects only important information to prevent functional “overload” of the finite capabilities of the cortical circuitry. Thus, some stimuli cause immediate behavioral responses; some leave a lasting impression on neural networks; and others may be overlooked entirely. In this paradigm, sensory cortices are not only input analyzers but also an information filtering and storage site. It appears that these properties of sensory cortices do not persist throughout the lifespan. Neonatal sensory cortices are more plastic and store information more readily than do their mature counterparts. The neonatal brain of rodents continually produces, develops, and refines sensory-input connections for days to weeks after birth, and the stability and persistence of these immature pathways is directly related to the activity levels they experience. During this early “critical period” of synapse formation and stabilization, very little filtering of information storage occurs; the immature sensory cortices rapidly individualize to their unique sensory environment through the persistence of active connections and the retraction and elimination of inactive ones. During this critical period, sensory cortices are “passive learners” of the surrounding sensory milieu. Once this period is over, however, storage of sensory information becomes filtered or gated, resulting in the substantially greater difficulty and less likely passive flow of sensory information affecting structure or connectivity of the primary sensory cortices. During this period, additional mechanisms (i.e. attention) are needed to grade incoming sensory information in order of importance and store only information associated with important tasks or experiences. During this time, sensory cortices become “associative learners” that modify their circuitry only when sensory information is behaviorally relevant.
The sensory connections that are reinforced with relative ease during the critical period or with more stringent requirements thereafter may have distinct purposes. Connections stabilized during the critical period may be responsible for laying down the basic topographic representational maps that order incoming sensory pathways, and those that are formed or strengthened later may result in perceptual learning of behaviorally relevant stimuli. During the development of the auditory cortex (ACx) of rodents, tonotopic arrangements are established and refined by 2 weeks of age (Sun and others, 2010), shortly after hearing onset (Ehret, 1976; Kraus and Aulbach-Kraus, 1981; Sally and Kelly, 1988). Passive exposure to sounds can influence receptive fields of cortical neurons and tonotopic maps in the ACx during a few days after hearing onset but not thereafter (Barkat and others, 2011; de Villers-Sidani and others, 2007; Insanally and others, 2009; Zhang and others, 2001). After this critical period is over, the tonotopic maps in the ACx can still be altered but only by behaviorally important sounds. For instance, during perceptual learning unique sound frequencies, intensities, or patterns can be associated with the presentation of either reward or punishment, making the conditioning sounds behaviorally relevant. It is well documented that receptive fields and tonotopic maps in the ACx of mature animals are influenced only by sounds that have been paired with these associative cues (Bakin and Weinberger, 1990; Blake and others, 2006; Polley and others, 2006; Diamond and Weinberger, 1986; Gao and Suga, 1998; Gao and Suga, 2000; Ji and Suga, 2003; Ji and others, 2005; Keuroghlian and Knudsen, 2007; Rutkowski and Weinberger, 2005; Suga and Ma, 2003) or with the electrical stimulation of circuits or sources of modulatory (mainly cholinergic) inputs (Bakin and Weinberger, 1996; Bao and others, 2001; Bjordahl and others, 1998; Chowdhury and Suga, 2000; Kilgard and Merzenich, 1998a; Ma and Suga, 2003; Ma and Suga, 2005; Weinberger, 2004). Thus, the ACx of animals readily detects sensory signals during both young (during critical period) and mature (after critical period) phases of development, but only during the critical period can these sensory signals alone cause a lasting effect on the cortical circuitry. These data allow for several inferences: First, the need for neuromodulators in experience-dependent cortical plasticity in animals aged beyond the critical period implies that attention-related mechanisms help gate cortical plasticity in adults (Keuroghlian and Knudsen, 2007). Second, the substrate of cortical plasticity is in place in the adult ACx. Third, adult cortical plasticity is prevented by gating mechanisms that are active when the animal does not pay attention to sounds or when the detected sounds are behaviorally irrelevant. Fourth, these gating mechanisms can be released by surges of acetylcholine or other neuromodulators once a sound becomes behaviorally important. Fifth, gating mechanisms are developmentally regulated. The termination of the critical period of cortical plasticity may be caused by an age-dependent emergence of such gating mechanisms that filter unnecessary or behaviorally irrelevant information from making changes in the finite computational circuitry that is available for storage of perceptual memories.
Here we will propose possible mechanisms of cortical plasticity gating, with a focus on the rodent primary ACx. We will also discuss a hypothesis that afferent thalamic inputs to the ACx are the sites of such gating. This hypothesis arose from our observations that TC synapses reliably transfer sensory information to sensory cortices in both young and mature animals but quickly lose their ability to undergo long-term synaptic plasticity after the early critical period. Synaptic plasticity remains largely preserved at other central synapses, including intracortical synapses in the ACx and other sensory cortices throughout and past development and is considered a leading cellular mechanism of various forms of learning and memory (Blundon and Zakharenko, 2008). Finally, we will review recent data that indicate that TC synapses, in fact, do not lose their plasticity in adulthood. Instead, similar to experience-dependent cortical plasticity, they acquire gating mechanisms that convert the synapses from passive learners to associative learners. We will discuss the emergence of cellular and molecular mechanisms of synaptic plasticity gating at TC synapses and suggest that this first synaptic relay station that conveys sensory information into the cortex has the ability to not only deliver information but also store it not only in the immature brain but in adults as well.
Critical periods of plasticity in sensory cortices
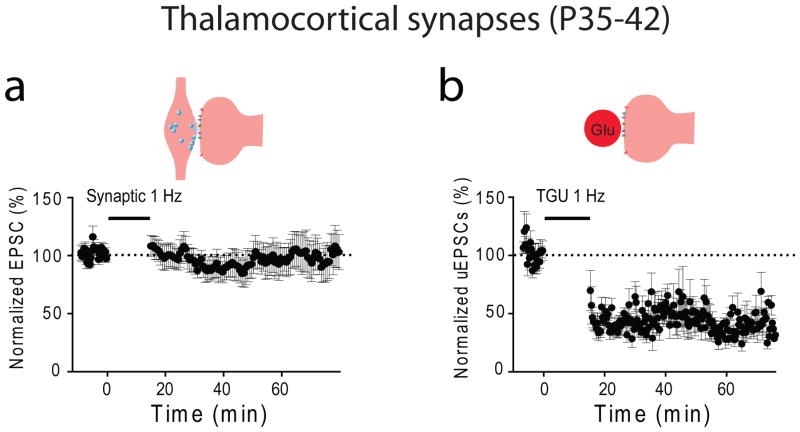
The concept of the critical period of synaptic plasticity at TC synapses emerged from experiments done in brain slices containing portions of the thalamus and sensory cortex (Figure 1). This preparation allows one to measure TC synaptic strength by electrically stimulating the thalamic radiation that contains TC projections and recording responses in thalamorecipient neurons in Layer (L) IV of the somatosensory cortex. Crair and Malenka (1995) showed the ease and simplicity with which the TC synapses of newborn rats adapt to previous synaptic activity. Specifically, in slices taken from rats aged postnatal day (P) 3 through P7, electric stimulation of the thalamic radiation paired with postsynaptic depolarization resulted in a rapid and long-lasting increase in synaptic strength, known as long-term potentiation (LTP) (Figure 2a). Conversely, long-term depression (LTD) of synaptic responses occurred in neonate LIV somatosensory neurons subjected to prolonged low-frequency stimulation of the thalamic radiation (Feldman and others, 1998) (Figure 2b). Repetition of these experiments in slices from animals just a few days older had entirely different results: stimulation of the thalamic radiation that yielded LTP or LTD in neonates produced no long-lasting changes in synaptic responses in older animals (Figures 2a, b). Similar results have been reported for LIV neurons of the visual cortex (Jiang and others, 2007). These experiments led to the notion of an early critical period: TC synaptic plasticity extinguishes within the first 2 weeks after birth (Figure 2c).
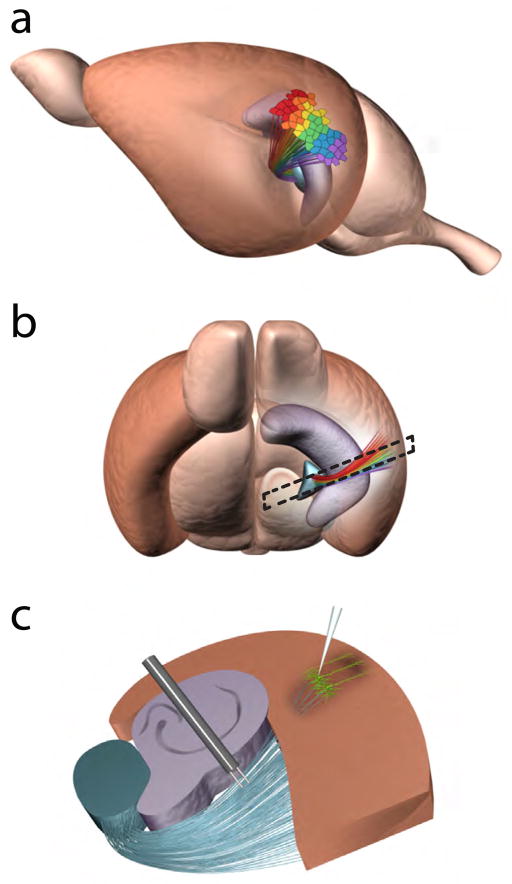
Figure 1. Thalamocortical projections form cortical maps in the primary ACx in mice.
(a, b) Cortical maps of sound frequencies in the primary ACx (brown region). The teal structure is the auditory thalamus (the ventral part of the medial geniculate, MGv) sending thalamocortical projections to the ACx, and the purple structure is the hippocampus. Different colors represent the tonotopy of thalamic projections and the primary ACx. Dashed lines in (b) represent the orientation and shape of the TC slice. (c) TC slice containing portions of the auditory thalamus, hippocampus, and ACx. LIII/IV pyramidal neurons (green) are the main thalamorecipient neurons in the primary ACx. Stimulating and recording electrodes are placed at the thalamic radiation and ACx, respectively. Note, the thalamic radiation contains multiple ascending and descending projections but only ascending thalamocortical projections are shown.
Figure 2. Critical periods for TC synaptic plasticity and cortical map plasticity in the ACx.
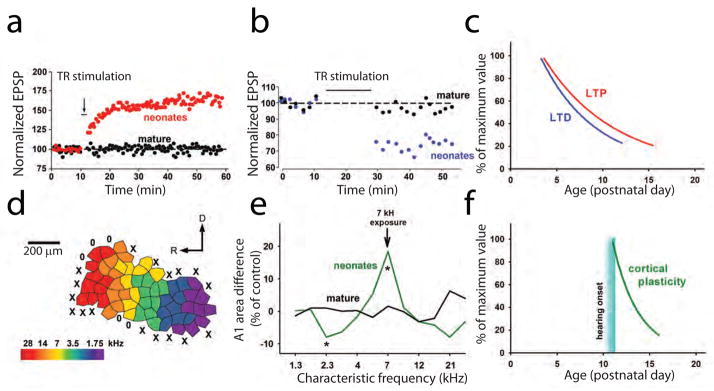
(a, b) TC LTP (a) and TC LTD (b) are readily expressed in young but not mature animals. (c) LTP and LTD expression as a function of age. This graph is based on data from previous studies (Crair and Malenka, 1995; Feldman and others, 1998; Jiang and others, 2007). (d) A representative cortical map of sound frequencies in the primary ACx of mice. (e) Changes in cortical map representation in the primary (A1) area of the ACx after 2 weeks of exposure to a 7-kHz sound in neonates and mature animals (* p<0.05) [adapted from (de Villers-Sidani and others, 2007)]. (f) Cortical map plasticity as a function of age. Abbreviations: D, dorsal; EPSP, excitatory postsynaptic potential; R, rostral; TR, thalamic radiation.
Critical periods have implications in early brain development as well. For decades, research has shown that the development and stabilization of neural pathways and synapses is ongoing during neonatal critical periods and that unstimulated synapses and pathways often retract or die (Shatz, 1996). In this way, the brain of the newborn animal quickly individualizes to its unique environmental experiences. The large-scale morphologic changes, as seen throughout this critical period during axonal and synaptic structural refinement, are no doubt metabolically costly. Therefore, having a prolonged critical period would be evolutionarily disadvantageous. Yet, there are costs associated with the termination of the critical period as well. Environmental stimuli that were not experienced by the neonate but are novel to the adult animal may be more difficult to incorporate into memory or even to perceive. For example, monocular deprivation for just a few weeks after birth results in the retraction and death of quieted neural pathways from the thalamus to the primary visual cortex, and as a result, adult animals experience permanent cortical blindness of the deprived eye (Wiesel and Hubel, 1965).
Although many in vitro experiments have described the disappearance of TC synaptic plasticity beyond an early critical period, in vivo experiments clearly show that cortical plasticity also has a critical period (Keuroghlian and Knudsen, 2007). Cortical maps in the ACx expand in response to the enrichment of the environment with a sound of a certain frequency in neonatal but not mature rodents (Figure 2d, e). These experiments take advantage of the fact that the rodent ACx is organized in a tonotopic pattern with low-frequency–responsive neurons located at the caudal region and high-frequency–responsive neurons distributed toward the rostral region (Figure 2d). The restructuring of these tonotopic maps is used as a readout of experience-dependent cortical plasticity. Passive exposure to a single-frequency conditioning tone given during the first 2 weeks after birth shifts the map’s frequency distribution toward the frequency of the conditioning tone (Figure 2e) but does not induce changes in auditory tonotopic maps when given to animals aged past this critical period (Barkat and others, 2011; de Villers-Sidani and others, 2007; Insanally and others, 2009; Zhang and others, 2001). Thus, in vivo cortical plasticity elicited by passive sound exposure has a critical period similar to that of in vitro TC plasticity, beginning at the age of hearing onset in rodents (approximately P10-P11) (Ehret, 1976; Kraus and Aulbach-Kraus, 1981) and ending within the first 2 weeks of life (Figure 2f).
Cortical plasticity, however, can be induced in adults, if they are given the appropriate training protocol (Keuroghlian and Knudsen, 2007). Unlike passive-conditioning experiments, associative-conditioning protocols that induce or mimic attention coincident with the presentation of conditioning tones do induce tonotopic reorganization in adult animals. For example, the pairing of tones with reward (Blake and others, 2006; Polley and others, 2006), punishment (Bakin and Weinberger, 1990), the activation of cholinergic projections from the nucleus basalis (Bakin and Weinberger, 1996; Edeline and others, 1994; Froemke and others, 2007; Hars and others, 1993; Kilgard and Merzenich, 1998b; Kilgard and Merzenich, 1998a; Ma and Suga, 2005), or the direct activation of cholinergic muscarinic receptors (Miasnikov and others, 2001; Thiel and others, 2002) all induce cortical plasticity in the adult ACx. [Note that pairing tones with other neuromodulators such as dopamine or norepinephrine also induces cortical plasticity (Bao and others, 2001; Edeline and others, 2011)]. Similar results are seen in other sensory cortices. In vivo cortical plasticity in adults has been associated with the modulation by cholinergic inputs or basal forebrain stimulation in the visual cortex (Bear and Singer, 1986; Brocher and others, 1992; Gordon and others, 1990; Gu and Singer, 1993) or somatosensory cortex (Juliano and others, 1990; Sachdev and others, 1998). In other words, activation of modulatory inputs releases the gating of experience-dependent cortical plasticity in animals after the critical period. One would expect that the synaptic mechanisms that underlie cortical plasticity in sensory cortices should have similar properties.
The concept of synaptic plasticity gating at thalamocortical synapses
A consensus on the cellular substrate of experience-dependent cortical plasticity has not yet been reached. Bidirectional, long-term synaptic plasticity at TC synapses was an attractive mechanism, until the concept of a critical period for synaptic plasticity at TC synapses made this hypothesis problematic. Although perceptual learning and cortical plasticity have a critical period, both can occur throughout the lifespan, whereas TC synaptic plasticity appears to be severely restricted in adults. Until recently, TC synaptic plasticity was considered strictly a neonatal feature. The most parsimonious conclusion drawn by the field was that TC synaptic plasticity is lost in adults; therefore, other synapses or circuits must underlie cortical map plasticity in adulthood. For instance, in vivo recordings from neurons in multiple cortical layers in the ACx of adult rats before and after conditioning suggested that corticocortical but not TC synapses are the sites of experience-dependent cortical plasticity (Froemke and others, 2007).
Corticocortical synapses do not undergo a sharp loss of LTP and LTD during development as do TC synapses. Developmental regulation of cortocortical plasticity is less dramatic than that of TC plasticity, i.e., it can be induced by the same experimental procedures in neonates and adults, does not have a well-defined critical period, and is present in sensory cortices long after the end of the early critical period described for TC synaptic plasticity (Amitai, 2001; Castro-Alamancos and Connors, 1996; Huang and others, 2012; Jiang and others, 2007; Kirkwood and Bear, 1994a). Therefore, the lack of similarity in the developmental regulation of corticocortical synaptic plasticity and that of cortical plasticity in vivo makes it currently difficult to recognize corticocortical synapses as the sites of experience-dependent cortical plasticity. Besides, corticocortical synapses are not a uniform entity, they are distributed throughout many cortical layers, several synapses downstream from thalamic neurons. This downstream position, within complex neuronal circuitry, predicts a stochastic pattern of presynaptic activity and low reproducibility of LTP or LTD at these synapses in response to sensory stimulation. TC synapses, because of their more upstream position within the cortical circuitry, reliably respond to sensory stimulation, thereby predicting more consistent induction of synaptic plasticity. Because of this consideration and the similarity between the critical periods for TC synaptic plasticity and experience-dependent cortical plasticity, TC synaptic plasticity remains an attractive candidate for cellular mechanism of cortical plasticity in sensory cortices.
To continue being considered a site of cortical plasticity, TC synaptic plasticity should persist into adulthood. Several reports indicate that TC plasticity persists, even after the previously defined critical period (Blundon and others, 2011; Cooke and Bear, 2010; Hogsden and Dringenberg, 2009a; Lee and Ebner, 1992; Yu and others, 2012; Chun and others, 2013). Moreover, similar to cortical plasticity, TC synaptic plasticity should be gated by modulatory inputs, especially by cholinergic inputs from the nucleus basalis. Removal or deactivation of such gating should enable bidirectional synaptic plasticity in the form of LTP and LTD at TC synapses of animals aged far beyond the critical period. This hypothesis predicts that removing the gating mechanisms would eliminate or dramatically extend the critical period for cortical map plasticity in sensory cortices.
LTD gating at thalamocortical synapses in adults
We recently tested the idea of synaptic plasticity gating at TC synapses in the ACx of mice matured beyond the critical period (Blundon and others, 2011). In this work, we attempted to induce LTD at the TC synapses of mature mice. Consistent with the idea of the critical period, electrical stimulation of the thalamic radiation failed to induce any form of plasticity in cortical neurons (Figure 3a). We hypothesized that LTD becomes gated after the critical period and this LTD gating could occur in an element of the TC circuitry that is presynaptic to thalamorecipient synapses in the ACx.
Figure 3. Thalamocortical synaptic plasticity is unmasked in mature animals by 2-hoton glutamate uncaging at a TC input of cortical neurons.
(a) Synaptic stimulation via the thalamic radiation fails to induce TC LTD in slices from mice matured beyond the critical period. (b) The same pattern of stimulation delivered to a single thalamic input of an LIII/IV pyramidal neuron by TGU produces LTD.
To bypass the presynaptic circuitry entirely, we used two-photon glutamate uncaging (TGU) to release glutamate directly onto an individual dendritic spine of an LIII/IV thalamorecipient neuron. Previous experiments in our laboratory identified morphologies and locations of dendritic spines that are the targets of functional thalamic inputs (Richardson and others, 2009). Using this information, we mimicked the pattern of electrical stimuli, which reliably induces LTD at other central synapses (1 Hz, 15 min) with the same pattern of TGU stimulation of individual dendritic spines that are the sites of thalamic inputs. Consistent with the hypothesis of presynaptic plasticity gating, this approach produced a robust LTD at TC synapses (Figure 3b). This experiment proved that TC synapses do not lose plasticity, even when electrical stimulation of the thalamic radiation fails to induce synaptic plasticity. It also demonstrated that TC LTD is expressed by postsynaptic mechanisms. Subsequent experiments showed that the expression mechanisms involve the activation of group I metabotropic glutamate receptors (mGluRs) and an increase in the concentration of postsynaptic calcium (Figure 4). Most importantly, these experiments indicated that TC synaptic plasticity still exists in adults and may therefore be a cellular mechanism of cortical plasticity. Thus, we endeavored to find more parallels between TC synaptic plasticity and cortical plasticity with the goal of learning more about the cellular and molecular mechanisms of perceptual learning.
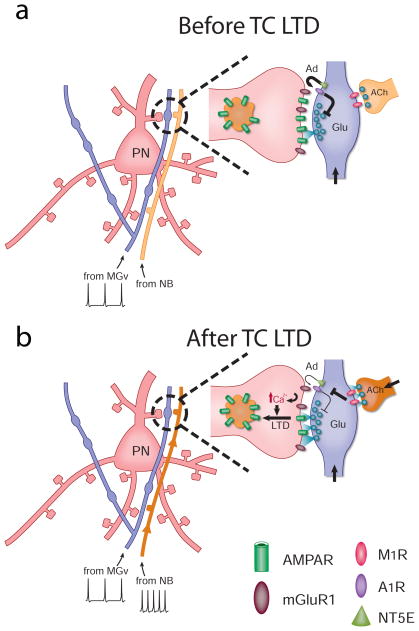
Figure 4. The model of TC LTD.
(a) Cholinergic synapses at thalamic projections are not active before LTD. Activity-dependent adenosine (Ad) release from thalamic projections gate glutamate (Glu) release and TC LTD. (b) Activation of cholinergic projections downregulates the adenosine machinery, sustains glutamate’s release from thalamic terminals, and releases gating mechanisms of TC LTD. TC LTD is expressed postsynaptically through mGluR-dependent mechanisms. Abbreviations: ACh, acetylcholine; MGv, ventral medial geniculate; NB, nucleus basalis; NT5E, ecto-5′-nucleotidase; PN, pyramidal neuron.
One of the parallels between TC synaptic plasticity and cortical plasticity is gating. Cortical plasticity in the ACx can be induced in neonates by passive exposure to certain sounds, but in adults, the same cortical plasticity occurs only if an animal attends to a sound or a sound delivery is paired with the activation of modulatory (mainly cholinergic) systems. In the context of gating, cholinergic activation can be seen as a switch that turns on plasticity when acoustic information arrives to the adult ACx. If this switch is turned off, acoustic information is delivered to the ACx but does not cause long-term changes in its circuitry. Recently, our laboratory provided an important parallel between cortical plasticity in vivo and synaptic plasticity in brain slices by showing that a similar switch gates plasticity at TC synapses (Blundon and others, 2011). Pairing cholinergic activation with low-frequency electrical stimulation of the thalamic radiation was sufficient to induce TC LTD far beyond the critical period.
Because a brain slice preparation is substantially more amendable to subcellular imaging and pharmacologic manipulations, the system makes it possible to investigate the molecular mechanism of synaptic plasticity gating in the ACx in more detail. Using direct imaging of presynaptic function (e.g., the FM 1-43 assay) (Zakharenko and others, 2001; Zakharenko and others, 2003) and direct testing of postsynaptic function (e.g., TGU), it became clear that gating mechanisms of synaptic plasticity operate at a presynaptic locus of a TC synapse. We concluded that cholinergic activation can sustain glutamate release from thalamic afferents (Blundon and others, 2011) that normally tends to quickly depress during repeated stimulation (Castro-Alamancos, 1997; Gil and others, 1997; Gil and others, 1999; Stratford and others, 1996). This effect is mediated by presynaptic M1 muscarinic receptors (M1Rs) (Figure 4). Without M1R activation, the stimulation of the thalamic radiation leads to the rapid depression of glutamate release from thalamic inputs and insufficient activation of mGluRs on dendritic spines of cortical neurons, and as a result, LTD is not induced. Similarly, cortical plasticity induced by pairing sounds with cholinergic activation depends on M1Rs (Zhang and others, 2006). Thus, presynaptic gating of plasticity at TC synapses is mechanistically comparable to that of cortical plasticity described in the adult ACx.
The amenability of slice preparations also allowed us to determine how M1Rs affect neurotransmitter release from thalamic inputs. We found that presynaptic M1Rs negatively regulate adenosine machinery (Blundon and others, 2011), a well-known negative regulator of neurotransmitter release at central synapses (Dunwiddie and Masino, 2001). Adenosine activates presynaptic A1 adenosine receptors (A1Rs) and suppresses the release of neurotransmitter. Through this double-negative regulation, M1R activation leads to higher sustained levels of glutamate release from thalamic terminals. Consistent with this model, the deletion of A1Rs removed presynaptic gating and allowed LTD at TC synapses by electrical stimulation of the thalamic radiation alone (Blundon and others, 2011), a method that is effective in wild-type mice only during the critical period.
Two mechanisms gate thalamocortical LTP in adults
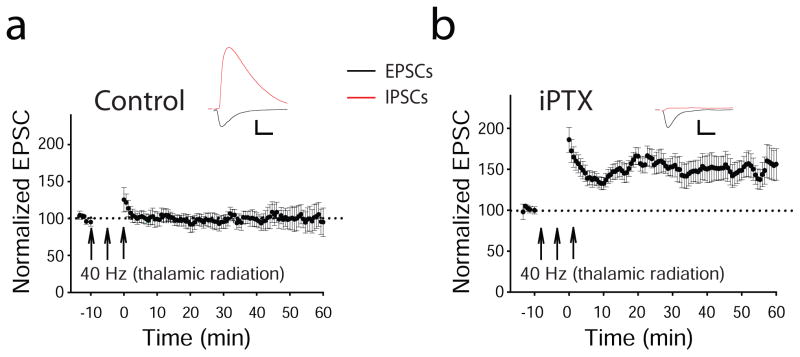
Shifts in the cortical maps of the ACx during associative conditioning typically show both increases in areas responsive to the conditioning-tone frequency and decreases in areas responsive to not conditioning-tone frequencies (Bakin and Weinberger, 1990; Weinberger, 2007a). If TC synaptic plasticity underlies these shifts in cortical maps, one might expect that bidirectional changes in synaptic strength (in the form of LTP and LTD) should be present at TC synapses. Until recently, the general consensus has been that TC LTP cannot be induced in slices from rodents older than 2 to 3 weeks (Crair and Malenka, 1995; Jiang and others, 2007). Thus, those in the field generally agree that TC synapses lose the capacity for LTP in adulthood. Recently, our laboratory revealed TC LTP in adult TC slices by describing and manipulating gating mechanisms that restrict this form of synaptic plasticity in mature animals (Chun and others, 2013). Consistent with the notion of the critical period for LTP at TC synapses, all types of stimulating protocols that readily induce LTP at other central synapses failed to induce LTP at TC synapses in slices from animals older than P30 (Figure 5a). However, stimulating protocols that mimic bursting activity of thalamic neurons in response to a sound were effective once intracortical inhibitory inputs to thalamorecipient neurons in the ACx were blocked either pharmacologically (Figure 5b) or physiologically (Chun and others, 2013). Further experiments revealed that similar to TC LTD, TC LTP in the ACx is expressed postsynaptically, requires activation of postsynaptic group I mGluRs, and depends on an increase in the concentration of intracellular calcium (Chun and others, 2013).
Figure 5. Cortical dysinhibition is required for TC LTP in mature animals.
(a) The 40-Hz tetanization of the thalamic radiation alone is not sufficient to induce TC LTP in mature animals. (b) Tetanization of the thalamic radiation coincident with deactivation of inhibitory inputs to LIII/IV pyramidal neurons via administration of intracellular picrotoxin (iPTX) is sufficient to induce TC LTP. Abbreviations: EPSC, excitatory postsynaptic currents; IPSCs, inhibitory postsynaptic currents. Scale bars: 100 pA, 10 ms.
It appears that mechanisms of expression of TC plasticity in the ACx differ from those of other sensory cortices. In contrast to the TC LTP and LTD expressed in the ACx, those in somatosensory or visual cortices depend on NMDARs (Barth and Malenka, 2001; Crair and Malenka, 1995; Gagolewicz and Dringenberg, 2011; Heynen and Bear, 2001; Dudek and Friedlander, 1996; Feldman and others, 1998; Feldman and others, 1999; Kirkwood and Bear, 1994b). It is not clear why the TC synapses in the ACx use a different postsynaptic mechanism for expression of synaptic plasticity. Nonetheless, these data add another feature to the list of morphologic and functional differences that set the ACx apart from other sensory cortices (King and Nelken, 2009).
Unmasking LTP in adult TC synapses further strengthens the hypothesis that TC synaptic plasticity serves as a substrate for cortical map plasticity during perceptual learning. Both TC LTD and LTP are gated in adulthood, with more complex gating for LTP than for LTD. TC LTP was revealed in adults by reducing an inhibitory tone in the ACx. In contrast, inhibiting GABAA transmission did not affect TC LTD in adults (Blundon and others, 2011). Both TC LTP and LTD in adults can be blocked by M1R inhibitors (Blundon and others, 2011; Chun and others, 2013). Whereas M1R appears to be a central signaling molecule in LTD gating (Figure 4), it was not immediately clear why M1Rs are required for TC LTP. Indeed, removing cortical inhibition is sufficient to induce TC LTP in mature mice by stimulating the thalamic radiation. It became clear more recently when it was shown that the thalamic radiation contains not only thalamic projections but also cholinergic projections emanating from neurons in the nucleus basalis (Figure 6a, b) en route to the ACx (Chun and others, 2013). Because TC LTP is conventionally induced by intense electrical activation of the thalamic radiation, it is conceivable that this induction protocol activates both glutamatergic and cholinergic projections to the ACx. Moreover, protocols for inducing LTP would be more prone to activate cholinergic projections than would the protocol for inducing LTD, because the former generally requires more robust stimulation of the thalamic radiation. These methodological considerations also suggest that electrical stimulation of the thalamic radiation does not accurately replicate information transfer from the thalamic neurons to the cortex in vivo. The optogenetic approach of controlling the activity in specific populations of neurons (Boyden and others, 2005) alleviated these concerns. By expressing a light-activated channelrhodopsin 2 in excitatory thalamic neurons, Chun and others activated TC projections alone in slices without affecting cholinergic projections. Unlike electrical stimulation, optogenetic stimulation of thalamic projections failed to induce LTP at adult TC synapses, even in the presence of cortical disinhibition (Figure 6c). However, when these conditions were paired with activating cholinergic receptors or inputs from the nucleus basalis, TC LTP was successfully unmasked in adults (Figure 6d). Removal of either cortical disinhibition or M1R-dependent cholinergic activation deemed LTP to fail (Chun and others, 2013), suggesting that TC LTP in adults is gated by two independent mechanisms (Figure 7). In this paradigm, LTP and LTD require the activation of presynaptic M1Rs that maintain glutamate release from thalamic terminals during trains of thalamic activity. In addition, TC LTP requires a second gating mechanism, i.e., a decrease in inhibitory tone in thalamorecepient cortical excitatory neurons.
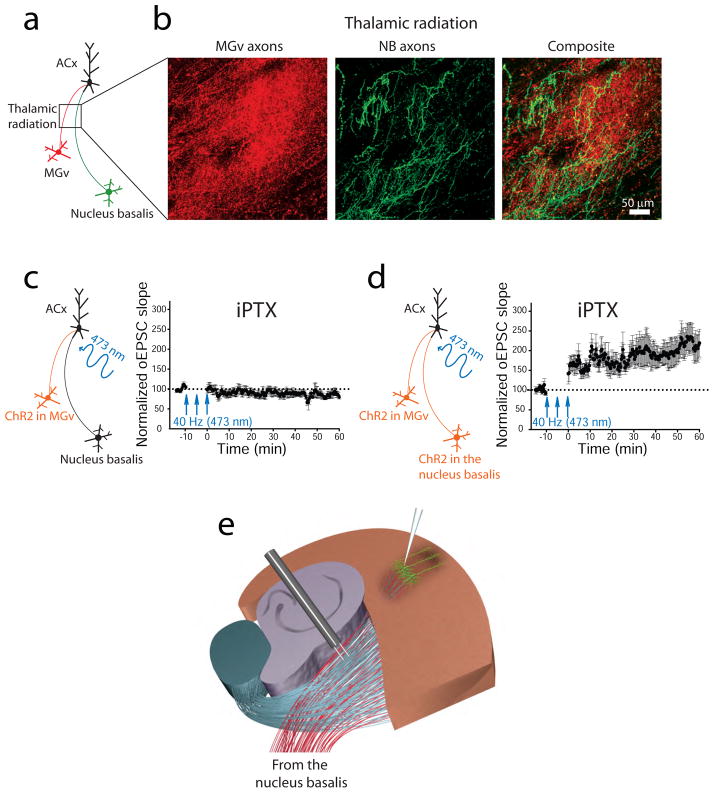
Figure 6. Thalamic radiation contains thalamic projections from the auditory thalamus and cholinergic projections from the nucleus basalis.
(a) Diagram shows the expression of a red fluorescent protein in the auditory thalamus (MGv) excitatory neurons and a green fluorescent protein in the nucleus basalis (NB) cholinergic neurons. (b) Thalamic and cholinergic projections are visualized in the thalamic radiation. (c, left) Diagram showing that channelrhodopsin 2 (ChR2) is expressed in the MGv excitatory neurons only. (c, right) A 40-Hz pattern of light-activated thalamic projections in the presence of cortical dysinhibition via picrotoxin (iPTX) is not sufficient to induce TC LTP of light-activated excitatory postsynaptic currents (oEPSCs) (d, left) Diagram showing that ChR2 is expressed in both the MGv excitatory neurons and NB cholinergic neurons (Chun and others, 2013). (d, right) Stimulation of thalamic projections paired with cholinergic activation of NB projections and cortical dysinhibition is sufficient for TC LTP of oEPSCs in the ACx. (e) The diagram of experimental slice preparation depicts that cholinergic afferents from the nucleus basalis are present in the thalamic radiation and can be activated during electrical stimulations of TC LTP.
Figure 7. Model of TC LTP.
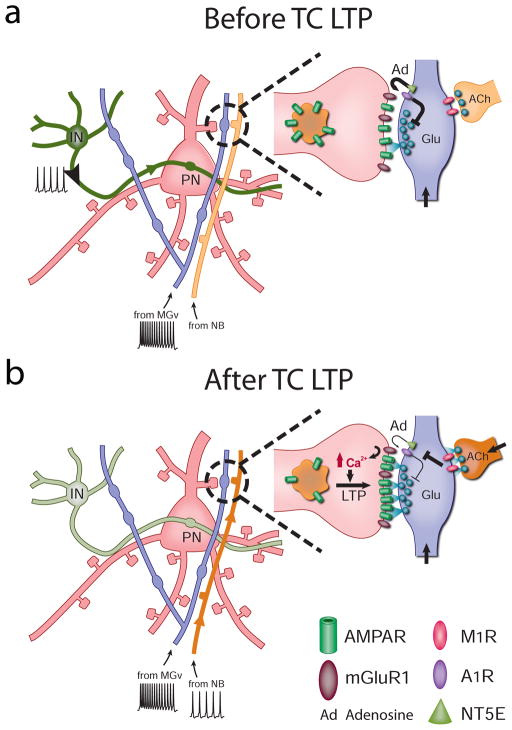
(a) Active cortical inhibition and inactive cholinergic synapses prevent TC LTP during trains of thalamic stimulations. (b) Cortical deactivation and activation of cholinergic synapses on thalamic projections release the gating of TC LTP in adults. Similar to TC LTD mechanisms, cholinergic activation leads to the downregulation of adenosine (Ad) release from thalamic projections, sustained glutamate (Glu) release at TC synapses, and the induction of postsynaptically expressed mGluR-dependent LTP. Abbreviations: ACh, acetylcholine; IN, cortical inhibitory neuron; MGv, ventral medial geniculate; NB, nucleus basalis; NT5E, ecto-5′-nucleotidase; PN, pyramidal neuron.
The distinction between gating and modulation
It is important to distinguish between the processes of synaptic gating and synaptic modulation. Cholinergic modulation of synaptic transmission and plasticity has been described for many central synapses. For instance, the magnitude of LTP or LTD at hippocampal excitatory synapses can change upon modulation by cholinergic agonists (Abe and others, 1994; Blitzer and others, 1990; Kumar, 2010; Maeda and others, 1994; McCutchen and others, 2006). Gating, on the other hand, is an all-or-none type of modulation. In the case of TC synapses, in the absence of M1R activation, glutamate release from thalamic terminals depresses so quickly during repeated stimulation that neither LTP nor LTD can be expressed. Although molecular mechanisms of M1R activation may be similar (or even the same) between TC and other glutamatergic synapses, the context of rapid depression of neurotransmitter release at adult TC synapses effectively turns cholinergic modulation into cholinergic gating at those synapses.
Cholinergic regulation of cortical map plasticity is of enormous importance. Only a few facts are known with certainty about the mechanisms of cortical map plasticity in the ACx, one of which is the important role of cholinergic projections originating from the nucleus basalis (Bakin and Weinberger, 1996; Kilgard and Merzenich, 1998a; Ma and Suga, 2005; Miasnikov and others, 2001; Recanzone and others, 1993; Weinberger, 1998; Weinberger, 2003). Therefore, determining the exact mechanisms of cholinergic regulation of TC circuitry is paramount. The cellular locus of cholinergic regulation during synaptic plasticity at TC synapses appears to be presynaptic. This conclusion is based on results of experiments in which presynaptic and postsynaptic functions were directly tested using subcellular imaging or manipulating individual synapses in slices. Historically, the effects of cholinergic or other modulatory transmitters on glutamatergic synaptic transmission or plasticity have been tested using electrophysiological tools that measure the net synaptic effect of modulation and are not sufficiently sensitive to distinguish an effect on a presynaptic or postsynaptic locus. Thus, an increase or decrease in synaptic transmission at glutamatergic synapses is misinterpreted and often simplified by either postsynaptic or presynaptic mechanisms. Direct imaging of presynaptic activity in individual thalamic terminals and testing the sensitivity of glutamatergic receptors in individual dendritic spines that are the postsynaptic sites of thalamic inputs on cortical neurons (Blundon and others, 2011; Chun and others, 2013) alleviate this uncertainty and facilitate studies on the effects of neuromodulators on glutamate release from thalamic projections and cortical neurons in sensory cortices.
Developmental aspects of thalamocortical gating
It is conceivable that the regulation of TC gating determines the mechanisms of the critical period for TC synaptic plasticity and underlies the critical period for cortical plasticity in sensory cortices. Numerous studies have concluded that plasticity at TC synapses is lost or drastically reduced after an early critical period (Feldman and others, 1998; Hogsden and Dringenberg, 2009b; Jiang and others, 2007; Kato and others, 1991), and some have suggested that the role of plasticity at these synapses is to influence developmental processes that end with termination of the critical period (Crair and Malenka, 1995; Kirkwood and others, 1995). Therefore, it has been assumed that cortical plasticity mechanisms are transferred to other synapses within local sensory pathways (Crair and Malenka, 1995; Jiang and others, 2007). However, cortical plasticity appears not be lost with age, but in adults, it becomes dependent on modulatory, attention-activated pathways. The result is that adult experiences are integrated into memory only if they are behaviorally relevant (Fritz and others, 2007; Weinberger, 2007b; Weinberger, 2007a). The nucleus basalis, one such attention-activated center, releases acetylcholine throughout the sensory cortices during the presentation of novel or relevant stimuli, and the role of acetylcholine in activating or enhancing cortical plasticity is now well established (Kilgard and Merzenich, 1998a; Metherate and Weinberger, 1990; Metherate and Ashe, 1993; Miasnikov and others, 2001; Weinberger, 2007b).
The emergence of gated cortical plasticity may coincide with the maturation of cholinergic fibers from the nucleus basalis; the cholinergic fibers accompany thalamic afferents to the sensory cortices and are fully developed in rodents by P14 (Mechawar and Descarries, 2001; Molnar and others, 1998). Furthermore, cholinergic activity increases during this early developmental stage (Hohmann and Berger-Sweeney, 1998; Robertson and others, 1991). Thus, the maturation of cholinergic innervations and activity in sensory cortices during early development may help establish gating mechanisms for synaptic plasticity at TC synapses.
Direct imaging of presynaptic function recently revealed that cholinergic activation increases the rate of sustained glutamate release at adult TC synapses during a train or burst of thalamic activity (Blundon and others, 2011), but did not modify the response to a single thalamic stimulation. Likewise, adenosine antagonists enhanced glutamate release during a train of thalamic stimulation but not during single-pulse stimulation. Adenosine acting through A1Rs is a well-established negative regulator of neurotransmitter release at excitatory synapses (Bayazitov and others, 2007; Dunwiddie and Fredholm, 1989; Dunwiddie and Masino, 2001). The adenosine machinery, in turn, can be regulated by muscarinic receptors (Bouron and Reuter, 1997; Oliveira and others, 2009; Worley and others, 1987), including presynaptic M1Rs at TC synapses (Blundon and others, 2011). Thus, the developmental trajectories of the expression of A1Rs, and/or the production of adenosine in thalamic neurons and the developmental dynamics of cholinergic inputs from the nucleus basalis may regulate age-dependence of the gating machinery of TC synaptic plasticity.
Future directions: adenosine as a regulator of cortical plasticity gating
Early investigations of the mechanisms of TC plasticity showed that the adenosine machinery on presynaptic thalamic terminals is a key component of plasticity gating. The activation of M1Rs removes TC plasticity gating through inhibition of adenosine signaling at TC synapses (Blundon and others, 2011; Chun and others, 2013). Pharmacological or genetic inhibition of adenosine production by ecto-5′-nucleotidase or adenosine signaling through A1Rs bypasses M1R-dependent gating and allows LTD and LTP at TC synapses after the critical period (Blundon and others, 2011; Chun and others, 2013). These results suggest that adenosine signaling in thalamic terminals in the ACx controls the gating of both TC LTP and LTD by regulating the maintenance of neurotransmitter release from thalamic projections during sustained activity required for LTP and LTD. Glutamate release at TC synapses decreases quickly during both high-frequency and low-frequency stimuli that are usually required for induction of LTP or LTD, respectively, and deletion or inhibition of A1Rs sustains the release from thalamic neurons. This leads to an activation of postsynaptic mGluRs that is sufficient to induce postsynaptic LTP or LTD. These results suggest that presynaptic adenosine signaling in thalamic neurons is an important target for extending TC plasticity beyond the early critical period. If TC synaptic plasticity underlies experience-dependent cortical plasticity, then manipulation of thalamic adenosine signaling may extend the critical period for cortical plasticity. Further in vivo experiments with a targeted deletion of A1Rs or adenosine production specifically in thalamic neurons at different developmental stages will provide a greater understanding of developmental aspects of cortical plasticity and perceptual memory.
Acknowledgments
Support
This work was supported in part by the National Institute of Mental Health of the National Institutes of Health (award numbers R01MH079079, R01MH095810) and by the American Lebanese Syrian Associated Charities (S.S.Z.).
We thank Angela McArthur for editing the manuscript, Joshua Stokes and Klo Spelshouse for assisting with the art work, and members of Zakharenko lab for their constructive comments.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other granting agencies.
References
- Abe K, Nakata A, Mizutani A, Saito H. Facilitatory but nonessential role of the muscarinic cholinergic system in the generation of long-term potentiation of population spikes in the dentate gyrus in vivo. Neuropharmacology. 1994;33:847–52. doi: 10.1016/0028-3908(94)90180-5. [DOI] [PubMed] [Google Scholar]
- Amitai Y. Thalamocortical synaptic connections: efficacy, modulation, inhibition and plasticity. Rev Neurosci. 2001;12:159–73. doi: 10.1515/revneuro.2001.12.2.159. [DOI] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Res. 1990;536:271–86. doi: 10.1016/0006-8993(90)90035-a. [DOI] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc Natl Acad Sci U S A. 1996;93:11219–24. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- Barkat TR, Polley DB, Hensch TK. A critical period for auditory thalamocortical connectivity. Nat Neurosci. 2011;14:1189–94. doi: 10.1038/nn.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth AL, Malenka RC. NMDAR EPSC kinetics do not regulate the critical period for LTP at thalamocortical synapses. Nat Neurosci. 2001;4:235–6. doi: 10.1038/85070. [DOI] [PubMed] [Google Scholar]
- Bayazitov IT, Richardson RJ, Fricke RG, Zakharenko SS. Slow presynaptic and fast postsynaptic components of compound long-term potentiation. J Neurosci. 2007;27:11510–21. doi: 10.1523/JNEUROSCI.3077-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Singer W. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature. 1986;320:172–6. doi: 10.1038/320172a0. [DOI] [PubMed] [Google Scholar]
- Bjordahl TS, Dimyan MA, Weinberger NM. Induction of long-term receptive field plasticity in the auditory cortex of the waking guinea pig by stimulation of the nucleus basalis. Behav Neurosci. 1998;112:467–79. doi: 10.1037//0735-7044.112.3.467. [DOI] [PubMed] [Google Scholar]
- Blake DT, Heiser MA, Caywood M, Merzenich MM. Experience-dependent adult cortical plasticity requires cognitive association between sensation and reward. Neuron. 2006;52:371–81. doi: 10.1016/j.neuron.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzer RD, Gil O, Landau EM. Cholinergic stimulation enhances long-term potentiation in the CA1 region of rat hippocampus. Neurosci Lett. 1990;119:207–10. doi: 10.1016/0304-3940(90)90835-w. [DOI] [PubMed] [Google Scholar]
- Blundon JA, Bayazitov IT, Zakharenko SS. Presynaptic gating of postsynaptically expressed plasticity at mature thalamocortical synapses. J Neurosci. 2011;31:16012–25. doi: 10.1523/JNEUROSCI.3281-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundon JA, Zakharenko SS. Dissecting the components of long-term potentiation. Neuroscientist. 2008;14:598–608. doi: 10.1177/1073858408320643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouron A, Reuter H. Muscarinic stimulation of synaptic activity by protein kinase C is inhibited by adenosine in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 1997;94:12224–9. doi: 10.1073/pnas.94.22.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–8. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Brocher S, Artola A, Singer W. Agonists of cholinergic and noradrenergic receptors facilitate synergistically the induction of long-term potentiation in slices of rat visual cortex. Brain Res. 1992;573:27–36. doi: 10.1016/0006-8993(92)90110-u. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA. Short-term plasticity in thalamocortical pathways: cellular mechanisms and functional roles. Rev Neurosci. 1997;8:95–116. doi: 10.1515/revneuro.1997.8.2.95. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Connors BW. Short-term synaptic enhancement and long-term potentiation in neocortex. Proc Natl Acad Sci U S A. 1996;93:1335–9. doi: 10.1073/pnas.93.3.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury SA, Suga N. Reorganization of the frequency map of the auditory cortex evoked by cortical electrical stimulation in the big brown bat. J Neurophysiol. 2000;83:1856–63. doi: 10.1152/jn.2000.83.4.1856. [DOI] [PubMed] [Google Scholar]
- Chun SK, Bayazitov IT, Blundon JA, Zakharenko SS. Thalamocortical LTP becomes gated after the early critical period in the auditory cortex. J Neurosci. 2013 doi: 10.1523/JNEUROSCI.4500-12.2013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SF, Bear MF. Visual experience induces long-term potentiation in the primary visual cortex. J Neurosci. 2010;30:16304–13. doi: 10.1523/JNEUROSCI.4333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature. 1995;375:325–8. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- de Villers-Sidani E, Chang EF, Bao S, Merzenich MM. Critical period window for spectral tuning defined in the primary auditory cortex (A1) in the rat. J Neurosci. 2007;27:180–9. doi: 10.1523/JNEUROSCI.3227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Weinberger NM. Classical conditioning rapidly induces specific changes in frequency receptive fields of single neurons in secondary and ventral ectosylvian auditory cortical fields. Brain Res. 1986;372:357–60. doi: 10.1016/0006-8993(86)91144-3. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Friedlander MJ. Developmental down-regulation of LTD in cortical layer IV and its independence of modulation by inhibition. Neuron. 1996;16:1097–106. doi: 10.1016/s0896-6273(00)80136-1. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Fredholm BB. Adenosine A1 receptors inhibit adenylate cyclase activity and neurotransmitter release and hyperpolarize pyramidal neurons in rat hippocampus. J Pharmacol Exp Ther. 1989;249:31–7. [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Hars B, Maho C, Hennevin E. Transient and prolonged facilitation of tone-evoked responses induced by basal forebrain stimulations in the rat auditory cortex. Exp Brain Res. 1994;97:373–86. doi: 10.1007/BF00241531. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Manunta Y, Hennevin E. Induction of selective plasticity in the frequency tuning of auditory cortex and auditory thalamus neurons by locus coeruleus stimulation. Hear Res. 2011;274:75–84. doi: 10.1016/j.heares.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Ehret G. Development of absolute auditory thresholds in the house mouse (Mus musculus) J Am Audiol Soc. 1976;1:179–84. [PubMed] [Google Scholar]
- Feldman DE, Nicoll RA, Malenka RC. Synaptic plasticity at thalamocortical synapses in developing rat somatosensory cortex: LTP, LTD, and silent synapses. J Neurobiol. 1999;41:92–101. [PubMed] [Google Scholar]
- Feldman DE, Nicoll RA, Malenka RC, Isaac JT. Long-term depression at thalamocortical synapses in developing rat somatosensory cortex. Neuron. 1998;21:347–57. doi: 10.1016/s0896-6273(00)80544-9. [DOI] [PubMed] [Google Scholar]
- Fritz JB, Elhilali M, David SV, Shamma SA. Does attention play a role in dynamic receptive field adaptation to changing acoustic salience in A1? Hear Res. 2007;229:186–203. doi: 10.1016/j.heares.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace for cortical receptive field plasticity. Nature. 2007;450:425–9. doi: 10.1038/nature06289. [DOI] [PubMed] [Google Scholar]
- Gagolewicz PJ, Dringenberg HC. NR2B-subunit dependent facilitation of long-term potentiation in primary visual cortex following visual discrimination training of adult rats. Eur J Neurosci. 2011;34:1222–9. doi: 10.1111/j.1460-9568.2011.07842.x. [DOI] [PubMed] [Google Scholar]
- Gao E, Suga N. Experience-dependent corticofugal adjustment of midbrain frequency map in bat auditory system. Proc Natl Acad Sci U S A. 1998;95:12663–70. doi: 10.1073/pnas.95.21.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao E, Suga N. Experience-dependent plasticity in the auditory cortex and the inferior colliculus of bats: role of the corticofugal system. Proc Natl Acad Sci U S A. 2000;97:8081–6. doi: 10.1073/pnas.97.14.8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil Z, Connors BW, Amitai Y. Differential regulation of neocortical synapses by neuromodulators and activity. Neuron. 1997;19:679–86. doi: 10.1016/s0896-6273(00)80380-3. [DOI] [PubMed] [Google Scholar]
- Gil Z, Connors BW, Amitai Y. Efficacy of thalamocortical and intracortical synaptic connections: quanta, innervation, and reliability. Neuron. 1999;23:385–97. doi: 10.1016/s0896-6273(00)80788-6. [DOI] [PubMed] [Google Scholar]
- Gordon B, Mitchell B, Mohtadi K, Roth E, Tseng Y, Turk F. Lesions of nonvisual inputs affect plasticity, norepinephrine content, and acetylcholine content of visual cortex. J Neurophysiol. 1990;64:1851–60. doi: 10.1152/jn.1990.64.6.1851. [DOI] [PubMed] [Google Scholar]
- Gu Q, Singer W. Effects of intracortical infusion of anticholinergic drugs on neuronal plasticity in kitten striate cortex. Eur J Neurosci. 1993;5:475–85. doi: 10.1111/j.1460-9568.1993.tb00514.x. [DOI] [PubMed] [Google Scholar]
- Hars B, Maho C, Edeline JM, Hennevin E. Basal forebrain stimulation facilitates tone-evoked responses in the auditory cortex of awake rat. Neuroscience. 1993;56:61–74. doi: 10.1016/0306-4522(93)90562-t. [DOI] [PubMed] [Google Scholar]
- Heynen AJ, Bear MF. Long-term potentiation of thalamocortical transmission in the adult visual cortex in vivo. J Neurosci. 2001;21:9801–13. doi: 10.1523/JNEUROSCI.21-24-09801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogsden JL, Dringenberg HC. Decline of long-term potentiation (LTP) in the rat auditory cortex in vivo during postnatal life: involvement of NR2B subunits. Brain Res. 2009a;1283:25–33. doi: 10.1016/j.brainres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Hogsden JL, Dringenberg HC. NR2B subunit-dependent long-term potentiation enhancement in the rat cortical auditory system in vivo following masking of patterned auditory input by white noise exposure during early postnatal life. Eur J Neurosci. 2009b;30:376–84. doi: 10.1111/j.1460-9568.2009.06835.x. [DOI] [PubMed] [Google Scholar]
- Hohmann CF, Berger-Sweeney J. Cholinergic regulation of cortical development and plasticity. New twists to an old story. Perspect Dev Neurobiol. 1998;5:401–25. [PubMed] [Google Scholar]
- Huang S, Trevino M, He K, Ardiles A, Pasquale R, Guo Y, Palacios A, Huganir R, Kirkwood A. Pull-push neuromodulation of LTP and LTD enables bidirectional experience-induced synaptic scaling in visual cortex. Neuron. 2012;73:497–510. doi: 10.1016/j.neuron.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insanally MN, Kover H, Kim H, Bao S. Feature-dependent sensitive periods in the development of complex sound representation. J Neurosci. 2009;29:5456–62. doi: 10.1523/JNEUROSCI.5311-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W, Suga N. Development of reorganization of the auditory cortex caused by fear conditioning: effect of atropine. J Neurophysiol. 2003;90:1904–9. doi: 10.1152/jn.00363.2003. [DOI] [PubMed] [Google Scholar]
- Ji W, Suga N, Gao E. Effects of agonists and antagonists of NMDA and ACh receptors on plasticity of bat auditory system elicited by fear conditioning. J Neurophysiol. 2005;94:1199–211. doi: 10.1152/jn.00112.2005. [DOI] [PubMed] [Google Scholar]
- Jiang B, Trevino M, Kirkwood A. Sequential development of long-term potentiation and depression in different layers of the mouse visual cortex. J Neurosci. 2007;27:9648–52. doi: 10.1523/JNEUROSCI.2655-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SL, Ma W, Bear MF, Eslin D. Cholinergic manipulation alters stimulus-evoked metabolic activity in cat somatosensory cortex. J Comp Neurol. 1990;297:106–20. doi: 10.1002/cne.902970108. [DOI] [PubMed] [Google Scholar]
- Kato N, Artola A, Singer W. Developmental changes in the susceptibility to long-term potentiation of neurones in rat visual cortex slices. Brain Res Dev Brain Res. 1991;60:43–50. doi: 10.1016/0165-3806(91)90153-a. [DOI] [PubMed] [Google Scholar]
- Keuroghlian AS, Knudsen EI. Adaptive auditory plasticity in developing and adult animals. Prog Neurobiol. 2007;82:109–21. doi: 10.1016/j.pneurobio.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998a;279:1714–8. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Plasticity of temporal information processing in the primary auditory cortex. Nat Neurosci. 1998b;1:727–31. doi: 10.1038/3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ, Nelken I. Unraveling the principles of auditory cortical processing: can we learn from the visual system? Nat Neurosci. 2009;12:698–701. doi: 10.1038/nn.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Bear MF. Hebbian synapses in visual cortex. J Neurosci. 1994a;14:1634–45. doi: 10.1523/JNEUROSCI.14-03-01634.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Bear MF. Homosynaptic long-term depression in the visual cortex. J Neurosci. 1994b;14:3404–12. doi: 10.1523/JNEUROSCI.14-05-03404.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Lee HK, Bear MF. Co-regulation of long-term potentiation and experience-dependent synaptic plasticity in visual cortex by age and experience. Nature. 1995;375:328–31. doi: 10.1038/375328a0. [DOI] [PubMed] [Google Scholar]
- Kraus HJ, Aulbach-Kraus K. Morphological changes in the cochlea of the mouse after the onset of hearing. Hear Res. 1981;4:89–102. doi: 10.1016/0378-5955(81)90038-1. [DOI] [PubMed] [Google Scholar]
- Kumar A. Carbachol-induced long-term synaptic depression is enhanced during senescence at hippocampal CA3-CA1 synapses. J Neurophysiol. 2010;104:607–16. doi: 10.1152/jn.00278.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Ebner FF. Induction of high frequency activity in the somatosensory thalamus of rats in vivo results in long-term potentiation of responses in SI cortex. Exp Brain Res. 1992;90:253–61. doi: 10.1007/BF00227236. [DOI] [PubMed] [Google Scholar]
- Ma X, Suga N. Augmentation of plasticity of the central auditory system by the basal forebrain and/or somatosensory cortex. J Neurophysiol. 2003;89:90–103. doi: 10.1152/jn.00968.2001. [DOI] [PubMed] [Google Scholar]
- Ma X, Suga N. Long-term cortical plasticity evoked by electric stimulation and acetylcholine applied to the auditory cortex. Proc Natl Acad Sci U S A. 2005;102:9335–40. doi: 10.1073/pnas.0503851102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Kaneko S, Satoh M. Roles of endogenous cholinergic neurons in the induction of long-term potentiation at hippocampal mossy fiber synapses. Neurosci Res. 1994;20:71–8. doi: 10.1016/0168-0102(94)90023-x. [DOI] [PubMed] [Google Scholar]
- McCutchen E, Scheiderer CL, Dobrunz LE, McMahon LL. Coexistence of muscarinic long-term depression with electrically induced long-term potentiation and depression at CA3-CA1 synapses. J Neurophysiol. 2006;96:3114–21. doi: 10.1152/jn.00144.2006. [DOI] [PubMed] [Google Scholar]
- Mechawar N, Descarries L. The cholinergic innervation develops early and rapidly in the rat cerebral cortex: a quantitative immunocytochemical study. Neuroscience. 2001;108:555–67. doi: 10.1016/s0306-4522(01)00389-x. [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH. Nucleus basalis stimulation facilitates thalamocortical synaptic transmission in the rat auditory cortex. Synapse. 1993;14:132–43. doi: 10.1002/syn.890140206. [DOI] [PubMed] [Google Scholar]
- Metherate R, Weinberger NM. Cholinergic modulation of responses to single tones produces tone-specific receptive field alterations in cat auditory cortex. Synapse. 1990;6:133–45. doi: 10.1002/syn.890060204. [DOI] [PubMed] [Google Scholar]
- Miasnikov AA, McLin D, III, Weinberger NM. Muscarinic dependence of nucleus basalis induced conditioned receptive field plasticity. Neuroreport. 2001;12:1537–42. doi: 10.1097/00001756-200105250-00047. [DOI] [PubMed] [Google Scholar]
- Molnar Z, Adams R, Blakemore C. Mechanisms underlying the early establishment of thalamocortical connections in the rat. J Neurosci. 1998;18:5723–45. doi: 10.1523/JNEUROSCI.18-15-05723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira L, Timoteo MA, Correia-de-Sa P. Negative crosstalk between M1 and M2 muscarinic autoreceptors involves endogenous adenosine activating A1 receptors at the rat motor endplate. Neurosci Lett. 2009;459:127–31. doi: 10.1016/j.neulet.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci. 2006;26:4970–82. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RJ, Blundon JA, Bayazitov IT, Zakharenko SS. Connectivity patterns revealed by mapping of active inputs on dendrites of thalamorecipient neurons in the auditory cortex. J Neurosci. 2009;29:6406–17. doi: 10.1523/JNEUROSCI.0258-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson RT, Mostamand F, Kageyama GH, Gallardo KA, Yu J. Primary auditory cortex in the rat: transient expression of acetylcholinesterase activity in developing geniculocortical projections. Brain Res Dev Brain Res. 1991;58:81–95. doi: 10.1016/0165-3806(91)90240-j. [DOI] [PubMed] [Google Scholar]
- Rutkowski RG, Weinberger NM. Encoding of learned importance of sound by magnitude of representational area in primary auditory cortex. Proc Natl Acad Sci U S A. 2005;102:13664–9. doi: 10.1073/pnas.0506838102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev RN, Lu SM, Wiley RG, Ebner FF. Role of the basal forebrain cholinergic projection in somatosensory cortical plasticity. J Neurophysiol. 1998;79:3216–28. doi: 10.1152/jn.1998.79.6.3216. [DOI] [PubMed] [Google Scholar]
- Sally SL, Kelly JB. Organization of auditory cortex in the albino rat: sound frequency. J Neurophysiol. 1988;59:1627–38. doi: 10.1152/jn.1988.59.5.1627. [DOI] [PubMed] [Google Scholar]
- Shatz CJ. Emergence of order in visual system development. Proc Natl Acad Sci U S A. 1996;93:602–8. doi: 10.1073/pnas.93.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford KJ, Tarczy-Hornoch K, Martin KA, Bannister NJ, Jack JJ. Excitatory synaptic inputs to spiny stellate cells in cat visual cortex. Nature. 1996;382:258–61. doi: 10.1038/382258a0. [DOI] [PubMed] [Google Scholar]
- Suga N, Ma X. Multiparametric corticofugal modulation and plasticity in the auditory system. Nat Rev Neurosci. 2003;4:783–94. doi: 10.1038/nrn1222. [DOI] [PubMed] [Google Scholar]
- Sun YJ, Wu GK, Liu BH, Li P, Zhou M, Xiao Z, Tao HW, Zhang LI. Fine-tuning of pre-balanced excitation and inhibition during auditory cortical development. Nature. 2010;465:927–31. doi: 10.1038/nature09079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel CM, Friston KJ, Dolan RJ. Cholinergic modulation of experience-dependent plasticity in human auditory cortex. Neuron. 2002;35:567–74. doi: 10.1016/s0896-6273(02)00801-2. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Physiological memory in primary auditory cortex: characteristics and mechanisms. Neurobiol Learn Mem. 1998;70:226–51. doi: 10.1006/nlme.1998.3850. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. The nucleus basalis and memory codes: auditory cortical plasticity and the induction of specific, associative behavioral memory. Neurobiol Learn Mem. 2003;80:268–84. doi: 10.1016/s1074-7427(03)00072-8. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Specific long-term memory traces in primary auditory cortex. Nat Rev Neurosci. 2004;5:279–90. doi: 10.1038/nrn1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Associative representational plasticity in the auditory cortex: a synthesis of two disciplines. Learn Mem. 2007a;14:1–16. doi: 10.1101/lm.421807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Auditory associative memory and representational plasticity in the primary auditory cortex. Hear Res. 2007b;229:54–68. doi: 10.1016/j.heares.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel T, Hubel D. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965;28:1029–40. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- Worley PF, Baraban JM, McCarren M, Snyder SH, Alger BE. Cholinergic phosphatidylinositol modulation of inhibitory, G protein-linked neurotransmitter actions: electrophysiological studies in rat hippocampus. Proc Natl Acad Sci U S A. 1987;84:3467–71. doi: 10.1073/pnas.84.10.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Chung S, Chen DY, Wang S, Dodd SJ, Walters JR, Isaac JT, Koretsky AP. Thalamocortical inputs show post-critical-period plasticity. Neuron. 2012;74:731–42. doi: 10.1016/j.neuron.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharenko SS, Patterson SL, Dragatsis I, Zeitlin SO, Siegelbaum SA, Kandel ER, Morozov A. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses. Neuron. 2003;39:975–90. doi: 10.1016/s0896-6273(03)00543-9. [DOI] [PubMed] [Google Scholar]
- Zakharenko SS, Zablow L, Siegelbaum SA. Visualization of changes in presynaptic function during long-term synaptic plasticity. Nat Neurosci. 2001;4:711–7. doi: 10.1038/89498. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Bao S, Merzenich MM. Persistent and specific influences of early acoustic environments on primary auditory cortex. Nat Neurosci. 2001;4:1123–30. doi: 10.1038/nn745. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hamilton SE, Nathanson NM, Yan J. Decreased input-specific plasticity of the auditory cortex in mice lacking M1 muscarinic acetylcholine receptors. Cereb Cortex. 2006;16:1258–65. doi: 10.1093/cercor/bhj067. [DOI] [PubMed] [Google Scholar]