Abstract
Previous research on the association between violence and biological stress regulation has been largely cross-sectional, and has also focused on childhood. Using longitudinal data from a low-income, high-risk, predominantly African-American sample (n = 266; 57 % female), we tested hypotheses about the influence of cumulative exposure to violence during adolescence and early adulthood on cortisol responses in early adulthood. We found that cumulative exposure to violence predicted an attenuated cortisol response. Further, we tested whether sex, mothers’ support, or fathers’ support moderated the effect of exposure to violence on cortisol responses. We found that the effect of cumulative exposure to violence on cortisol was modified by sex; specifically, males exposed to violence exhibited a more attenuated response pattern. In addition, the effect of cumulative exposure to violence on cortisol was moderated by the presence of fathers’ support during adolescence. The findings contribute to a better understanding of how cumulative exposure to violence influences biological outcomes, emphasizing the need to understand sex and parental support as moderators of risk.
Keywords: Contextual stressors, Violence exposure, Stress response, Cortisol, Adolescence
Introduction
Examining biological correlates of stress is useful when seeking to understand how exposure to risk influences developmental outcomes. The biological stress response is one mechanism of how contextual stress can get under the skin and subsequently lead to health problems. The hypothalamic–pituitary–adrenal (HPA) axis is the primary system mediating the biological stress response (Walker et al. 2011), and HPA axis dysregulation is associated with poor health and mental health outcomes (Cohen et al. 2000; Herbert 2013; Sapolsky 1998; Sephton et al. 2000). Exposure to violence is associated with both hyper-activation and hypo-activation of the HPA axis (Gunnar and Vazquez 2001; Rao et al. 2008), and adolescents are particularly at risk of exposure to violence (Lambert et al. 2005). Yet, most studies in this area have been either cross-sectional or retrospective, and many have focused on early child development. Although adolescents are often exposed to violence in community settings, less is known about how exposure to violence during adolescence and early adulthood relates to later HPA axis functioning. In the present study, we examined the longitudinal associations between exposure to violence during adolescence and early adulthood on cortisol responsivity in early adulthood.
Biological Stress Response
Cortisol, a hormone released by the adrenal gland in response to both physical and psychological stressors, is the most widely used indicator of HPA axis activation (Schmeelk-Cone et al. 2003). When functioning normally, the HPA axis has a diurnal rhythm with an early morning cortisol peak and a decline in cortisol over the course of the day. The diurnal rhythm plays a critical role in maintaining physiological and metabolic homeostasis (Sapolsky et al. 2000). Importantly, the HPA axis is also activated in response to stress. When an individual experiences psychological stress, the system produces cortisol, allowing the individual to act in response to a stress or challenge by activating a “fight or flight” response, thus encouraging arousal. When functioning normally, the HPA axis stops producing cortisol once the stressful experience has passed and cortisol returns to baseline (Chrousos and Gold 1992). Thus, the HPA axis and the stress hormone, cortisol, play a key role in the maintenance and control of both resting and stress-related metabolic functions (Sapolsky et al. 2000).
The terms stress responsivity and stress reactivity are sometimes used interchangeably to describe the threshold, dampening, and reactivation of biological arousal (Boyce et al. 1992). High reactivity (or hyperreactivity) refers to a greater activation of arousal with exposure to a stressor and a slower time return to baseline levels, whereas low reactivity (or hyporeactivity) refers to a lack of response after exposure to a stressor (Compas and Reeslund 2009; Gunnar and Vazquez 2001; Heim et al. 2000). In the present study, we focus more on the role of stress responsivity, as this term more appropriately describes our study protocol.
Chronic Stress, Biological Stress Response, and Health
The biological stress response is proposed as a key mechanism through which psychosocial stress can affect health. Lower socioeconomic status, for example, is associated with higher levels of psychological stress, which ultimately influences the HPA axis and its regulation (Hackman et al. 2012). The biological stress response may become dysregulated when an individual experiences chronic stressors beyond his or her control and lacks sources of support with which to mitigate the effects of such stressors (McEwen 1998; Miller et al. 2007; Yehuda et al. 1993). Over time, the diurnal cortisol rhythm may become increasingly altered, such that basal cortisol levels are either atypically high or low (McEwen 1998; Yehuda et al. 1993). Thus, although an increase in cortisol is adaptive when individuals are faced with acute stress, frequent and prolonged activation of this response is maladaptive (Sapolsky 1993, 1998).
The attenuation hypothesis suggests that, under conditions of chronic stress, the HPA axis may adapt to prolonged hypersecretion of cortisol by downregulating, resulting in a period of hyposecretion (Trickett et al. 2010). This adaptive mechanism is designed to decrease allostatic load; or the physiological damage caused by repeated mobilization of the stress response system, and occurs when the body must make repeated physiological adjustments to stressors in an effort to maintain homeostasis (Susman 2006). Researchers have found, for example, that the stress response may become blunted when children are consistently exposed to consistent, high levels of contextual stress (Blair 2010). This maladaptive biological stress response may increase vulnerability to health and mental health disorders (Miller et al. 2007).
Exposure to Violence and Biological Stress Response
Understanding how exposure to stress during adolescence shapes subsequent cortisol responsivity is an important, yet understudied question. Adolescence is a developmental period characterized by biological and hormonal changes, and the HPA axis develops and matures from childhood through early adulthood (Walker et al. 2001). Adolescence is also a period characterized by changes in exposure to risk. Low-income adolescents are likely to experience multiple psychosocial stressors, including family conflict and exposure to community violence (McLoyd et al. 2009). Experiencing uncontrollable negative life events becomes more prevalent in early adolescence (around age 13) compared to childhood and continues to increase until youth reach adulthood (Ge et al. 2001a, b). Stressful experiences are typically out of the adolescent’s control, and thus may shape biological stress responses and influence later cortisol patterns. Thus, stressors experienced during adolescence may exert lasting influences on biological stress responses, as well as brain structures and functions (Walker et al. 2011). Further, adolescents and young adults living in violent communities may experience “pathological adaptations”, which include a desensitization to violence (Garbarino et al. 2002). In sum, the biological stress response may be one mechanism through which exposure to stressors influence negative neurobiological outcomes in both adolescence and early adulthood (Evans and Kim 2007).
On the one hand, exposure to violence and contextual stressors is associated with elevated cortisol levels, and hyperresponsivity. Adolescents raised in poverty, who report high levels of perceived stress, for example, exhibit elevated basal cortisol levels (Brenner et al. 2012; Evans and Kim 2007; Pruessner et al. 1999; Schreier and Evans 2003). Neighborhood disadvantage (Hackman et al. 2012) and exposure to violence (Peckins et al. 2012) are associated with increased reactivity in male adolescents (but not female). Abuse, trauma, and maltreatment are also associated with elevated cortisol levels (Rao et al. 2008) in both adolescents and adults. Although most people adapt quickly when exposed to minor, stressful events, some display longer lasting elevations in cortisol levels. This effect is typically interpreted as a chronic cortisol response, which causes damage over the long-term (Compas et al. 1989).
On the other hand, exposure to violence and contextual stressors is also associated with lower cortisol levels, and hyporesponsivity. Chronic family stress, cumulative contextual stress, and maltreatment during childhood and adolescence have also been associated with attenuated or blunted cortisol responsivity (Carpenter et al. 2009; Gunnar and Vazquez 2001; Roy 2004; Susman and Dorn 2009). In addition, researchers who have studied African American adolescent boys reported that witnessing violence and experiencing victimization were linked to lower baseline cortisol levels (Dulin-Keita et al. 2010; Kliewer 2006). Adolescent boys reporting chronic exposure to high levels of stress also exhibit cortisol hyporeactivity (Moss et al. 1999), providing further evidence for the habituation hypothesis. Finally, children and adolescents with both conduct disorder and depression are more likely to exhibit hyporeactive stress patterns when presented with a stressful task (Fairchild et al. 2008; Hankin et al. 2010), suggesting that high-risk groups may be more likely to exhibit such reactivity. Although exposure to violence during adolescence and early adulthood is associated with concurrent cortisol profiles (Gunnar and Vazquez 2001; Rao et al. 2008), the direction of these findings remains inconclusive.
Parental Support
Parent-adolescent relationships remain important social and emotional resources extending beyond childhood (Collins and Steinberg 2006). Supportive parent–adolescent relationships provide stability within the dyad, and function similarly to those for children. Across childhood and adolescence, parents serve as a secure base for exploring the environment safely. Adolescents who perceive their parents as supportive typically exhibit better psychosocial adjustment (Kamptner 1988), and also engage in less health-risk behaviors (Steinberg 2001).
Parents serve an important role in both buffering and exacerbating the effects of extrafamilial stressors on mental health outcomes (Laursen and Collins 2009; Wills and Cleary 1996). The role of parental support may also be more pronounced in high-crime neighborhoods, as youth have more opportunities for exposure to risk outside of the home (Leventhal et al. 2009; Tolan et al. 2003). Thus, it is important to consider the potential moderating role of parental support in buffering risk from exposure to violence on the biological stress response. Specifically, we consider mothers’ and fathers’ support as potential moderators of the exposure to violence-cortisol association. We hypothesize that parental support during early adolescence will buffer risk from exposure to violence, thus promoting the development of an adaptive stress response.
Sex Differences
Researchers have reported sex differences in HPA axis functioning, yet the direction of these findings remains inconclusive. In many studies, males demonstrate greater increases in cortisol compared to females in response to contextual stress (Kudielka and Kirschbaum 2005; Peckins et al. 2012; Vigil et al. 2010). In adolescent boys, this finding might be due to boys engaging in more risk-taking behavior (Macpherson et al. 2003). Yet, adult men also demonstrate greater increases in cortisol in response to psychological stress, suggesting a lasting sex difference across developmental periods (Peeters et al. 2003).
Conversely, adolescent girls may have heightened stress sensitivity, thus increasing their risk for developing internalizing disorders when exposed to contextual stress (Natsuaki et al. 2009; Peeters et al. 2003; Shih et al. 2006), indicating that stress may actually be more damaging for females. Furthermore, the findings from the Moving to Opportunity program revealed that adolescents girls who moved to less violent neighborhoods exhibited fewer mental health problems (e.g., distress, anxiety, substance use, and delinquency), while their male counterparts did not exhibit such changes due to the program (Leventhal et al. 2009). These results suggest that adolescent girls may be more sensitive to the effects of neighborhood exposure to violence, compared to adolescent boys, and may also be more likely to internalize contextual exposure to risk. In sum, we still need a better understanding of sex differences as they relate to the link between exposure to violence and biological stress responses.
Previous research has focused on the environment of poverty more broadly, and on the effects of cumulative contextual exposure to risk. Although we know that chronic exposure to stress during childhood is associated with adverse cortisol profiles, less is known about the specific role of violence, particularly during adolescence and early adulthood. Yet, exposure to violence may be particularly detrimental to the regulation of cortisol, as such exposure may generate chronic, pervasive fear of the threat of violence (Martinez and Richters 1993). Exposure to violence during adolescence may also be particularly critical, as this is a developmental period when children are spending increasingly more time outside of the family context, and are engaging more in their communities. Further, rates of both witnessing violence and violent victimization continue to increase from adolescence to early adulthood, making both periods especially salient for studying the effects of chronic exposure to violence (Scarpa 2003). Thus, we examine whether exposure to violence during adolescence and early adulthood provides a unique effect on cortisol, against the backdrop of chronic contextual stressors, which are often experienced by our Flint-based participants.
We must also consider the role of potential moderators, in order to determine what types of factors protect (or harm) adolescents exposed to violence from developing dysregulated cortisol responses. As adolescence is a critical period characterized by changes in biology and contextual stressors, we focus on the influence of moderating factors during adolescence on biological outcomes in early adulthood. Previous research on stress regulation has also been largely cross-sectional, which limits researchers from investigating the neurobiological consequences of exposure to violence, rather than the co-occurrence of such. This longitudinal approach also allows us to better understand the complex nature of the impact of exposure to violence across developmental periods.
Current Study
We focused on a sample from a highly disadvantaged area, thus likely to be exposed to higher levels of violence and contextual stress, compared with a normative sample. First, we examined whether cumulative exposure to violence (violence victimization and witnessing violence) across adolescence and early adulthood predicted cortisol responses in early adulthood. Based on previous findings, we hypothesized that cumulative exposure to violence during adolescence would be associated with a hyporesponsive cortisol pattern in early adulthood. Specifically, participants exposed to violence were predicted to exhibit signs of an attenuated stress response. We also tested interactions to determine whether the associations between exposure to violence predictors and cortisol were moderated by sex, mother support, or father support. We hypothesized that girls exposed to violence would exhibit a more attenuated cortisol patterns in response to a stressful task. Further, we hypothesized that parental support during early adolescence would buffer the negative effect of exposure to violence on cortisol outcomes. Specifically, we predicted that parental support would promote adaptive cortisol patterns in participants exposed to violence.
Study Context
The present study participants were recruited from Flint, Michigan. In the past, Flint was a prosperous metropolitan area due to the manufacturing jobs provided by General Motors factories. Yet, since 1970, Flint has lost over 70,000 auto industry jobs, and now has one of the highest unemployment rates in the US (United States Bureau of Labor Statistics 2013). Flint also has the second highest rate of violent crime in the country (US Department of Justice 2012). Due to Flint’s notable economic decline and high rates of violence, our sample is likely to have experienced higher rates of contextual stressors, compared to a normative sample. Thus, in this study, we focus on an understudied, at-risk population with regards to exposure to risk and cortisol.
Methods
Participants
We analyzed data collected as part of an ongoing longitudinal study of youth, at risk for high-school dropout (Zimmerman and Schmeelk-Cone 2003). Participants were recruited from the 9th grades of the 4 public high schools in Flint, Michigan, and were followed into early adulthood. To be eligible for the study, participants had to have a grade point average of 3.0 or below at the end of 8th grade. Participants were not selected for participation if they were diagnosed by the school as having either emotional or developmental impairments.
The present study included 266 participants who had cortisol data and data on our study variables across waves 1–7. The cortisol collection procedure is described in detail below. Participants were between ages 13.9 and 16.9 years at wave 1 (mean = 14.9; SD = .64) and between ages 21.9 and 24.3 years at wave 7 (mean = 22.1; SD = .66). We measured demographic variables at wave 1, as such measures were only collected at baseline and are assumed to be stable over time. We assessed cumulative exposure to violence across waves 1–7, as rates of victimization and exposure to community violence are typically highest during adolescence and early adulthood (Kracke and Hahn 2008). In addition, we focused on cortisol at wave 7, as this wave of data represents the transition to early adulthood. In addition, early adulthood is also a developmental period when the brain begins to reach maturity, and biological changes begin to decrease (Steinberg 2008). The analytic sample was 80 % African American, 17 % Caucasian, and 3 % Mixed Race. The analytic sample was 43 % Male and 57 % Female.
Procedure
This study received approval from both the University of Michigan’s Institutional Review Board (UM- IRB # H03-0001 309) and from the school staff where data were collected. Participants completed structured face-to-face interviews, conducted by trained interviewers. Interviews averaged 60-min in duration. Self-report questionnaires (paper-and-pencil format) were administered following the interview to collect information about alcohol and substance use, sexual behavior, and other sensitive topics. Participants who were not enrolled in school were interviewed at home or in a location specified by the participants. In years following high-school completion, participants were also interviewed at home or in another specified location.
Cortisol Collection
We collected saliva samples for cortisol assay at three designated time points during wave 7 interviews. Following all consent procedures, respondents rinsed their mouths with water. For each sample, participants collected saliva in their mouths for one minute, and then exporated slowly through a stray into a cryotube. All participants were also interviewed after 11:00 a.m. to control for changes due to diurnal rhythm. The first saliva collection was taken approximately 10 min after the interview process (e.g., consent procedures) had begun, reflecting the participants’ baseline state prior to the start of the substantive interview. The second saliva collection was taken at the interview mid-point, approximately 22 min after the first collection. The third and final collection took place at the end of the interview, approximately 30 min after the second collection. Finally, the saliva samples were placed on ice and refrigerated until transport to an −80 °F freezer for storage. Saliva was only collected from those participants who reported no food or drink or tobacco in the previous hour. Participants with blood protein contamination greater than 3 mg/DL were excluded. The pregnant participants were also excluded, as their samples were significantly higher than the rest due to hormonal changes.
Cortisol was specifically assessed by high sensitivity salivary cortisol enzyme immunoassay by Salimetrics, Incorporated. The saliva samples were thawed and centrifuged at 1,500 rpm for 15 min before assay. The assay follows standard enzyme immunoassay procedures as previously described (Klimes-Dougan et al. 2001). The intra-and inter-assay coefficient of variations ranged from 3.88 to 7.12 % and 6.69 to 6.88 %, respectively. The lower limit of sensitivity of this assay is .007 μg/DL (Schmeelk-Cone et al. 2003).
Measures
Cortisol
We utilized salivary cortisol as our measure of the stress response, as it reflects the free portion of cortisol in plasma recommended by many researchers (Kirschbaum and Hellhammer 1989). The saliva was collected during wave 7 at three points during an in-person paper-and-pencil interview concerning sensitive information (e.g., substance use, sexual risk behavior, violent behavior, and discrimination). Such questions were thought to be somewhat stressful for participants, based on their sensitive nature. Cortisol values were log transformed to reduce the skewness of the distribution. To make the repeated measurements of salivary cortisol more useful, we assessed the cortisol response by calculating area under the curve with respect to increase [AUC (I)]. This allowed us to assess changes in cortisol secretion over time (Fekedulegn et al. 2007).
Cumulative Exposure to Violence (ETV)
Cumulative ETV was calculated by standardizing each subscale (witnessing violence and violence victimization) across each wave (waves 1–7), and adding scores to represent cumulative exposure to violence across adolescence and early adulthood. The specific subscales are described in detail below.
Violence Victimization
Victimization was measured with 3 items (Cronbach’s alpha = .60). The participants were asked to report the frequency of experiencing events over the last 12-months. The response options ranged from 1 (0 times) to 5 (4 or more times). The participants reported how often they had “someone threaten to hurt you”, “someone physically assault or hurt you” and “someone take something from you using physical force”. Victimization was assessed at waves 1–7.
Witnessing Violence
Witnessing Violence was measured with 2 items. The participants were asked to report the frequency of experiencing events over the last 12-months. The response options ranged from 1 (0 times) to 5 (4 or more times). The participants reported how often they had “seen someone commit a violent crime where a person was hurt”, and “seen someone get shot, stabbed or beaten up.” Witnessing was also assessed at waves 1–7.
Mother and Father Support
Mother and father support were each measured with 5 items (Cronbach’s alpha = .88–.94, for mother and father support respectively) from the Parental Support Scale (Procidano and Heller 1983). The participants were asked the extent to which they endorsed statements about their relationships with their mother and father (assessed separately). Response options ranged from 1 (not true), to 5 (very true). The scale was designed to assess emotional support, problem solving and moral support from parents. The participants reported whether, “my mother (or father) enjoys hearing about what I think”, “my mother (or father) is good at helping me solve problems”, and “I rely on my mother (or father) for moral support”. Mother and father support were assessed at wave 2.
Control Variables
First, we controlled for interview start time in order to reduce potential confounding related to diurnal changes in cortisol. Next, in order to reduce potential confounding due to race, we controlled for race assessed at wave 1. The participants were asked to self-identify their race. Race was re-coded as a binary variable such that 0 = White and Mixed Race and 1 = African American. As males are more likely than females to be exposed to violence (Singer et al. 1995), we also controlled for sex in the initial analyses, and examined sex as a moderator in later analyses. Sex was coded such that 0 = Female and 1 = Male.
Analytic Strategy
We conducted a hierarchical regression analysis to examine the unique contributions of cumulative exposure to violence, mother support and father support to cumulative exposure to violence. At each step in the analysis, we also examined the contribution of participant race, sex, and interview start time to reduce potential confounding. All three covariates were initially entered into all regression equations at step 1. Next, we examined the effect of cumulative exposure to violence at step 2. At step 3, we examined the direct effects of mother and father support. At step 4, we introduced the interaction terms testing mother support and father support as moderators of the association between cumulative exposure to violence and cortisol responses. In step 5, we tested the interaction between sex and cumulative exposure to violence to examine whether the link between exposure to violence and cortisol was moderated by participant sex. In step 6, we tested a three-way interaction between exposure to violence, father support, and sex in order to examine whether the two-way interaction observed between cumulative exposure to violence and father support was modified by sex. Finally, we centered our predictors and moderators in order to probe the interactions further. The significant interactions were also graphed in order to facilitate meaningful interpretation.
Results
Preliminary Analyses
Means and Standard Deviations
The means and standard deviations for all key study variables are presented in Table 1.
Table 1.
Descriptive statistics
| Variable | Range | Minimum | Max | Mean (SD) |
|---|---|---|---|---|
| Interview start time | 793 | 729 | 1,522 | 1,043.53 (194.22) |
| Cumulative ETV | 47.57 | −9.36 | 38.31 | .42 (.50) |
| Mother support | 4 | 1 | 5 | 4.00 (.96) |
| Father support | 4 | 1 | 5 | 3.13 (1.30) |
| Cortisol sample time 1 | 1.86 | −1.71 | .15 | −.78 (.36) |
| Cortisol sample time 2 | 2.20 | −2.00 | .20 | −.90 (.39) |
| Cortisol sample time 3 | 2.30 | −2.13 | .17 | −.96 (.40) |
| Cortisol AUC | 67.85 | −54.18 | 13.67 | −3.74 (6.71) |
N = 266. Max = maximum, SD = standard deviation; Interview start time measured in minutes; Cumulative ETV = Cumulative exposure to violence; Cortisol AUC = Cortisol area under the curve with respect to increase
Intercorrelations
After calculating descriptive statistics, we examined bivariate correlations among the study variables (see Table 2). Correlation analyses revealed that most of the associations were non-significant. The associations that were statistically significant were typically small, suggesting few issues of overlap. We also examined collinearity statistics, and all tolerance statistics were over 0.40, indicating no major issues with multicollinearity (Cohen et al. 2000).
Table 2.
Correlations
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| 1. Interview start time | 1.00 | |||||
| 2. Race | .05 | 1.00 | ||||
| 3. Sex | .04 | −.00 | 1.00 | |||
| 4. Cumulative ETV | −.05 | −.09 | .24** | 1.00 | ||
| 5. Mother support | .01 | .04 | .07 | −.23* | 1.00 | |
| 6. Father support | .06 | .03 | .16** | −.05 | .28** | 1.00 |
| 7. Cortisol AUC | −.03 | .02 | .01 | −.13* | .17** | .15* |
Tests of significant Pearson correlations are one-tailed. Cumulative ETV = Cumulative exposure to violence; Cortisol AUC = Cortisol area under the curve with respect to increase
p < .05;
p < .01;
p < .001
Attrition Analyses
In order to address issues related to attrition across the seven waves, we conducted t-tests comparing participants in wave 1 to participants in wave 7 on all demographics and key study variables (e.g., race, sex, mother support, father support, violence victimization, and witnessing violence). Participants differed based on demographic variables (sex and race), yet there were no significant differences based on grouping by parental support variables or exposure to violence variables. The independent samples test results for sex and race were t = 2.97, p < .05, and t = −2.02, p < .01, respectively. Thus, despite sample attrition, we found no evidence that attrition influenced the representativeness of the parental support or exposure to violence variables.
Main Effects
Main effects and interaction findings are reported in Table 3. Results from step 1 indicated no significant associations between interview start time, race, and sex, with cortisol responses; this model was also non-significant. Introducing cumulative exposure to violence in step 2 resulted in improved model fit. Further, cumulative exposure to violence was negatively association with cortisol area under the curve with respect to increase. Introducing mother and father support in step 3 improved the fit of the model further and resulted in a significant overall model fit. In step 3, we found a trend towards significance for cumulative exposure to violence, mother support, and father support (p < .10) as direct effects.
Table 3.
Main effects and interactions
| 1 | 2 | 3 | 4 | 5 | 6 | ||
|---|---|---|---|---|---|---|---|
| Step 1: Covariates | Interview start time | −.03 | −.04 | −.05 | −.04 | −.04 | −.05 |
| Race | .02 | .00 | .00 | −.02 | −.01 | −.01 | |
| Sex | .01 | .05 | .01 | .02 | .01 | .03 | |
| Step 2: Cumulative exposure to violence (ETV) | Cumulative ETV | −.15* | −.11+ | −.38 | −.17 | .28 | |
| Step 3: Parental support | Mother support | .12+ | .13* | .12 | .12 | ||
| Father support | .11+ | .11 | .12 | .09 | |||
| Step 4: ETV × Parental support interactions | ETV × Mother support | −.01 | −.08 | −.56 | |||
| ETV × Father support | .31* | .33* | .36* | ||||
| Step 5: ETV × Sex interaction | ETV × Sex | −.20* | −.72* | ||||
| Step 6: ETV × Sex × Father support interaction | ETV × Sex × Father support | .15 | |||||
| Model F | .13 | 1.40 | 2.32* | 2.24* | 2.51** | 2.53** | |
| Model R2 | .00 | .02 | .05 | .07 | .08 | .09 | |
| Change in R2 | .02* | .03* | .01 | .02* | .01 |
ETV = Cumulative exposure to violence
p <.05;
p <.01;
p <.10
Interactions
In step 4, we examined two 2-way interactions between mother support, father support, and cumulative exposure to violence. The interaction between mother support and cumulative exposure to violence was not significant in predicting cortisol. Yet, the interaction between father support and cumulative exposure to violence was indeed significant. Thus, the association between cumulative exposure to violence and cortisol responses varied based on the level of father support. We grouped father support by tertiles in order to probe the interaction further. A graphical illustration of this interaction is shown in Fig. 1. A simple slopes analysis revealed that at low levels of father support, cumulative exposure to violence was associated with a steeper decline in cortisol (e.g., a more attenuated cortisol response) (b = −.27; t(256) = −3.02, p < .01). The interaction finding was not significant at high and moderate levels of father support, however.
Fig. 1.

Graphical representation of interaction between cumulative exposure to violence and father support
In step 5, we also found that sex moderated the effect of cumulative exposure to violence on cortisol (see Table 3). We probed this interaction further in order to facilitate interpretation of the results (see Fig. 2). After analyzing simple slopes, we found that the simple slope for males was negative and significant (b = −.17; t(258) = −2.52, p = .01). This interaction finding suggests that males exposed to violence exhibit a more attenuated cortisol response compared to females; in fact, for females the exposure to violence interaction was not significant. Further, the interaction between exposure to violence and father support remained significant even after accounting for variance explained by the exposure to violence and sex interaction. Including the interaction between cumulative exposure to violence and sex resulted in a significantly improved model fit.
Fig. 2.
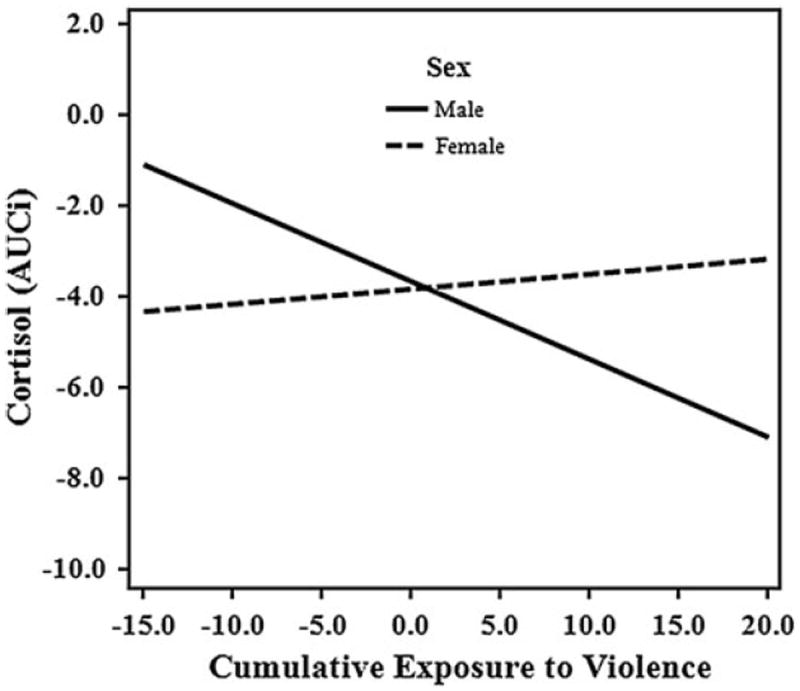
Graphical representation of interaction between cumulative exposure to violence and sex
Given the significant two-way interactions we found, we tested an exploratory three-way interaction between father support, sex, and exposure to violence in step 6. The final model did not improve the overall model fit, nor was the three-way interaction a significant predictor of cortisol.
Summary of Findings
Cumulative exposure to violence assessed across waves 1–7 was associated independently with cortisol area under the curve with respect to increase at wave 7; specifically, exposure to violence was associated with lower cortisol values, indicating an attenuated cortisol response. Next, we examined interactions and found that lack of father support during adolescence exacerbated the effects of exposure to violence on cortisol responsivity. At low levels of father support, exposure to violence was more strongly associated with an attenuated cortisol response. In addition, we found that sex modified the association between cumulative exposure to violence and cortisol AUC (I). Contrary to our hypotheses, males exposed to violence produced less cortisol during the course of the interview compared with females. Finally, we examined an exploratory three-way interaction in order to determine if our finding for the role of father support differed by sex. We did not find evidence of such an effect.
Discussion
We still need a better understanding of the influence of cumulative exposure to violence on cortisol responses, particularly during developmental periods of adolescence and early adulthood. Furthermore, we know even less about the roles of parental support and sex in modifying such associations. Considering the importance of understanding the effects of exposure to violence on cortisol patterns, along with identifying potential moderators, we focused on associations between the aforementioned constructs in the present study.
In the present study, we examined the role of cumulative exposure to violence during adolescence and early adulthood predicting cortisol responses in early adulthood. Our findings indicated that exposure to violence was indeed associated with cortisol responses over time. Specifically, we found that exposure to cumulative violence was associated with an attenuated cortisol response, a finding that was particularly salient for males. We also found that the effect of cumulative exposure to violence was moderated by fathers’ support, indicating that lack of fathers’ support during adolescence served as a risk factor for participants exposed to violence. Thus, we found evidence for the long-term neurobiological effects of cumulative exposure to violence on cortisol responses, moderated by both participants’ sex and fathers’ support.
Our findings emphasize the importance of identifying contextual risk factors that may alter cortisol responses across time, further emphasizing the need to understand how these processes operate in a high-risk sample. Our findings also underscore the role of participants’ sex and fathers’ support during adolescence in modifying such associations. The present study expands on previous research concerning the influence of exposure to violence during childhood by focusing on periods of adolescence and early adulthood. We focus on a high-risk sample from Flint, Michigan, a sample experiencing more chronic violence than a normative sample. To date, few researchers have examined moderators of the link between exposure to violence and biological outcomes. Our finding for the role of father support in exacerbating maladaptive cortisol functioning has important implications.
Exposure to Violence, Cortisol, and Sex Differences
Our findings indicate that cumulative exposure to violence may have long-term neurobiological effects; specifically we point to an attenuated cortisol response. This type of decreasing cortisol profile in response to a stressful situation is consistent with the attenuation hypothesis, which suggests that, although the stress response may initially increase with repeated exposure to chronic stressors, it may decrease over time as a protective mechanism, and future stress responses may be subsequently altered (Gunnar and Vazquez 2001; Susman 2006). Thus, youth exposed to chronic exposure to violence during adolescence may develop desensitization as a (mal)adaptive mechanism. We speculate that this hyporesponsive pattern in early adulthood may have emerged from a hypperresponsive pattern throughout childhood and adolescence. Specifically, adolescents who display cortisol hyperresponsivity in response to stressful tasks may develop a dysregulated stress response as they are exposed to more violence over time. Thus, this hyporesponsive pattern may indicate a cortisol response system that has become less and less capable of adaptively coping with stress, ultimately resulting in HPA-axis suppression derived from this cumulative exposure to violence.
In order to examine the role of sex in modifying the effect of cumulative exposure to violence, we tested this interaction and found that the attenuation effect was most pronounced for males. This finding indicates that adolescent boys who face extensive contextual adversity, as reflected in our cumulative exposure to violence measure, may have been likely to experience chronic HPA axis arousal with little chance for recovery of the system. This chronic HPA axis arousal during adolescence could result in the system burning itself out over time, thus impairing the individual’s ability to mount a cortisol response (McEwen 1998). Sustained hyperresponsivity in adolescent boys exposed to violence may also lead to a hyporesponsive cortisol pattern by the time they reach early adulthood. Finally, our findings regarding the attenuated cortisol response in adolescent boys exposed to violence may shed unique light on sex differences and adolescent development, particularly in terms of coping with exposure to violence.
Parental Support
Social support is a powerful mechanism that can protect against the negative effects of exposure to stress by providing a child security and predictability, despite adverse circumstances. For children, a secure attachment relationship with a primary caregiver can moderate the association between negative life events and cortisol responses, providing an important buffer (Nachmias et al. 1996). In adolescents and young adults, however, this association has not been examined. Interestingly, we did not find evidence that social support from mothers during adolescence buffered the effect of exposure to violence on cortisol responses. Yet, we did find evidence for the role of father support in moderating the deleterious effect of exposure to violence. Specifically, low levels of father support during adolescence were found to exacerbate the attenuation effect from exposure to violence.
Although previous studies have shown that fathers’ involvement is associated with positive developmental outcomes (Lamb and Tamis-Lemonda 2004), less is known about the role of fathers in shaping the stress response. Researchers have shown an association between fathers’ negativity and cortisol responsivity in infants and toddlers (Mills-Koonce et. al. 2011); yet, the specific role of fathers’ support during adolescence and early adulthood has not yet been examined. Further, the presence of fathers’ support may be most beneficial for adolescents and young adults living in disadvantaged, stressful contexts (Kruger et al. 2014). Our findings underscore the critical importance of fathers’ support in promoting adaptive HPA-axis functioning for youth exposed to violence.
An indirect explanation for our finding may be that lack of fathers’ support increases the emotional and psychological stress experienced by mothers (Cabrera et al. 2000), which is likely to detrimentally influence stress regulation in children. In addition, lack of support from fathers may signal a lack of paternal investment to youth, thus contributing to maladaptive cortisol functioning. Conversely, the presence of father support may reduce the perceived threat or fear of violence in adolescents exposed to violence. Thus, support from fathers may reduce the subjective impact of exposure to violence, by providing youth with a safe, consistent base. Taken together, these findings contribute to a better understanding of the complex role that fathers can plan in adolescent and early adulthood development by improving cortisol functioning.
Disadvantage
As previously noted, our sample was recruited from a community exposed to particularly high rates of disadvantage and violence. We know that adolescents who live in disadvantaged neighborhoods are more likely to be exposed to chronic contextual stressors (Latkin and Curry 2003) including violence. Such adolescents are also more likely to exhibit poor stress regulation patterns. Stress responsivity has also been linked to a multitude of health problems (e.g., immune suppression, cancer, cardiovascular problems) (Brenner et al. 2012; Chandola et al. 2006) and mental health problems (e.g., aggression, antisocial behavior, depression) (Dockray et al. 2009; Ge et al. 2001a, b; McBurnett et al. 2000). Unequal exposure to contextual stressors contributes to health and mental health disparities (Bobak and Marmot 1996; Dohrenwend 2000). Thus, the alteration of biological processes in response to exposure to chronic stressors may be central in understanding health disparities and also in designing prevention programs to reduce the impact of contextual risk on negative neurobiological outcomes.
Limitations
Several limitations of our study require attention. First, we relied on self-report data for all of our exposure to violence and social support measures. Although self-report may reduce variance because of the potential for social desirability, our findings were fairly consistent with theory and previous research, suggesting that the results are not completely explained by response bias.
Second, it is important to note that, although it may have provoked a moderate degree of psychological stress, our interview was not designed to elicit acute physiological stress. Thus, we did not assess cortisol responses to acute stress in the current study, but rather responses to a moderately stressful task. Cortisol dysregulation does indeed happen in response to minor stressors, however (Graber and Sontag 2009). In fact, chronic cortisol responses to daily stressors, which may cause damage over the long term, are suggested when cortisol levels remain attenuated (Compas et al. 1989). It is also important to note that cortisol is mobilized when individuals are effortfully engaged in a task (D’Angiulli et al. 2012; Tops et al. 2006); thus, an attenuated response over time may reflect a lack of engagement in the interview, which may be interpreted as a maladaptive, withdrawn behavior. Finally, our rates of responsivity are similar to that reported in previous research (Dockray et al. 2009; Schmeelk-Cone et al. 2003; Susman 2006), suggesting consistency with other studies.
In the present study, we analyzed seven waves of data, collected across 9 years. Although the longitudinal nature of this study can be interpreted as a strength, one limitation to be noted is that not all constructs were available across all waves. Specifically, cortisol was only measured during waves 6 and 7; we chose to analyze cortisol data from wave 7 as allowed us to maximize the longitudinal nature of our study. Further, mother and father support were not assessed separately until wave 2; subsequently, we chose to analyze wave 2 data as it was the earliest wave of mother and father support data available. This allowed us to capture parental support during a critical period of adolescence.
Future Directions and Prevention Implications
Future research on sex differences in the association between exposure to violence and biological outcomes remains useful. We still need a better understanding of how to promote positive developmental outcomes in adolescents and young adults exposed to chronic violence. Understanding how sex and parental support influence the association between exposure to violence and biological outcomes would also allow us to effectively design prevention programs to promote adaptive stress regulation in youth exposed to violence.
Prevention programs designed to target both parents and youth may be particularly effective. Parents’ ability to cope with traumatic events strongly predicts their children’s reactions to contextual stressors (Garbarino et al. 2002; Zahr 1996). Thus, teaching both parents and their children how to adaptively cope with stress may help them to maintain a better homeostatic balance when faced with stressful situations. In addition, there is a notable association between parent–child attachment and HPA axis functioning, which has prevention implications. In fact, relational interventions that encourage parent–child attachment may enhance children’s abilities to regulate cortisol (Dozier et al. 2008). Family interventions that encourage parents to provide warm, supportive, responsive care for their children may encourage adaptive cortisol functioning. In addition, the present findings underscore the role of weak father support in potentially exacerbating risk from exposure to violence. Thus, intervening at the family-level may be a particularly salient context to protect youth from developing maladaptive stress responses.
In addition, individual-level programs designed to promote emotional regulation and mindfulness may be helpful in regulating cortisol. Mindfulness training and emotion regulation programs have been shown to improve cortisol regulation in those exposed to trauma (McCraty et al. 1998; Smith et al. 2011). Thus, programs designed to promote emotion regulation and mindfulness may improve coping skills in the face of adversity and stress, ultimately improving cortisol functioning.
Perhaps the most significant contribution of our study is the demonstration of persistent, noxious effects of chronic exposure to violence for youth raised in a disadvantaged, urban environment. Parental support is typically a salient buffering resource for youth, and we found additional evidence for the role of fathers’ support in exacerbating risk for youth in our sample from the effects of exposure to violence. Future research examining other individual assets or social resources that help male adolescents overcome the detrimental consequences of exposure to violence is needed. We also need a better way of identifying those youth who are exposed to violence early on, in order to provide them with the necessary programs and services. As many adolescents are exposed to violence within their neighborhoods, families and schools may be effective contexts for identification of those exposed to violence (Garbarino et al. 2002).
Conclusion
We found evidence for the influence of cumulative exposure to violence during adolescence and early adulthood on cortisol responsivity in early adulthood. Focusing on a uniquely high-risk sample, we found that participants exposed to chronic violence demonstrate signs of an attenuated stress response. Our findings also indicate that the attenuation effect of exposure to violence on cortisol responsivity was more relevant for males. In addition, lack of father support during adolescence served a detrimental role in contributing to maladaptive cortisol functioning. Thus, our results suggest that chronic exposure to violence can have lasting deleterious effects, which include physiological consequences. Prevention efforts designed to prevent maladaptive cortisol regulation in populations exposed to high rates of violence would be useful. Research is also needed to understand why exposure to violence during adolescence may be more detrimental to boys, compared with girls.
Acknowledgments
This research was funded by the National Institute on Drug Abuse, Grant Number DA007484. The research reported here does not necessarily reflect the views or policies of the National Institute on Drug Abuse. The research reported here was also supported by the Michigan Youth Violence Prevention Center, Cooperative Agreement Number 5U01CE001957-02 from the Centers for Disease Control and Prevention, and the Michigan Injury Center, Grant Number 1R49CE002099. The research reported here does not necessarily represent the official position of the Centers for Disease Control and Prevention. Finally, the authors would like to acknowledge Benjamin Zimmerman, who provided research assistance on the project.
Biographies
Dr. Sophie M. Aiyer is a Postdoctoral Research Fellow in the Youth Violence Prevention Center at the University of Michigan School of Public Health. Dr. Aiyer’s research experiences to date share a common goal of understanding how ecological and individual factors shape developmental outcomes in child and adolescent populations. Her research also examines the etiology, development, and prevention of antisocial behavior. Dr. Aiyer earned her Ph.D. in Education from the University of Virginia, and her Master of Health Science in Public Health from the Johns Hopkins School of Public Health.
Dr. Justin E. Heinze is a Research Investigator at the University of Michigan School of Public Health. Dr. Heinze’s research focuses on developmental transitions, social exclusion and ostracism, issues of gender and sexuality, and longitudinal methodology. His current projects examine the social determinants of health and risk behavior in adolescence and emerging adulthood. Dr. Heinze earned his Ph.D. in Educational Psychology from the University of Illinois-Chicago.
Dr. Alison L. Miller is an Assistant Research Professor at the University of Michigan School of Public Health, and an Assistant Research Scientist in the Center for Human Growth and Development. Dr. Miller is a developmental psychologist who studies risk and resilience in children and families. Her research focuses on child bio-behavioral regulation, family functioning, and social context. Dr. Miller earned both her Master of Arts and Ph.D. in Developmental Psychology from the University of Michigan.
Dr. Sarah A. Stoddard is an Assistant Professor at the University of Michigan School of Nursing. Dr. Stoddard’s research focuses on understanding the interaction between individual factors and social and environmental factors (e.g., neighborhood characteristics), and how together they shape the psychosocial development and health trajectories of at-risk urban youth. Dr. Stoddard earned her Ph.D. in Nursing from the University of Minnesota.
Dr. Marc A. Zimmerman is a Professor and Chair of Health Behavior and Health Education in the University of Michigan School of Public Health. Dr. Zimmerman is also the Director of the CDC-funded Prevention Research Center of Michigan, and the CDC-funded Youth Violence Prevention Center. His research focuses on health and resiliency of adolescents, and on empowerment theory. His work on adolescent health examines how positive factors in adolescent’s lives help them overcome risks they face. Dr. Zimmerman earned his Ph.D. in Psychology from the University of Illinois, and his Master of Science in Community Psychology from the University of Oregon.
Footnotes
Authors’ contributions SMA conceived of, designed, and coordinated the study. SMA also drafted the manuscript and contributed to the statistical analyses and interpretation of the data. JEH assisted with the conception and design of the study, performed the statistical analysis, and assisted with interpretation of the data. JEH also assisted with revising the manuscript. ALM assisted with the study design and also helped with drafting the manuscript. SAS and MAZ contributed to the conception of the study and reviewed the manuscript. Finally, all authors read and approved of the final manuscript. The authors on the manuscript have agreed to the byline order and to submission of the manuscript in this form.
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Sophie M. Aiyer, Email: smaiyer@umich.edu, Health Behavior Health Education, University of Michigan School of Public Health, 3706 SPH I, 1415 Washington Heights, Ann Arbor, MI 48109, USA.
Justin E. Heinze, Health Behavior Health Education, University of Michigan School of Public Health, 3706 SPH I, 1415 Washington Heights, Ann Arbor, MI 48109, USA
Alison L. Miller, Health Behavior Health Education, University of Michigan School of Public Health, 3706 SPH I, 1415 Washington Heights, Ann Arbor, MI 48109, USA
Sarah A. Stoddard, Health Behavior Health Education, University of Michigan School of Public Health, 3706 SPH I, 1415 Washington Heights, Ann Arbor, MI 48109, USA
Marc A. Zimmerman, Health Behavior Health Education, University of Michigan School of Public Health, 3706 SPH I, 1415 Washington Heights, Ann Arbor, MI 48109, USA
References
- Blair C. Stress and the development of self-regulation in context. Child Development Perspectives. 2010;4:181–188. doi: 10.1111/j.1750-8606.2010.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobak M, Marmot M. East-West mortality divide and its potential explanations: Proposed research agenda. European Journal of General Practice. 1996;2:8. doi: 10.1136/bmj.312.7028.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT, Barr RG, Zeltzer LK. Temperament and the psychobiology of childhood stress. Pediatrics. 1992;90:483–486. [PubMed] [Google Scholar]
- Brenner AB, Zimmerman MA, Bauermeister JA, Caldwell CH. The physiological expression of living in disadvantaged neighborhoods for youth. Journal of Youth and Adolescence. 2012:1–15. doi: 10.1007/s10964-012-9838-8. NIHMSID: NIHMS416568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera N, Tamis-LeMonda CS, Bradley RH, Hofferth S, Lamb ME. Fatherhood in the twenty-first century. Child Development. 2000;71:27–136. doi: 10.1111/1467-8624.00126. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Tyrka AR, Ross NS, Khoury L, Anderson GM, Price LH. Effect of childhood emotional abuse and age on cortisol responsivity in adulthood. Biological Psychiatry. 2009;66:69–75. doi: 10.1016/j.biopsych.2009.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandola T, Brunner E, Marmot M. Chronic stress at work and the metabolic syndrome: Prospective study. BMJ. 2006;332:521–525. doi: 10.1136/bmj.38693.435301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. JAMA: The Journal of the American Medical Association. 1992;267:1244–1252. doi: 10.1001/jama.1992.03480090092034. [DOI] [PubMed] [Google Scholar]
- Cohen S, Hamrick NM, Rodriguez MS, Feldman PJ, Rabin BS, Manuck SB. The stability of and intercorrelations among cardiovascular, immune, endocrine, and psychological reactivity. Annals of Behavioral Medicine. 2000;22:171–179. doi: 10.1007/BF02895111. [DOI] [PubMed] [Google Scholar]
- Collins WA, Steinberg L. Adolescent development in interpersonal context. In: Damon W, Eisenberg N, editors. Handbook of child psychology: Socioemotional processes. Vol. 4. New York: Wiley; 2006. pp. 1003–1067. [Google Scholar]
- Compas BE, Howell DC, Phares V, Williams RA, Ledoux N. Parent and child stress and symptoms: An integrative analysis. Developmental Psychology. 1989;25:550–559. [Google Scholar]
- Compas BE, Reeslund KL. Processes of risk and resilience during adolescence. Handbook of Adolescent Psychology. 2009 doi: 10.1002/9780470479193.adlpsy001017. [DOI] [Google Scholar]
- D’angiulli A, Van Roon PM, Weinberg J, Oberlander TF, Grunau RE, Hertzman C, et al. Frontal EEG/ERP correlates of attentional processes, cortisol and motivational states in adolescents from lower and higher socioeconomic status. Frontiers in Human Neuroscience. 2012;6:306. doi: 10.3389/fnhum.2012.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray S, Susman EJ, Dorn LD. Depression, cortisol reactivity, and obesity in childhood and adolescence. Journal of Adolescent Health. 2009;45:344–350. doi: 10.1016/j.jadohealth.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrenwend BP. The role of adversity and stress in psychopathology: Some evidence and its implications for theory and research. Journal of Health and Social Behavior. 2000;41:1–19. [PubMed] [Google Scholar]
- Dozier M, Peloso E, Lewis E, Laurenceau JP, Levine S. Effects of an attachment-based intervention on the cortisol production of infants and toddlers in foster care. Development and Psychopathology Special Issue: Integrating Biological Measures into the Design and Evaluation of Prevention Interventions. 2008;20:845–859. doi: 10.1017/S0954579408000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulin-Keita A, Casazza K, Fernandez JR, Goran MI, Gower B. Do neighborhoods matter? Neighborhood disorder and long-term trends in serum cortisol levels. Journal of Epidemiology and Community Health. 2010;66:24–29. doi: 10.1136/jech.2009.092676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Kim P. Childhood poverty and health cumulative exposure to risk and stress dysregulation. Psychological Science. 2007;18:953–957. doi: 10.1111/j.1467-9280.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- Fairchild G, van Goozen SH, Stollery SJ, Brown J, Gardiner J, Herbert J, et al. Cortisol diurnal rhythm and stress reactivity in male adolescents with early-onset or adolescenceonset conduct disorder. Biological Psychiatry. 2008;64:599–606. doi: 10.1016/j.biopsych.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekedulegn DB, Andrew ME, Burchfiel CM, Violanti JM, Hartley TA, Charles LE, et al. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosomatic Medicine. 2007;69:651–659. doi: 10.1097/PSY.0b013e31814c405c. [DOI] [PubMed] [Google Scholar]
- Garbarino J, Bradshaw CP, Vorrasi JA. Mitigating the effects of gun violence on children and youth. The Future of Children. 2002;12:73–85. [PubMed] [Google Scholar]
- Ge X, Conger RD, Elder GH. Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Developmental Psychology. 2001a;37:404–416. doi: 10.1037//0012-1649.37.3.404. [DOI] [PubMed] [Google Scholar]
- Ge X, Conger RD, Elder GH., Jr The relation between puberty and psychological distress in adolescent boys. Journal of Research on Adolescence. 2001b;11:49–70. doi: 10.1111/1532-7795.00003. [DOI] [Google Scholar]
- Graber JA, Sontag LM. Internalizing problems during adolescence. In: Lerner RM, Steinberg L, editors. Handbook of adolescent psychology: Individual bases of adolescent development. 3. Vol. 1. New Jersey: Wiley; 2009. [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;13:515–538. doi: 10.1016/S0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Hackman DA, Betancourt LM, Brodsky NL, Hurt H, Farah MJ. Neighborhood disadvantage and adolescent stress reactivity. Frontiers in Human Neuroscience. 2012;6:277. doi: 10.3389/fnhum.2012.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Badanes LA, Abela JR, Watamura SE. Hypothalamic–pituitary–adrenal axis dysregulation in dysphoric children and adolescents: Cortisol reactivity to psychosocial stress from preschool through middle adolescence. Biological Psychiatry. 2010;68:484–490. doi: 10.1016/j.biopsych.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/S0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Herbert J. Cortisol and depression: Three questions for psychiatry. Psychological Medicine. 2013;43:449–469. doi: 10.1017/S0033291712000955. [DOI] [PubMed] [Google Scholar]
- Kamptner NL. Identity development in late adolescence: Causal modeling of social and familial influences. Journal of Youth and Adolescence. 1988;17:493–514. doi: 10.1007/BF01537827. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research. An overview Neuropsychobiology. 1989;22:150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- Kliewer W. Exposure to violence and cortisol responses in urban youth. International Journal of Behavioral Medicine. 2006;13:109–120. doi: 10.1207/s15327558ijbm1302_2. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: Individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Development and Psychopathology. 2001;13:695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- Kracke K, Hahn H. Nature and extent of childhood exposure to violence: What we know, why we don’t know more and why it matters. Journal of Emotional Abuse. 2008;8:29–49. [Google Scholar]
- Kruger DJ, Aiyer SM, Caldwell CH, Zimmerman MA. Local scarcity of adult men predicts youth assault rates. Journal of Community Psychology. 2014;42:119–125. doi: 10.1002/jcop.21597. [DOI] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: A review. Biological Psychology. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Lamb ME, Tamis-Lemonda CS. The role of the father: An introduction. In: Lamb ME, editor. The role of the father in child development. 4. New York: John Wiley & Sons; 2004. pp. 1–31. [Google Scholar]
- Lambert SF, Ialongo NS, Boyd RC, Cooley MR. Risk factors for community exposure to violence in adolescence. American Journal of Community Psychology. 2005;36:29–48. doi: 10.1007/s10464-005-6231-8. [DOI] [PubMed] [Google Scholar]
- Latkin CA, Curry AD. Stressful neighborhoods and depression: A prospective study of the impact of neighborhood disorder. Journal of Health and Social Behavior. 2003:34–44. [PubMed] [Google Scholar]
- Laursen B, Collins WA. Parent—child relationships during adolescence. Handbook of Adolescent Psychology. 2009 doi: 10.1002/9780470479193.adlpsy002002. [DOI] [Google Scholar]
- Leventhal T, Dupéré V, Brooks-Gunn J. Neighborhood influences on adolescent development. Handbook of Adolescent Psychology. 2009 doi: 10.1002/9780470479193.adlpsy002013. [DOI] [Google Scholar]
- Macpherson L, Reynolds EK, Daughters SB, Wang F, Cassidy J, Mayes LC, et al. Positive and negative reinforcement underlying risk behavior in early adolescents. Prevention Science. 2010;11:331–342. doi: 10.1007/s11121-010-0172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez P, Richters JE. The NIMH community violence project II. Children’s distress symptoms associated with exposure to violence. Psychiatry. 1993;56:22–33. doi: 10.1080/00332747.1993.11024618. [DOI] [PubMed] [Google Scholar]
- McBurnett K, Lahey BB, Rathouz PJ, Loeber R. Low salivary cortisol and persistent aggression in boys referred for disruptive behavior. Archives of General Psychiatry. 2000;57:38. doi: 10.1001/archpsyc.57.1.38. [DOI] [PubMed] [Google Scholar]
- McCraty R, Barrios-Chopin B, Rozman D, Atkinson M, Watkins AD. The impact of a new emotional self-management program on stress, emotions, heart rate variability, DHEA, and cortisol. Integrative Physiological and Behavioral Science. 1998;33:151–171. doi: 10.1007/BF02688660. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- McLoyd VC, Kaplan R, Purtell KM, Bagley E, Hardaway CR, Smalls C. Poverty and socioeconomic disadvantage in adolescence. Handbook of Adolescent Psychology. 2009 doi: 10.1002/9780470479193.adlpsy002014. [DOI] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic–pituitary–adrenocortical axis in humans. Psychological Bulletin. 2007;133:25. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Moss HB, Vanyukov M, Yao J, Irillova G. Salivary cortisol responses in prepubertal boys: The effects of parental substance abuse and association with drug use behavior during adolescence. Biological Psychiatry. 1999;45:1293–1299. doi: 10.1016/s0006-3223(98)00216-9. [DOI] [PubMed] [Google Scholar]
- Nachmias M, Gunnar M, Mangelsdorf S, Parritz RH, Buss K. Behavioral inhibition and stress reactivity: The moderating role of attachment security. Child Development. 1996;67:508–522. doi: 10.1111/j.1467-8624.1996.tb01748.x. [DOI] [PubMed] [Google Scholar]
- Natsuaki MN, Klimes-Dougan B, Ge X, Shirtcliff EA, Hastings PD, Zahn-Waxler C. Early pubertal maturation and internalizing problems in adolescence: Sex differences in the role of cortisol reactivity to interpersonal stress. Journal of Clinical Child & Adolescent Psychology. 2009;38:513–524. doi: 10.1080/15374410902976320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckins MK, Dockray S, Eckenrode JL, Heaton J, Susman EJ. The longitudinal impact of exposure to violence on cortisol reactivity in adolescents. Journal of Adolescent Health. 2012;51:366–372. doi: 10.1016/j.jadohealth.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters F, Nicholson NA, Berkhof J. Cortisol responses to daily events in major depressive disorder. Psychosomatic Medicine. 2003;65:836–841. doi: 10.1097/01.psy.0000088594.17747.2e. [DOI] [PubMed] [Google Scholar]
- Procidano ME, Heller K. Measures of perceived social support from friends and from family: Three validation studies. American Journal of Community Psychology. 1983;11:1–24. doi: 10.1007/BF00898416. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening. Psychosomatic Medicine. 1999;61:197–204. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- Rao U, Hammen C, Ortiz LR, Chen LA, Poland RE. Effects of early and recent adverse experiences on adrenal response to psychosocial stress in depressed adolescents. Biological Psychiatry. 2008;64:521–526. doi: 10.1016/j.biopsych.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy MP. Patterns of cortisol reactivity to laboratory stress. Hormones and Behavior. 2004;46:618–627. doi: 10.1016/j.yhbeh.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Endocrinology alfresco: Psychoendocrine studies of wild baboons. Recent Progress in Hormone Research. 1993;48:437. doi: 10.1016/b978-0-12-571148-7.50020-8. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Molecular neurobiology: The stress of Gulf War syndrome. Nature. 1998;393(6683):308–309. doi: 10.1038/30606. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/er.21.1.55. [DOI] [PubMed] [Google Scholar]
- Scarpa A. Community exposure to violence in young adults. Trauma Violence Abuse. 2003;4:210–227. doi: 10.1177/1524838003004003002. [DOI] [PubMed] [Google Scholar]
- Schmeelk-Cone KH, Zimmerman MA, Abelson JL. The buffering effects of active coping on the relationship between SES and cortisol among African American young adults. Behavioral Medicine. 2003;29:85–94. doi: 10.1080/08964280309596061. [DOI] [PubMed] [Google Scholar]
- Schreier A, Evans GW. Adrenal cortical response of young children to modern and ancient stressors. Current Anthropology. 2003;44:306–309. doi: 10.1086/367974. [DOI] [Google Scholar]
- Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. Journal of the National Cancer Institute. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- Shih JH, Eberhart NK, Hammen CL, Brennan PA. Differential exposure and reactivity to interpersonal stress predict sex differences in adolescent depression. Journal of Clinical Child and Adolescent Psychology. 2006;35:103–115. doi: 10.1207/s15374424jccp3501_9. [DOI] [PubMed] [Google Scholar]
- Singer MI, Anglin TM, Song LY, Lunghofer L. Adolescents’ exposure to violence and associated symptoms of psychological trauma. JAMA: The Journal of the American Medical Association. 1995;273:477–482. doi: 10.1001/jama.1995.03520300051036. [DOI] [PubMed] [Google Scholar]
- Smith BW, Ortiz JA, Steffen LE, Tooley EM, Wiggins KT, Yeater EA, et al. Mindfulness is associated with fewer PTSD symptoms, depressive symptoms, physical symptoms, and alcohol problems in urban firefighters. Journal of Consulting and Clinical Psychology. 2011;79:613–617. doi: 10.1037/a0025189. [DOI] [PubMed] [Google Scholar]
- Steinberg L. We know these things: Parent adolescent relationships in retrospect and prospect. Journal of Research on Adolescence. 2001;11:1–19. [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susman EJ. Psychobiology of persistent antisocial behavior: Stress, early vulnerabilities and the attenuation hypothesis. Neuroscience and Biobehavioral Reviews. 2006;30:376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Susman EJ, Dorn LD. Puberty: Its role in development. In: Lerner RM, Steinberg L, editors. Handbook of adolescent psychology. New York: John Wiley & Sons; 2009. pp. 116–151. [Google Scholar]
- Tolan PH, Gorman-Smith D, Henry DB. The developmental ecology of urban males’ youth violence. Developmental Psychology. 2003;39:274–290. doi: 10.1037/0012-1649.39.2.274. [DOI] [PubMed] [Google Scholar]
- Tops M, Boksem MA, Wester AE, Lorist MM, Meijman TF. Task engagement and the relationships between the error-related negativity, agreeableness, behavioral shame proneness and cortisol. Psychoneuroendocrinology. 2006;31:847–858. doi: 10.1016/j.psyneuen.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Trickett PK, Noll JG, Susman EJ, Shenk CE, Putnam FW. Attenuation of cortisol across development for victims of sexual abuse. Development and Psychopathology. 2010;22:165–175. doi: 10.1017/S0954579409990332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Bureau of Labor Statistics. Economy at a Glance: Flint, Michigan. 2013 http://www.bls.gov/eag/eag.mi_flint_msa.htm.
- U.S. Department of Justice-Federal Bureau of Investigation. Uniform Crime Report: Crime in the United States, 2011 2012 [Google Scholar]
- Vigil JM, Geary DC, Granger DA, Flinn MV. Sex differences in salivary cortisol, alpha-amylase, and psychological functioning following Hurricane Katrina. Child Development. 2010;81:1228–1240. doi: 10.1111/j.1467-8624.2010.01464.x. [DOI] [PubMed] [Google Scholar]
- Walker E, Trotman H, Brasfield J, Esterberg M, Larsen M. The dynamic genome and mental health: The role of genes and environments in youth development. New York: Oxford University Press, Inc; 2011. Stress hormones, genes, and neuronal signaling in adolescence. The perfect storm for vulnerability to psychosis. [Google Scholar]
- Walker EF, Walder DJ, Reynolds F. Developmental changes in cortisol secretion in normal and at-risk youth. Development and Psychopathology. 2001;13:721–732. doi: 10.1017/s0954579401003169. [DOI] [PubMed] [Google Scholar]
- Wills TA, Cleary SD. How are social support effects mediated? A test with parental support and adolescent substance use. Journal of Personality and Social Psychology. 1996;71:937. doi: 10.1037/0022-3514.71.5.937. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Resnick H, Kahana B, Giller EL. Long-lasting hormonal alterations to extreme stress in humans: Normative or maladaptive? Psychosomatic Medicine. 1993;55:287–297. doi: 10.1097/00006842-199305000-00006. [DOI] [PubMed] [Google Scholar]
- Zahr LK. Effects of war on the behaviour of Lebanese pre-school children: The influence of home environment and family functioning. American Journal of Orthopsychiatry. 1996;66:401–408. doi: 10.1037/h0080190. [DOI] [PubMed] [Google Scholar]
- Zimmerman MA, Schmeelk-Cone KH. A longitudinal analysis of adolescent substance use and school motivation among African American youth. Journal of Research on Adolescence. 2003;13:185–210. [Google Scholar]


