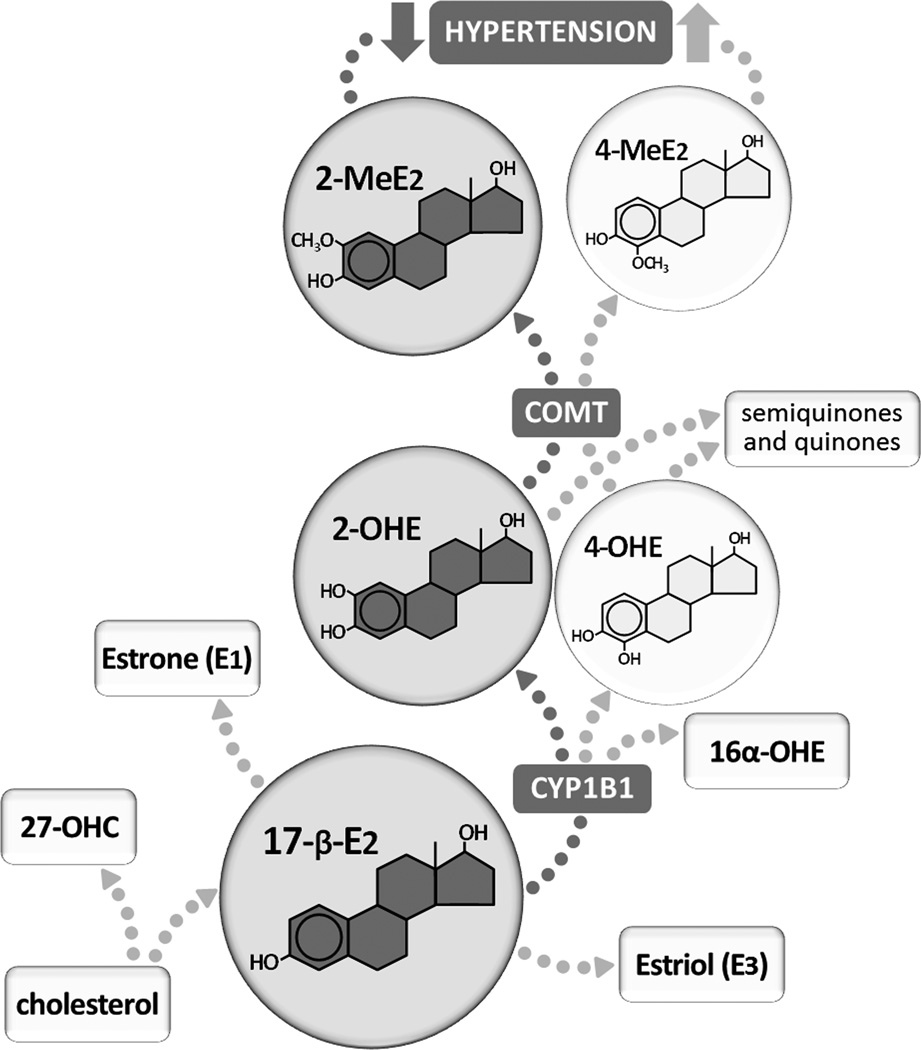
The influence of estrogen on blood pressure is complicated and sometimes contradictory. Premenopausal women have a lower incidence of hypertension, but recent clinical trials suggest that postmenopausal hormone replacement therapy does not necessarily decrease the risk of cardiovascular disease. Sexually dimorphic hypertension is evident in multiple animal models yet only some indicate a critical role for estrogen.1 These contradictions emphasize the importance of identifying the critical molecular signaling pathways in the cardiovascular system that are activated by estrogen and pursuing alternative pathways for their activation. One important factor that has been neglected in the study of estrogen’s cardiovascular effects is the conversion of 17-β-estradiol, the most commonly studied ovarian estrogen, to metabolites that are capable of exerting discrete physiological effects (Figure).
Figure.
In a study by Jennings et al in this issue of Hypertension, the ability of endogenous estrogen to counteract the blood pressure response to angiotensin II infusion is a result of the metabolism of 17-β-estradiol to 2-hydroxyestradiol (2-OHE) by cytochrome P450 1B1 (CYP1B1) and subsequent conversion by catechol-O-methyl transferase (COMT) to 2-methoxyestradiol (2-MeE2). In contrast, production of 4-hydroxyestradiol (4-OHE) and 4-methoxyestradiol (4-MeE2) exacerbates hypertension in this model. Other 17-β-estradiol precursors or metabolites that may impact cardiovascular function include 27-hydroxycholesterol (27-OHC), 16α-hydroxyestradiol (16α-OHE), estrone (E1), and estriol (E3).
In this issue of Hypertension, Jennings et al utilize a mouse model of angiotensin (Ang) II-induced hypertension to demonstrate that the protective cardiovascular effects historically attributed to the predominant estrogen 17-β-estradiol may instead reflect distinct actions by the metabolite 2-methoxyestradiol (2-MeE2). The critical enzymes include cytochrome P450 1B1 (CYP1B1), which metabolizes 17-β-estradiol to the catechol estrogen 2-hydroxyestradiol (2-OHE2), and catechol-O-methyl transferase (COMT), which subsequently converts 2-OHE2 to 2-MeE2. In comparison to wild type controls, female CYP1B1 knockout mice display a significantly greater pressor response to Ang II accompanied by vascular and cardiac remodeling, vascular dysfunction, and oxidative stress. This genetic approach is nicely complemented by studies using the selective CYP1B1 inhibitor tetramethoxystilbene, which similarly increases the Ang II response. The ability of both CYP1B1 gene deletion and pharmacological inhibition to enhance Ang II-induced hypertension is absent in ovariectomized mice, emphasizing the necessity of endogenous estrogen as a substrate for CYP1B1’s protective actions.
Protection from Ang II-induced hypertension is restored in CYP1B1 knockout mice by administration of the CYP1B1 product 2-OHE and its COMT-derived metabolite 2-MeE2. Interestingly, treatment with another catechol estrogen, 4-hydroxyestradiol (4-OHE), does not reduce blood pressure in Ang II-infused CYP1B1 knockout mice and exacerbates the Ang II response in wild-type females. The increased pressure may result from the actions of 4-OHE and/or its metabolite 4-MeE2. The contradictory actions of these two catechol estrogens indicate that in wild type females, 17-β-estradiol is predominantly metabolized to 2-OHE and 2-MeE2 which have beneficial actions on blood pressure. Whether processes such as disease or aging alter the predominant metabolic pathway and lead to increased production of detrimental metabolites is not yet known.
2-MeE2 may have direct actions on the renin-angiotensin system or may counteract hypertension via other mechanisms. Studies by Dubey and Jackson have established a role for this metabolite in estrogen’s protective cardiovascular effects, including vasodilation and inhibition of vascular smooth muscle cell growth.2 Although the estrogen receptor subtype that mediates the beneficial actions of this metabolite is still unknown, membrane-initiated signaling events occurring independently of the classical steroid receptors ERα and ERβ have been implicated. Recently, the ability of 2-MeE2 to decrease angiotensin receptor binding was found to be mediated by the G protein-coupled estrogen receptor (GPR30/GPER).3 Additional studies using this Ang II-infusion model utilizing estrogen receptor knockout mice or pharmacological inhibitors will reveal the estrogen receptor subtype that binds 2-MeE2 and facilitates its protective actions during Ang II-dependent hypertension.
Formation of the catechol estrogens 2-OHE and 4-OHE via CYP1B1 and subsequent conversion by COMT to methoxyestradiols is only one metabolic pathway for 17-β-estradiol. This hormone is also subjected to 16α-hydroxylation, and all of the catechol estrogens (2-OHE, 4-OHE, and 16α-OHE) can be oxidized to form semiquinones and quinones. 17β-hydroxysteroid dehydrogenase converts 17-β-estradiol to estrone (E1), which can also be converted to catechol and methoxy estrones. During pregnancy, 16-hydroxydehydroepiandrosterone sulfate in the placenta converts 17-β-estradiol to estriol (E3). Furthermore, cholesterol metabolites that are upstream of 17-β-estradiol and formed independently of the enzymes 17α-hydroxylase and aromatase, may be important in estrogen’s cardiovascular effects. Cholesterol is directly converted by cholesterol 27-hydroxylase to the metabolite 27-hydroxycholesterol (27-OHC), which acts as both an estrogen receptor agonist and antagonist, depending on the tissue.4 Finally, catechol estrogens may directly participate in the formation of prostaglandins through the process of cooxidation in the absence of any receptor binding or activation.5
For these findings to be translated into clinical therapies, the relative amounts of estrogen metabolites that are present in females and the influence of aging and disease on the metabolic pathway need to be established. In pulmonary arterial hypertension, CYP1B1 is upregulated and its metabolic products contribute to disease progression.6 Urinary quinone metabolites are more prevalent in women at high risk or newly diagnosed with breast cancer and are postulated to act as endogenous carcinogens.7 Recent advances in mass spectrometry permit urinary profiling of estrogen metabolites8, which can be applied to samples obtained from large epidemiologic studies to determine their associations with genetic, racial, pharmacological, pathological, or environmental factors. Similar studies should be conducted in animal models and may help to explain strain differences in the response to estrogen. Furthermore, establishing estrogen receptor affinities for each metabolite in addition to their discrete physiological effects will help determine which metabolites are beneficial for cardiovascular health.
Recognition of the importance of estrogen metabolites combined with improvements in technology enabling their accurate measurement should encourage the development of more sophisticated treatments for menopausal symptoms. The current clinical standard, Premarin, is a combination of ten different equine estrogens obtained from the urine of pregnant mares. Half of these estrogens are not endogenous to humans and contain one or two additional double bonds in ring B.9 Not surprisingly, these equine estrogens have unique pharmacokinetic and receptor binding profiles which may contribute to the conflicting results of clinical trials. More sophisticated pharmaceuticals which selectively replace certain estrogen metabolites or are resistant to metabolism need to be developed. Alternatively, combining estrogens with enzyme inhibitors or selective estrogen receptor modulators (SERMs) such as bazedoxifene may prove to be beneficial for cardiovascular function while avoiding estrogen’s deleterious effects.10
ACKNOWLEDGMENTS
I would like to thank Dr. John McLachlan for his insight in the preparation of this manuscript.
SOURCE(S) OF FUNDING
Dr. Lindsey is funded by the National Institutes of Health grant R00 HL103974 and startup funds from the Tulane University Department of Pharmacology.
Footnotes
CONFLICT(S) OF INTEREST/DISCLOSURE(S)
None
REFERENCES
- 1.Sandberg K, Ji H. Sex differences in primary hypertension. Biol Sex Differ. 2012;3:7. doi: 10.1186/2042-6410-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubey RK, Jackson EK. Potential vascular actions of 2-methoxyestradiol. Trends Endocrinol Metab. 2009;20:374–379. doi: 10.1016/j.tem.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koganti S, Snyder R, Gumaste U, Karamyan VT, Thekkumkara T. 2-Methoxyestradiol binding of GPR30 down-regulates angiotensin AT1 receptor. Eur J Pharmacol. 2014;723:131–140. doi: 10.1016/j.ejphar.2013.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umetani M, Domoto H, Gormley AK, Yuhanna IS, Cummins CL, Javitt NB, Korach KS, Shaul PW, Mangelsdorf DJ. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med. 2007;13:1185–1192. doi: 10.1038/nm1641. [DOI] [PubMed] [Google Scholar]
- 5.Degen GH, McLachlan JA, Eling TE, Sivarajah K. Cooxidation of steroidal and non-steroidal estrogens by purified prostaglandin synthase results in a stimulation of prostaglandin formation. J Steroid Biochem. 1987;26:679–685. doi: 10.1016/0022-4731(87)91039-9. [DOI] [PubMed] [Google Scholar]
- 6.White K, Johansen AK, Nilsen M, Ciuclan L, Wallace E, Paton L, Campbell A, Morecroft I, Loughlin L, McClure JD, Thomas M, Mair KM, MacLean MR. Activity of the estrogen-metabolizing enzyme cytochrome P450 1B1 influences the development of pulmonary arterial hypertension. Circulation. 2012;126:1087–1098. doi: 10.1161/CIRCULATIONAHA.111.062927. [DOI] [PubMed] [Google Scholar]
- 7.Gaikwad NW, Yang L, Pruthi S, Ingle JN, Sandhu N, Rogan EG, Cavalieri EL. Urine biomarkers of risk in the molecular etiology of breast cancer. Breast Cancer (Auckl) 2009;3:1–8. doi: 10.4137/bcbcr.s2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eliassen AH, Ziegler RG, Rosner B, Veenstra TD, Roman JM, Xu X, Hankinson SE. Reproducibility of fifteen urinary estrogens and estrogen metabolites over a 2- to 3-year period in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2009;18:2860–2868. doi: 10.1158/1055-9965.EPI-09-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhavnani BR. The saga of the ring B unsaturated equine estrogens. Endocr Rev. 1988;9:396–416. doi: 10.1210/edrv-9-4-396. [DOI] [PubMed] [Google Scholar]
- 10.Kim JH, Meyers MS, Khuder SS, Abdallah SL, Muturi HT, Russo L, Tate CR, Hevener AL, Najjar SM, Leloup C, Mauvais-Jarvis F. Tissue-selective estrogen complexes with bazedoxifene prevent metabolic dysfunction in female mice. Molecular Metabolism. 2014;3:177–190. doi: 10.1016/j.molmet.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]