Abstract
Background
A subset of patients with Alzheimer’s disease (AD) present with early and prominent language deficits. It is unclear whether the burden of underlying β-amyloid pathology is associated with language or general cognitive impairment in these subjects.
Methods
Here, we assess the relationship between cortical β-amyloid burden on [11C]Pittsburgh compound B (PiB) PET and performance on the Montreal Cognitive Assessment (MoCA), the Wechsler Memory Scale-Third Edition (WMS-III), the Boston Naming Test (BNT), and the Western Aphasia Battery (WAB) using regression and correlation analyses in subjects presenting with aphasia that showed β-amyloid deposition on PiB PET.
Results
The global PiB ratio was inversely correlated with MoCA (p = 0.02) and the WMS-III Visual Reproduction (VR) subtest (VR I, p = 0.02; VR II, p = 0.04). However, the correlations between PiB ratio, BNT (p = 0.13), WAB aphasia quotient (p = 0.11), and WAB repetition scores (p = 0.34) were not significant.
Conclusion
This study demonstrates that an increased cortical β-amyloid burden is associated with cognitive impairment, but not language deficits, in AD subjects presenting with aphasia. The results suggest that β-amyloid deposition may partly contribute to impaired cognition in such patients while language dysfunction may be influenced by other pathologic mechanisms, perhaps downstream pathways of β-amyloid deposition.
Keywords: Dementia, Aphasia, PET, Beta-amyloid, PiB
Introduction
Beta-amyloid deposition is one of the hallmark pathological features defining Alzheimer’s disease (AD). Most patients with AD present with early and prominent loss of episodic memory, as well as additional cognitive impairment. However, there is a subset of AD patients in which language impairment is the earliest and most prominent feature of the presenting syndrome [1]. It is unclear whether there is any association between β-amyloid deposition and the severity of language or general cognitive impairment in such patients. Here, we utilize [11C] Pittsburgh compound B (PiB) imaging to examine the relationship between cortical β-amyloid burden and performance on tests of language and general cognition in a large cohort of AD patients that presented with early and prominent language impairment.
Methods
Thirty-five subjects who presented with a language disorder and showed β-amyloid deposition on [11C] PiB-PET were included in this prospective study. All 35 subjects had a chief complaint of prominent language deficits and features suggestive of aphasia at the time of presentation. None of these patients had memory complaints prior to developing language symptoms. They underwent a cognitive evaluation by a behavioral neurologist (KAJ) and a speech evaluation by one of two speech pathologists (JRD & EAS). Testing included the Montreal Cognitive Assessment (MoCA) battery to assess general cognitive function, the Wechsler Memory Scale-Third Edition (WMS-III) Visual Reproduction (VR) subtest to assess visual memory, the short version of the Boston Naming Test (BNT) to assess naming, the Western Aphasia Battery aphasia quotient (WAB-AQ) to assess aphasia severity, and the WAB repetition subscore to assess sentence repetition. Cortical β-amyloid burden was determined by a global PiB ratio, calculated as previously described, with a cut-point of 1.5 considered positive [2]. The association between global PiB ratio and performance on clinical measures of cognition and language was assessed using regression and multivariate correlation analyses. Statistical analyses were performed utilizing JMP software, version 9.0 (SAS Institute Inc, Cary, NC). A p value <0.05 was considered statistically significant.
This study was approved by the Mayo IRB. Informed consent was obtained from the patients and their family members or significant others.
Results
The subjects included 17 men and 18 women. Of the 35 subjects, 3 were left-hand dominant. Demographic characteristics of the subjects, including age, education level, and disease duration, are included in Table 1. All 35 subjects had language characteristics of anomia, poor sentence repetition, and phonological errors in spontaneous speech. None of the 35 subjects had apraxia of speech or loss of single word meaning. All subjects met the criteria for primary progressive aphasia. By design, all subjects demonstrated elevated β-amyloid deposition in the brain with a median PiB ratio of 2.08 (Table 1). The degrees of cognitive decline and aphasia were variable among these individuals as presented in Table 1.
Table 1.
Demographic, cognitive, and language characteristics
| Category | Median (Interquartile range) |
|---|---|
| Age (yr) | 68 (57 – 73) |
| Education (yr) | 14 (12 – 17) |
| Disease Duration (yr) | 3.5 (2 – 5) |
| PiB ratio | 2.08 (1.99 – 2.33) |
| MoCA | 15 (7 – 20) |
| WMS-III VR I z-score* | −2 (−2.5 – −1) |
| WMS-III VR II z-score* | −1.3 (−1.8 – −0.5) |
| BNT | 7 (2 – 10) |
| WAB-AQ | 81.7 (69.5 – 88.4) |
| WAB repetition | 7.8 (6.8 – 8.6) |
WMS-III VR z-scores were obtained from 33 subjects. Two subjects could not complete the test.
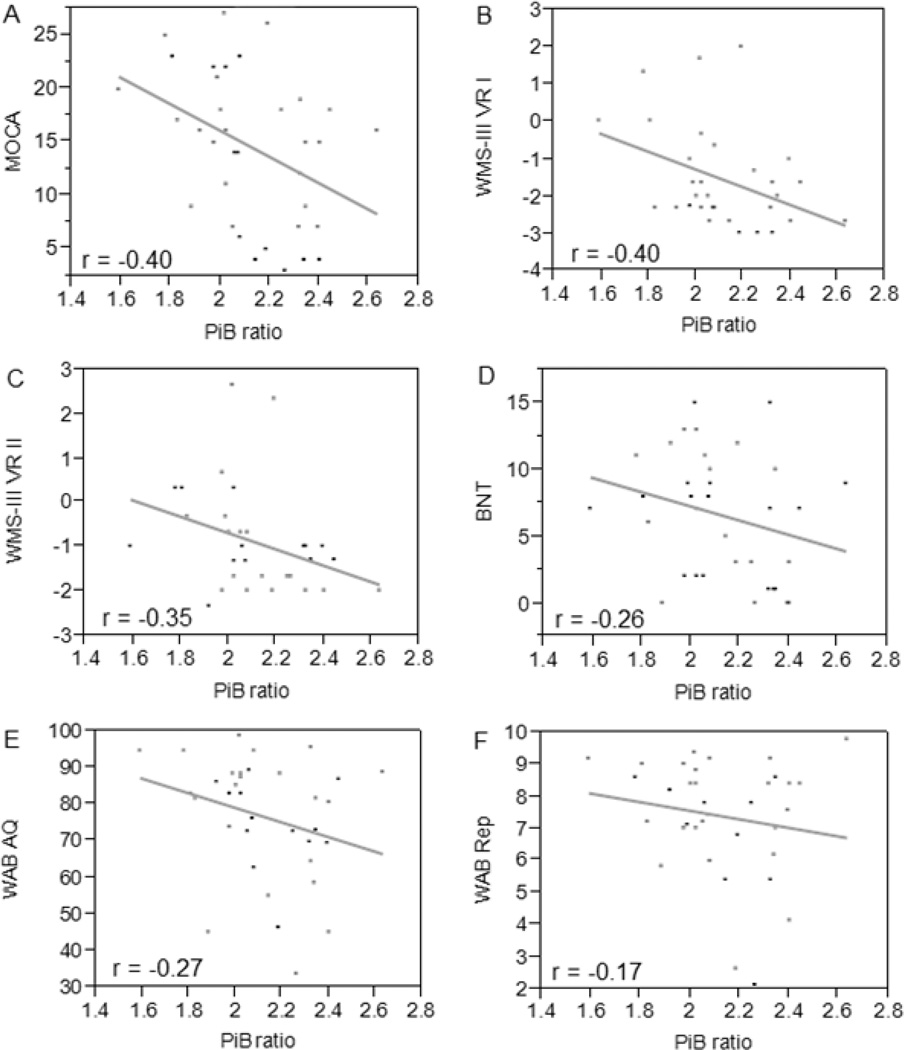
Figure 1 displays the relationships between PiB ratio, MoCA, WMS-III VR, BNT, WAB-AQ, and WAB repetition scores. There was a significant inverse correlation between the global PiB ratio and performance on the MoCA (β = −12.33, t(34) = −2.48, p = 0.02) and the WMS-III VR (VR I z-score, β = −2.38, t(32) = −2.45, p = 0.02; VR II z-score, β = −1.85, t(32) = −2.09, p = 0.04). Age, education level, and disease duration had no influence on PiB ratio (age, β = 0.004, t(34) = 1.01, p = 0.32; education, β = 0.004, t(34) = 0.30, p = 0.76; disease duration, β = 0.02, t(34) = 0.89, p = 0.38), MoCA scores (age, β = −0.12, t(34) = −0.94, p = 0.35; education, β = −0.20, t(34) = −0.43, p = 0.67; disease duration, β = −1.22, t(34) = −1.74, p = 0.09), WMS-III VR I z-scores (age, β = −0.01, t(32) = −0.52, p = 0.61; education, β = −0.03, t(32) = −0.39, p = 0.70; disease duration, β = −0.05, t(32) = −0.33, p = 0.74), or WMS-III VR II z-scores (age, β = −0.02, t(32) = −0.68, p = 0.50; education, β = −0.06, t(32) = −0.75, p = 0.46; disease duration, β = −0.10, t(32) = −0.69, p = 0.50). In addition, the correlations between PiB and measures of aphasia, including BNT (β = −5.35, t(34) = −1.53, p = 0.13), WAB-AQ (β = −19.94, t(34) = −1.64, p = 0.11), and WAB repetition scores (β = −1.34, t(34) = −0.97, p = 0.34), were not significant.
Figure 1. The relationship between amyloid, cognition, and language.
Higher PiB ratio is associated with lower MoCA (A) and WMS-III VR z-scores (B, C), whereas no correlation was observed between PiB ratio, BNT (D), WAB-AQ (E), and WAB repetition scores (F). The r values indicate correlation coefficient from the multivariate correlation analysis.
Conclusions
The present study demonstrates that increased cortical β-amyloid burden measured by [11C] PiB-PET is associated with worse cognition in patients with AD presenting with early and prominent language impairment. In typical AD patients that present with loss of episodic memory, a higher β-amyloid load has similarly been shown to negatively correlate with cognitive function [3]. Similar results were also shown in healthy elderly individuals with subjective cognitive complaints [4]. These findings, along with the results of our study, suggest that amyloid burden may be in part responsible for cognitive impairment in patients with AD, independent of the primary presenting symptoms. Interestingly, language deficits in our subjects were not significantly associated with degree of amyloid burden, suggesting that other pathologic mechanisms may contribute to language dysfunction in these subjects.
The mechanisms by which β-amyloid affects cognitive deterioration in AD could not be determined in this study. Given that β-amyloid deposition in the brain is seen in otherwise healthy elderly individuals [4], it is likely that β-amyloid deposition occurs in early stages of cognitive dysfunction in AD. The accumulation of β-amyloid plaques may initiate downstream cascades involving other degenerative proteins, such as tau, as well as inflammatory processes that can lead to further cognitive impairment. Although language deficits in these subjects may not be directly affected by β-amyloid deposition, they may be linked to these downstream events of β-amyloid deposition. A future longitudinal study involving molecular and pathological approaches will be informative for examining the effects of AD pathology on the progression of cognitive deficits in AD patients presenting with early and prominent language deficits.
Acknowledgement
We would like to acknowledge the Mayo Clinic Center for Translational Science Activities (CTSA) for statistical guidance and Elsie and Marvin Dekelboum Family Foundation for the PET scanner.
Study funding
The study was supported by NIH R01-DC010367.
Dr. Whitwell receives research support from the NIH (NIDCD and NIA) and the Alzheimer’s Association, and has served as a consultant for Bristol-Myers Squibb. Dr. Lowe serves as a consultant for Bayer Schering Pharma and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals, the NIH (NIA, NCI), the MN Partnership for Biotechnology and Medical Genomics, the Leukemia & Lymphoma Society, and the Alzheimer’s Association. Drs. Duffy, Strand, and Machulda receive research support from the NIH (NIDCD). Dr. Jack serves as a consultant for Janssen, Bristol-Meyer-Squibb, General Electric, Siemens, and Johnson and Johnson and is involved in clinical trials sponsored by Allon and Baxter, Inc. He receives research funding from the National Institutes of Health and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation. Dr. Josephs receives research support from the NIH (NIDCD and NIA), and the Alzheimer’s Association.
Footnotes
Disclosure statement
Dr. Jung and Mr. Senjem have no disclosures.
References
- 1.Josephs KA, Whitwell JL, Duffy JR, et al. Progressive aphasia secondary to Alzheimer disease vs FTLD pathology. Neurology. 2008;70:25–34. doi: 10.1212/01.wnl.0000287073.12737.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jack CR, Jr, Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain. 2008;131:665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villemagne VL, Pike KE, Chételat G, et al. Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Annals of Neurology. 2011;69:181–192. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perrotin A, Mormino EC, Madison CM, Hayenga AO, Jagust WJ. Subjective cognition and amyloid deposition imaging: a Pittsburgh Compound B positron emission tomography study in normal elderly individuals. Archives of Neurology. 2012;69:223–229. doi: 10.1001/archneurol.2011.666. [DOI] [PMC free article] [PubMed] [Google Scholar]