Abstract
Growing evidence suggests that adverse childhood experiences (ACEs) increase the risks for coronary heart disease and hypertension in mid and late adulthood. We previously reported that early life stress induces a hyper-reactive endothelin (ET)-dependent cardiovascular phenotype in a rat model. In the present study, we evaluated whether exposure to ACEs is associated with greater peripheral resistance, arterial stiffness, blood pressure, or elevated circulating ET-1 levels in humans. In 221 healthy adolescents and young adults (mean age: 21; age range: 13–29), we found a graded association of ACE exposure with plasma ET-1 levels, of which on average 18% and 24% were higher in subjects with 1 ACE, and ≥2 ACEs compared to those with no ACEs (P=0.001). The subjects with moderate/severe exposure to ACEs (≥2 ACEs) had significantly higher total peripheral resistance index (+12%), diastolic blood pressure (+5%) and pulse wave velocity (+9%) compared with those who were not exposed. These associations were independent of age, race, gender, body mass index and childhood socioeconomic status. Our results indicate that early life stress promotes cardiovascular disease risk, specifically detrimental vascular and cardiac function, detectable in very young adulthood.
Keywords: early life stress, endothelin-1, peripheral resistance, pulse wave velocity, blood pressure, adverse childhood experiences
INTRODUCTION
Childhood adversity, characterized by abuse, neglect and household dysfunction, is a national problem that exerts a significant impact on individuals, families and society. 1, 2 Growing evidence suggests that traumatic experiences in childhood are associated with health decline in adulthood. 3 The first large-scale adverse childhood experiences (ACE) study and subsequent studies have found that exposure to ACEs increased the incidence of ischemic heart disease, autoimmune disorders and premature mortality. 4–6 More recently, another large longitudinal study of >60,000 women suggested that childhood maltreatment increased later risks for diabetes and hypertension. 7, 8 In addition, studies in Finland have found that children who were evacuated abroad temporarily without parents during World War II had higher risks for coronary heart disease and hypertension in late adulthood. 9 These epidemiological studies, as well as a recent meta-analysis, 10 indicate that poor health outcomes in adulthood may stem from non-optimal growth and exceptional conditions during early life. However, the underlying mechanisms remain unclear.
Animal studies have found that rodents separated from their mothers during the hyporesponsive period, a widely used model to manipulate early life stress (ELS), displayed alterations of the central nervous, endocrine and immune systems as adults. 11–13 Most recently, a study from our group using this maternal separation model in rats indicated that ELS increased basal endothelin-1 (ET-1) levels as well as blood pressure reactivity through the ET-1 pathway. 14 ET-1, an endothelium-derived peptide, is a potent endogenous vasoconstrictor and exerts its major effect on blood pressure and basal vascular tone through ETA and ETB receptors. 15 Exaggerated ET-1 levels have been linked to a number of biological activities including elevated blood pressure, decreased cardiac output (CO) and increased pulse wave velocity (PWV), a measure of arterial stiffness. 16 However, the role of ELS on circulating ET-1 levels and systemic hemodynamic parameters has not been examined in humans.
We hypothesized that ACEs are associated with detrimental hemodynamic parameters and elevated plasma ET-1 levels in adolescents and young adults. In the present study we tested this hypothesis by determining whether exposure to ACEs is associated with plasma ET-1 levels, blood pressure, total peripheral resistance index, cardiac output index, or pulse wave velocity in a cohort of adolescents and young adults. We further evaluated whether circulating ET-1 levels may be a mediator between ACEs and vascular or cardiac dysfunction.
METHODS
Subjects
The present study comprised subjects from a longitudinal cohort that was established in 1989 to study the development of cardiovascular risk factors. 17 It included similar number of African American (AA) and European American (EA) youth with evaluations conducted annually from visit 1 to 13 and every two years from visit 13 to 16. All the subjects were recruited from the southeastern United States and were overtly healthy, free of any acute or chronic illness, and not taking any prescription medications based on parental or self-report. Study design, selection criteria and the criteria to classify subjects as AA or EA for the longitudinal study have been described previously. 17
The Institutional Review Board at the Medical College of Georgia had given approval for the study. Informed consent was provided by all subjects or by parents if subjects were <18 years. With the exception of ACE assessment, which was conducted on visit 15, all the other measurements used in the current study were from visit 12. There were 537 subjects recruited at visit 12 and basal plasma ET-1 levels were measured in 322 of these subjects, as well as the PWV and hemodynamic measurements. At visit 15, the subjects were over 19 years old and their exposure to ACEs during the first 18 years of life were assessed. Of the 322 subjects, 232 were followed-up at visit 15 and 221 of them answered the ACE questionnaire. In total, there were 221 subjects who had both measurements of ET-1 and ACE assessments, including 112 EAs (61 males and 51 females) and 109 AAs (44 males and 65 females). Compared to the subjects excluded (N=316) who had missing values of either ET-1 or ACE assessments, the subjects included in the present study (N=221) showed no differences in demographics, including age, gender and ethnicity.
Hemodynamic Measurements
At visit 12, on arrival at the laboratory, the subject participant was escorted to a quiet, temperature-controlled room where anthropometric data and hemodynamic measures were obtained by using established protocols. 18 The study participants were then instrumented for the recording of blood pressure (BP) by Dinamap (model 1864 SX) and cardiac output by Impedance Cardiograph (BioZICG Monitor, CardioDynamics, San Diego, CA). Cardiac output (CO) was indexed by body surface area (i.e., CO index). Total peripheral resistance (TPR) index was calculated as mean arterial pressure/CO index expressed in Wood units (mmHg/L/min/m2). After attachment of the BP cuff to the right arm, the subject was asked to lie on a hospital bed in a supine position. The left elbow was stabilized with an armboard; a 21-gauge butterfly needle was inserted into the antecubital vein, and a three-way plastic stopcock was attached. One ml of 0.9% saline was infused at 2 to 3 minute intervals to help maintain patency. Immediately after the needle was inserted, a 5-ml blood sample was drawn, transferred to a 10-ml prechilled EDTA tube vacutainer, and maintained on ice. Basal plasma ET-1 levels were determined using this blood sample. Then the subject was given standardized instructions to relax as completely as possible for 15 minutes. Hemodynamic measurements were taken at 11, 13, and 15 minutes and the average of the 3 measurements was used.
Measurement of Pulse Wave Velocity
Aortoradial (radial) PWV was measured noninvasively with applanation tonometry (Millar Instruments) and analysis software (SphygmoCor, AtCor Medical, Sydney, Australia). 19 Pressure waves were recorded at the common carotid and radial arteries for the radial PWV. PWV was then automatically calculated from measurements of pulse transit time and the distance traveled by the pulse between the two recording sites: PWV=Distance (meters)/Transit Time (seconds).
Plasma ET-1 Measurement
Basal concentrations of plasma ET-1 were determined with ELISA (QuantiGlo, R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions except that the standard curve was limited to a maximum of 6 pg/ml. The reported cross-reactivity of the antibody was <0.02% for all big ETs, 7.8% for ET-3, and 27.4% for ET-2. Samples were thawed at room temperature, inverted three times, and centrifuged for 5 minutes at 1500 g at 4°C. All samples and standards were processed in duplicate. Unknown sample data were fitted to a standard curve with commercially available software (Prism 2.0, Graph-Pad Software, San Diego). The intra-assay variability was 4.2%.
Assessment of Childhood Socioeconomic Status
Childhood socioeconomic status (SES) was indexed by father’s education level, because this measure remained highly stable across the years of this longitudinal cohort and was available for all subjects used in the present study. We used the father’s education as measured at the midpoint of visit 1–12 as a representative for the whole study period. Father’s education was measured in years on a 7-point scale that ranged from less than high school to postgraduate education and was subsequently divided into 3 categories: low (<12 years), medium (≥12 and <16 years) and higher (≥16 years). Although not available in all subjects (with 9 missing values), we also calculated Hollingshead Four Factor Social Status Index 20 on the basis of parental education level and occupation, with a higher value indicating a higher SES.
Assessment of Adverse Childhood Experiences
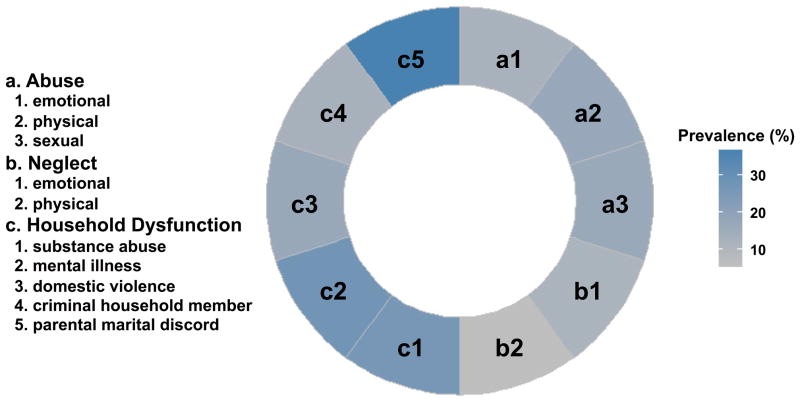
The assessment of participants’ exposure to ACEs covered the first 18 years of their lives. We adapted the questions used in the ACE study. 5 This questionnaire consists of 28 items divided into 3 categories and 10 subscales, including childhood abuse (emotional, physical and sexual), neglect (emotional and physical), and growing up with household dysfunction (substance abuse, mental illness, domestic violence, criminal household member, and parental marital discord). The definition and score of ACEs have been described in the Supplemental Table S1. The ACE score (the number of 10 ACE subscales reported) was used to assess the cumulative effect of multiple ACEs, by classifying respondents into three groups: no exposure (0 ACEs, n=67), mild (1 ACE, n=62), moderate/severe (≥2 ACEs, n=92) exposure. Knowledge of the ET-1 levels or hemodynamic variables in the participants was not examined until after obtaining ACE scores.
Statistical Analysis
All analyses were done using STATA software. Plasma ET-1 levels, BP, CO index and TPR index were log-transformed to obtain a better approximation of the normal distribution. The difference of prevalence of ACE scores between genders and races were examined by using Χ2 test. Linear regression models were used to estimate the associations of ACE scores with ET-1 levels, BP, CO index, TPR index and PWV. The ethnicity and gender differences were further tested by including the interaction of ACE scores with race and sex, respectively. The relationships of ET-1 levels with hemodynamic measures and PWV were also examined by using partial correlation analyses. The potential mediation effect of ET-1 was examined by using the Sobel test. 21 Covariates in all models included age, sex, race, BMI, and father’s education at visit 12.
RESULTS
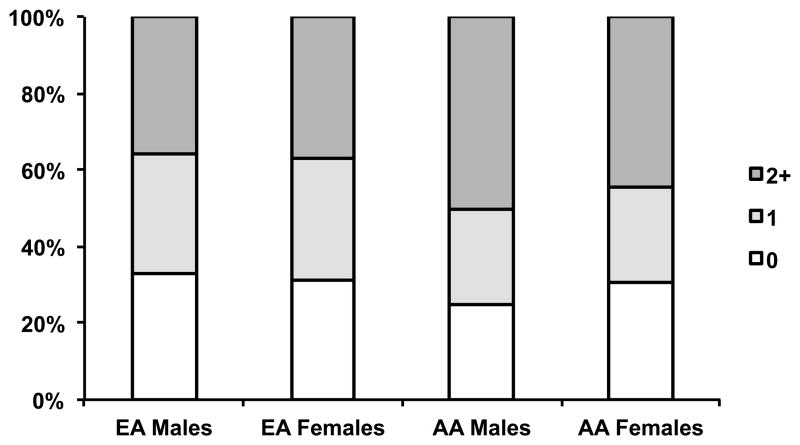
The mean age (±SD) of the participants at visit 12 was 21.3±2.84 (age range: 13.2 – 29.5). Table 1 presents the general characteristics of the subjects by ethnicity and gender. AAs had significantly greater BMI and lower childhood SES than EAs. Males had higher plasma ET-1 levels than females. In both genders, AAs had significantly higher SBP, DBP, TPR index and PWV than EAs. Compared with EAs, AAs had lower CO index. The prevalence of each individual ACE subscale is shown in Figure 1, with the lowest prevalence of physical neglect (5.88%) and the highest prevalence of parental marital discord (36.7%). Overall, about 70% of participants reported at least one ACE, with ACE count categories 1 and ≥2 representing 28.1% and 41.6% respectively (Supplemental Table S1). As shown in Figure 2, the prevalence of ACE scores was not significantly different between genders and races, although AA males had a slightly higher, but not significant, prevalence (75%).
Table 1.
Descriptive Characteristics of Study Participants
| Characteristic | EA Males | EA Females | AA Males | AA Females | Sex, P | Race, P |
|---|---|---|---|---|---|---|
| N | 61 | 51 | 44 | 65 | ||
| Age, y | 20.8 (3.1) | 21.3 (2.6) | 22.0 (3.2) | 21.4 (2.4) | 0.85 | 0.11 |
| BMI, kg/m2 | 25.0 (5.5) | 26.1 (6.6) | 29.9 (7.5) | 29.9 (9.5) | 0.27 | <0.001 |
| SES | ||||||
| Father’s education level, y | 14.2 (2.7) | 13.9 (1.9) | 13.1 (2.6) | 12.9 (2.0) | 0.08 | 0.001 |
| Mother’s education level, y | 13.4 (2.0) | 13.9 (1.9) | 13.8 (2.2) | 13.3 (1.9) | 0.65 | 0.40 |
| Hollingshead Index | 43.1 (14.0) | 43.8 (12.6) | 39.6 (15.9) | 36.4 (13.5) | 0.30 | 0.003 |
| ET-1, pg/ml | 1.39 (0.60) | 1.28 (0.64) | 1.63 (0.73) | 1.27 (0.69) | 0.015 | 0.45 |
| SBP, mmHg | 114.2 (10.6) | 107.5 (8.8) | 119.8 (10.9) | 112.4 (10.9) | <0.001 | 0.005 |
| DBP, mmHg | 58.9 (6.6) | 60.9 (5.7) | 62.4 (7.7) | 65.4 (7.8) | 0.002 | <0.001 |
| CO Index, L/min/m2 | 2.96 (0.79) | 3.07 (0.88) | 2.78 (0.69) | 2.75 (0.61) | 0.97 | 0.014 |
| TPR Index, mmHg/L/min/m2 | 27.8 (7.1) | 26.8 (7.5) | 31.0 (7.8) | 30.8 (6.6) | 0.99 | <0.001 |
| Radial PWV, m/s | 6.36 (1.30) | 6.82 (0.97) | 7.43 (1.09) | 7.38 (1.42) | 0.11 | <0.001 |
Figure 1.
Prevalence of each category of adverse childhood experiences (ACEs).
Figure 2.
Proportion of participants reporting 0, 1, and ≥2 adverse childhood experiences (ACEs). EA, European Americans; AA, African Americans.
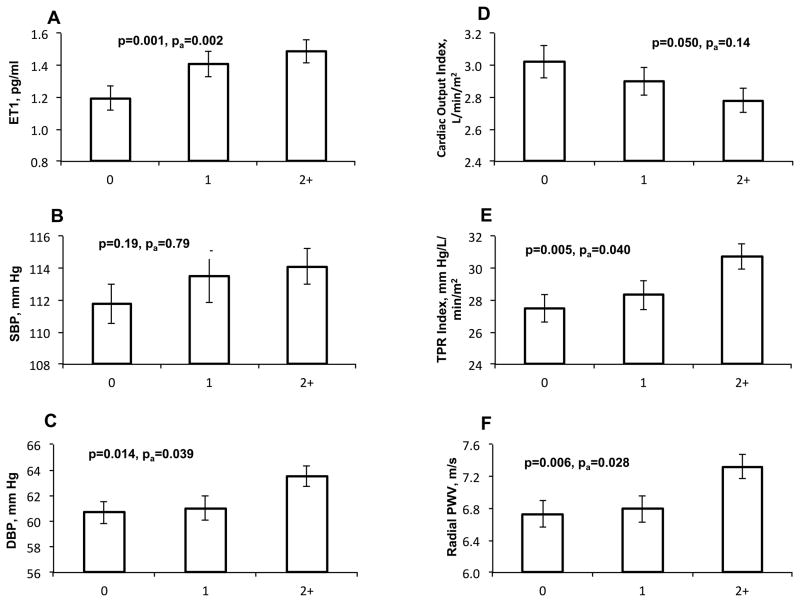
Exposure to ACEs was significantly associated with plasma ET-1 levels, DBP, CO index, TPR index and radial PWV, but not with SBP. Figure 3 depicts a graded association of ACE scores with plasma ET-1 levels, of which on average 18% and 24% were higher in subjects with 1 ACE, and ≥2 ACEs than in those with no ACEs (P=0.001). The participants in two exposure groups also showed 3% and 12% higher TPR index than those with no ACEs (P=0.002). The subjects with ≥2 ACEs had significantly higher DBP (5%) and PWV (9%) compared with those who were not exposed, but lower CO index (8%). After controlling for covariates these associations were attenuated but still significant for ET-1, DBP, TPR index and PWV (Figure 3). Further adjustment for Hollingshead scores instead of father’s education for childhood SES did not change the results. There were no significant interactions of ACE scores with gender and ethnicity on these measurements, indicating that exposure to ACEs had similar effect on ET-1 levels and hemodynamic parameters in males and females, as well as in AAs and EAs.
Figure 3.
Average levels of plasma ET-1 (A), SBP (B), DBP (C), CO index (D), TPR index (E), and radial PWV (F) according to ACE exposures (0, 1, and 2+). The error bars indicate the standard error. P and pa were p values with and without adjustment for age, sex, race, BMI and father’s education, respectively. Further adjustment for Hollingshead scores instead of father’s education for childhood SES did not change the results.
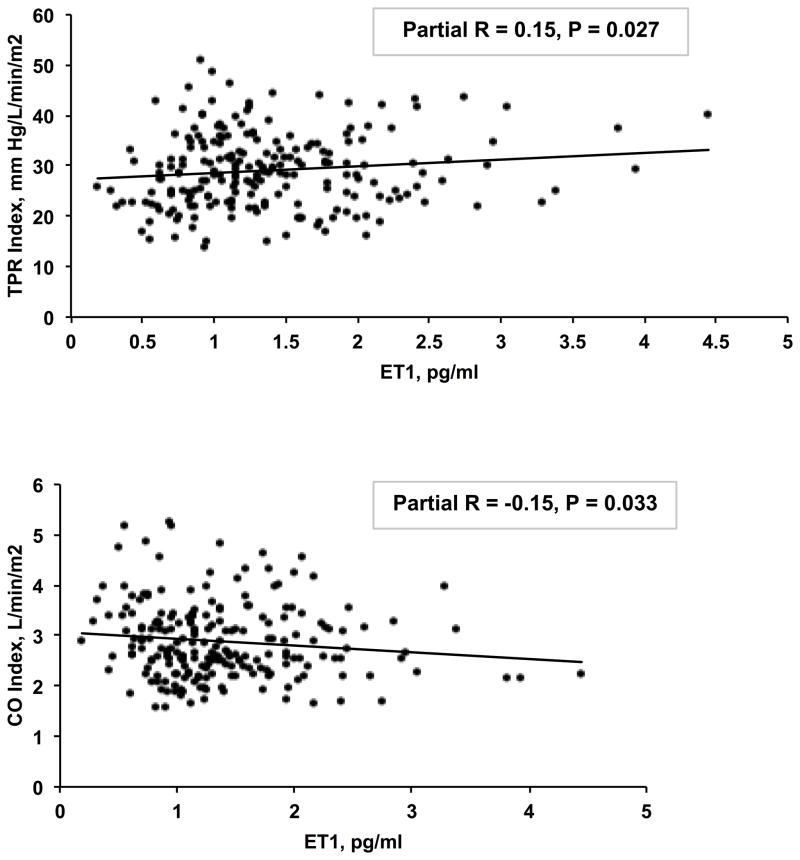
We did not observe the associations of ET-1 with DBP and PWV. However, plasma ET-1 levels were significantly associated with TPR index (partial R = 0.15, P=0.027) and CO index (partial R = −0.15, P=0.033), after adjusting for covariates (Figure 4). The mediation model showed that the associations of ACE scores with TPR index and CO index were partially mediated by ET-1 (19% and 25% respectively), although the Sobel tests were borderline significant (P=0.067 and 0.074).
Figure 4.
Associations of plasma ET-1 level with TPR index (A) and CO index (B). The partial correlations and p values were adjusted for age, sex, race, BMI and father’s education. Further adjustment for Hollingshead scores instead of father’s education for childhood SES did not change the results.
DISCUSSION
The major findings of this novel study revealed that exposure to ACEs were associated with increased circulating ET-1, DBP, TPR index and PWV in healthy adolescents and young adults. These observations indicate that ELS in humans promotes cardiovascular disease risk, specifically detrimental vascular and cardiac function, detectable during very young adulthood.
Early Life Stress and Adult CVD Risk
Recent retrospective and prospective studies have demonstrated ELS as a factor predisposing to coronary heart disease (CHD) and hypertension in adulthood. For instance, the Dong et al study included men and women 56 years of age on average and found a dose-response relation of ACEs to self-reported CHD. 5 Similar association was observed between childhood abuse and CVD events in a longitudinal cohort of women 43 to 60 years of age. 7 The same study also reported that women exposed to severe physical and/or sexual abuse prior to age 18 were more likely to develop hypertension. 8 In our study of youth and young adults aged 13 to 29 years old, higher DBP, TPR index and PWV were observed in subjects with moderate/severe exposure to ACEs compared to those with no ACE exposure. Efficient functioning of the vasculature is an essential component for cardiovascular health and vascular dysfunction is linked to the etiology and pathophysiology of CVD. 15 Elevated blood pressure maintained by increased TPR has been associated with increased risk for cardiovascular events and death in both normotensive and hypertensive individuals. 22 Higher PWV, indicating greater aortic stiffness, has also been recognized as an independent risk factor for CHD, stroke, CVD mortality, and recently reported as a possible causative mediator of hypertension. 23–27 Future studies will be designed to examine the relationship of higher PWV and blood pressure in this longitudinal cohort. Our novel findings indicated that these cardiovascular risk factors and probable mediators were observed in early 20-year-olds with moderate-to-severe ACEs.
Several mechanisms underlying these associations have been proposed, including the hypothalamic-pituitary-adrenal (HPA) axis and inflammation. Former childhood war evacuees had altered responsiveness of the HPA axis to psychosocial stress in late adulthood. 28 Altered cortisol responses have also been observed among children exposed to violence. 29 Other studies have reported that adult individuals exposed to childhood maltreatment had elevated levels of inflammation biomarkers. 30–32 These findings are supported by experimental evidence in animal models, in which greater HPA axis reactivity to stress 33 and vascular inflammation 34 were found in pups separated from their mothers compared to normally-reared pups.
Early Life Stress, ET Pathway, and Hemodynamic Variables
In the present study in humans, we observed an association between ELS and plasma ET-1 levels. It is well known that ET-1 is a very potent vasoconstrictor in the cardiovascular system. 35 ET-1 also has inotropic 36 and pro-inflammatory properties 37, and stimulates plasma pituitary hormones 38. Dysregulation of the ET pathway is considered to occur early during the development of atherosclerosis and vascular complications. Elevated plasma ET-1 levels have been found in patients with CHD, heart failure, renal disease and pulmonary arterial hypertension. 15 These studies, including ours, 14 have suggested that the ET pathway may underlie the link between ELS and later development of CVD.
Most recently, our study in rats 14 suggested that maternal separation (MatSep), rodent model of early life stress, induces increased circulating ET-1 levels strikingly similar to the humans exposed to adversity early in life observed in our current study. We also demonstrated that MatSep induces down-regulation of ETB receptors in the vasculature. Thus, we propose that the down-regulation in ETB receptors results in the dysfunctional clearance of ET-1 and increased circulating ET-1 levels. Furthermore, we showed that the rats exposed to early life stress demonstrated an enhanced pressor response to acute air jet stress that was blunted with ET receptor blockade. Taken together, these results indicate that the enhanced plasma levels of ET-1 most likely exert systemic hemodynamic effects in this rat model.
ET-1 is a critical regulator of hemodynamics. Infusion of ET-1 in healthy subjects increased peripheral resistance and PWV, and decreased cardiac output; while ET receptor blockade prevented these changes. 16 We observed a positive correlation of plasma ET-1 levels with TPR index, and a negative correlation with cardiac output index. Mediation model indicated that the correlations between ACEs and these two hemodynamic variables could be partially explained by the increased ET-1 levels (20–25%). Thus, deciphering the possible mechanism(s) of the elevated plasma ET-1 levels is of interest. Future studies are planned to explore the ELS-induced mechanism of higher plasma ET-1 levels, which may be related to increased ET-1 production, decreased clearance of ET-1 via ETB receptor dysfunction, or both.
A recent clinical trial also suggested a potential protective role of ET receptor blockade in chronic kidney disease patients by reducing blood pressure and arterial stiffness (reduced PWV). 39 However, in our study, we did not find correlations of plasma ET-1 levels with blood pressure and PWV. As noted, in the present study we measured carotid-radial PWV, but not carotid-femoral PWV as measured in previous studies. Therefore, the lack of association may reflect different mechanical properties between the peripheral small vascular vessels and central aortic vessels.
The associations of ELS with plasma ET-1 levels and hemodynamic parameters may be confounded by covariates. Previous studies have reported ethnicity and sex differences in plasma ET-1 levels. 40–42 Consistently, in the present study, we observed higher basal plasma ET-1 levels in males than females (P<0.05). AAs exhibited higher ET-1 levels compared with EAs, but not statistically significant. Higher levels of SBP, DBP, TPR index and PWV were found in AAs than EAs (P values <0.01), confirming previous findings. 19, 43, 44 However, adjustment of ethnicity and sex did not change the associations between ELS and these variables. Furthermore, no significant interactions of ACEs with ethnicity and sex were found on these measurements, indicating that ELS had similar effects on hemodynamic functions in males and females, as well as in AAs and EAs.
Previous reports showed that children with a history of physical abuse were more likely to have higher BMI as adults than those with no exposure to abuse. 45 The long-term health effect of low childhood SES has also been well documented. 46 Thus, the associations that we found may be confounded by BMI and childhood SES. After adjustment for these two factors, the associations of ELS with plasma ET-1 levels and hemodynamic parameters were attenuated but remained significant, suggesting that adverse experiences prior to age 18 may affect cardiovascular health in young adults through a mechanism independent of BMI and childhood SES. As noted, we further adjusted for Hollingshead score, which incorporated parental education levels and occupations, and the results were virtually unchanged.
Limitations of the Study
Our study has several limitations. First, similar to previous large cohort studies, the individual’s experiences of childhood adversity were collected based on retrospective self-report. Because of the sensitive nature of questions about ACEs and affective problems, the responses probably represent an under-reporting of their actual occurrence. However, given the established relationship between ELS and the risk for CVD development, the under-reporting of the adversities, if exists, should have weakened the associations we found here, not exaggerated them. Second, the plasma ET-1 levels, hemodynamic variables and PWV were measured at visit 12, 6 years before collection of the ACEs information. The cohort at visit 12 included both adolescents (age < 18 years old) and young adults (age ≥ 18 years old). Therefore, subjects reporting ACEs at visit 15 may not have been exposed to those ACEs at visit 12. Similar to the under-reporting, this misclassification (no-exposure to exposure) should have weakened the associations. Moreover, only 21 adolescents (aged 13–17) were included at visit 12. Exclusion of these adolescents did not change the results. The plasma ET-1 levels were measured at only one time point, thus it is warranted to investigate the longitudinal effect of ELS on plasma ET-1 levels in the future. In addition, there are several stressors associated with the measurements, such as venipuncture and undergoing the hemodynamic testing. Although the subjects were exposed to the same environment for the tests at each visit and each subject knew what to expect, there are other acute and chronic stressors that may also be present and associated with ACE. This study was not designed to parse out these distinctions between the various types of stressors. Finally, the sample size of the present study was relatively small, which may imply effect overestimation.
PERSPECTIVES
To date, the prolonged and lasting effects of ELS have been established on the development of CVD in mid and late adulthood. Our results provide the first evidence that ELS may induce unfavorable vascular and cardiac function detectable in early adulthood. In addition, the novel findings of this study suggest that the ET pathway is a new potential mechanism underlying the association between ELS and cardiovascular health. Identification and prevention of vascular complications in young adults with experiences of childhood adversity may provide an important avenue for the prevention of CVD in later adult life.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is New?
Humans exposed to adverse childhood experiences were associated with increased circulating endothelin-1 levels, diastolic blood pressure, total peripheral resistance index and pulse wave velocity in healthy adolescents and young adults. These associations were independent of age, race, gender, body mass index and childhood socioeconomic status.
What is Relevant?
These observations indicate that early life stress in humans promotes cardiovascular disease risk, specifically detrimental vascular and cardiac function, detectable during very young adulthood. These findings suggest that a possible mechanism for the early life stress-mediated CVD risk is the endothelin pathway. Understanding of the mechanisms underlying early life stress and CVD risk would enable strategies for prevention and control of vascular and cardiac pathologies later in life.
Summary
We evaluated whether exposure to adverse childhood experiences (ACEs) was associated with greater peripheral resistance, arterial stiffness, blood pressure, or elevated circulating ET-1 levels in humans. In 221 healthy adolescents and young adults (mean age: 21; age range: 13–29), we found a graded association of ACE exposure with plasma ET-1 levels. The subjects with moderate/severe exposure to ACEs (≥2 ACEs) had significantly higher total peripheral resistance index, diastolic blood pressure and pulse wave velocity compared with those who were not exposed. These associations were independent of age, race, gender, body mass index and childhood socioeconomic status. Our results indicate that early life stress promotes cardiovascular disease risk, specifically detrimental vascular and cardiac function, detectable in very young adulthood.
Acknowledgments
We are grateful to Amy Dukes for the outstanding technical support.
SOURCES OF FUNDING
This research was supported in part by the National Institutes of Health Program Project Grant on Stress-Related Mechanisms of Hypertensive Risk (P01 HL69999 to GAH, FAT, GKK, DMP, and JSP). SS is funded by the American Heart Association (09SDG2140117) and the NIH (HL106333-01A1).
Footnotes
DISCLOSURES
None
References
- 1.Gilbert R, Widom CS, Browne K, Fergusson D, Webb E, Janson S. Burden and consequences of child maltreatment in high-income countries. Lancet. 2009;373:68–81. doi: 10.1016/S0140-6736(08)61706-7. [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychopathology in the National Comorbidity Survey Replication (NCS-R) III: associations with functional impairment related to DSM-IV disorders. Psychol Med. 2010;40:847–859. doi: 10.1017/S0033291709991115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anda RF, Dong M, Brown DW, Felitti VJ, Giles WH, Perry GS, Valerie EJ, Dube SR. The relationship of adverse childhood experiences to a history of premature death of family members. BMC Public Health. 2009;9:106. doi: 10.1186/1471-2458-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110:1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- 6.Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, Croft JB. Cumulative childhood stress and autoimmune diseases in adults. Psychosom Med. 2009;71:243–250. doi: 10.1097/PSY.0b013e3181907888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rich-Edwards JW, Spiegelman D, Lividoti Hibert EN, Jun HJ, Todd TJ, Kawachi I, Wright RJ. Abuse in childhood and adolescence as a predictor of type 2 diabetes in adult women. Am J Prev Med. 2010;39:529–536. doi: 10.1016/j.amepre.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riley EH, Wright RJ, Jun HJ, Hibert EN, Rich-Edwards JW. Hypertension in adult survivors of child abuse: observations from the Nurses’ Health Study II. J Epidemiol Community Health. 2010;64:413–418. doi: 10.1136/jech.2009.095109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alastalo H, Raikkonen K, Pesonen AK, Osmond C, Barker DJ, Kajantie E, Heinonen K, Forsen TJ, Eriksson JG. Cardiovascular health of Finnish war evacuees 60 years later. Ann Med. 2009;41:66–72. doi: 10.1080/07853890802301983. [DOI] [PubMed] [Google Scholar]
- 10.Wegman HL, Stetler C. A meta-analytic review of the effects of childhood abuse on medical outcomes in adulthood. Psychosom Med. 2009;71:805–812. doi: 10.1097/PSY.0b013e3181bb2b46. [DOI] [PubMed] [Google Scholar]
- 11.Vig R, Gordon JR, Thebaud B, Befus AD, Vliagoftis H. The effect of early-life stress on airway inflammation in adult mice. Neuroimmunomodulation. 2010;17:229–239. doi: 10.1159/000290039. [DOI] [PubMed] [Google Scholar]
- 12.Biagini G, Pich EM, Carani C, Marrama P, Agnati LF. Postnatal maternal separation during the stress hyporesponsive period enhances the adrenocortical response to novelty in adult rats by affecting feedback regulation in the CA1 hippocampal field. Int J Dev Neurosci. 1998;16:187–197. doi: 10.1016/s0736-5748(98)00019-7. [DOI] [PubMed] [Google Scholar]
- 13.Hilakivi-Clarke LA, Turkka J, Lister RG, Linnoila M. Effects of early postnatal handling on brain beta-adrenoceptors and behavior in tests related to stress. Brain Res. 1991;542:286–292. doi: 10.1016/0006-8993(91)91580-t. [DOI] [PubMed] [Google Scholar]
- 14.Loria AS, D’Angelo G, Pollock DM, Pollock JS. Early life stress downregulates endothelin receptor expression and enhances acute stress-mediated blood pressure responses in adult rats. Am J Physiol Regul Integr Comp Physiol. 2010;299:R185–191. doi: 10.1152/ajpregu.00333.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khimji AK, Rockey DC. Endothelin--biology and disease. Cell Signal. 2010;22:1615–1625. doi: 10.1016/j.cellsig.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Vuurmans TJ, Boer P, Koomans HA. Effects of endothelin-1 and endothelin-1 receptor blockade on cardiac output, aortic pressure, and pulse wave velocity in humans. Hypertension. 2003;41:1253–1258. doi: 10.1161/01.HYP.0000072982.70666.E8. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Poole JC, Treiber FA, Harshfield GA, Hanevold CD, Snieder H. Ethnic and gender differences in ambulatory blood pressure trajectories: results from a 15-year longitudinal study in youth and young adults. Circulation. 2006;114:2780–2787. doi: 10.1161/CIRCULATIONAHA.106.643940. [DOI] [PubMed] [Google Scholar]
- 18.Kapuku GK, Treiber FA, Davis HC, Harshfield GA, Cook BB, Mensah GA. Hemodynamic function at rest, during acute stress, and in the field: predictors of cardiac structure and function 2 years later in youth. Hypertension. 1999;34:1026–1031. doi: 10.1161/01.hyp.34.5.1026. [DOI] [PubMed] [Google Scholar]
- 19.Ge D, Young TW, Wang X, Kapuku GK, Treiber FA, Snieder H. Heritability of arterial stiffness in black and white American youth and young adults. Am J Hypertens. 2007;20:1065–1072. doi: 10.1016/j.amjhyper.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hollingshead A. Four Factor Index of Social Status. New Haven, Conn: Department of Sociology; 1981. [Google Scholar]
- 21.Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. In: Leinhardt S, editor. Sociological methodology. Washington, DC: American Sociological Association; 1982. [Google Scholar]
- 22.Fagard RH, Pardaens K, Staessen JA, Thijs L. Prognostic value of invasive hemodynamic measurements at rest and during exercise in hypertensive men. Hypertension. 1996;28:31–36. doi: 10.1161/01.hyp.28.1.31. [DOI] [PubMed] [Google Scholar]
- 23.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 24.Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 25.Meaume S, Benetos A, Henry OF, Rudnichi A, Safar ME. Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of age. Arterioscler Thromb Vasc Biol. 2001;21:2046–2050. doi: 10.1161/hq1201.100226. [DOI] [PubMed] [Google Scholar]
- 26.Pesonen AK, Raikkonen K, Feldt K, Heinonen K, Osmond C, Phillips DI, Barker DJ, Eriksson JG, Kajantie E. Childhood separation experience predicts HPA axis hormonal responses in late adulthood: a natural experiment of World War II. Psychoneuroendocrinology. 2010;35:758–767. doi: 10.1016/j.psyneuen.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 27.Cicchetti D, Rogosch FA. Diverse patterns of neuroendocrine activity in maltreated children. Dev Psychopathol. 2001;13:677–693. doi: 10.1017/s0954579401003145. [DOI] [PubMed] [Google Scholar]
- 28.Danese A, Moffitt TE, Harrington H, et al. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med. 2009;163:1135–1143. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65:409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci. 2007;25:3091–3098. doi: 10.1111/j.1460-9568.2007.05522.x. [DOI] [PubMed] [Google Scholar]
- 32.Loria AS, Pollock DM, Pollock JS. Early life stress sensitizes rats to angiotensin II-induced hypertension and vascular inflammation in adult life. Hypertension. 2010;55:494–499. doi: 10.1161/HYPERTENSIONAHA.109.145391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez-Pascual F, Busnadiego O, Lagares D, Lamas S. Role of endothelin in the cardiovascular system. Pharmacol Res. 2011;63:463–472. doi: 10.1016/j.phrs.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 34.MacCarthy PA, Grocott-Mason R, Prendergast BD, Shah AM. Contrasting inotropic effects of endogenous endothelin in the normal and failing human heart: studies with an intracoronary ET(A) receptor antagonist. Circulation. 2000;101:142–147. doi: 10.1161/01.cir.101.2.142. [DOI] [PubMed] [Google Scholar]
- 35.Browatzki M, Schmidt J, Kubler W, Kranzhofer R. Endothelin-1 induces interleukin-6 release via activation of the transcription factor NF-kappaB in human vascular smooth muscle cells. Basic Res Cardiol. 2000;95:98–105. doi: 10.1007/s003950050170. [DOI] [PubMed] [Google Scholar]
- 36.Malendowicz LK, Nussdorfer GG, Meneghelli V, Nowak M, Markowska A, Majchrzak M. Effects of endothelin-1 on the rat pituitary-adrenocortical axis under basal and stressful conditions. Endocr Res. 1997;23:349–364. doi: 10.1080/07435809709031862. [DOI] [PubMed] [Google Scholar]
- 37.Dhaun N, MacIntyre IM, Kerr D, et al. Selective endothelin-A receptor antagonism reduces proteinuria, blood pressure, and arterial stiffness in chronic proteinuric kidney disease. Hypertension. 2011;57:772–779. doi: 10.1161/HYPERTENSIONAHA.110.167486. [DOI] [PubMed] [Google Scholar]
- 38.Treiber FA, Kapuku GK, Davis H, Pollock JS, Pollock DM. Plasma endothelin-1 release during acute stress: role of ethnicity and sex. Psychosom Med. 2002;64:707–713. doi: 10.1097/01.psy.0000021952.59258.1c. [DOI] [PubMed] [Google Scholar]
- 39.Evans RR, Phillips BG, Singh G, Bauman JL, Gulati A. Racial and gender differences in endothelin-1. Am J Cardiol. 1996;78:486–488. doi: 10.1016/s0002-9149(96)00344-x. [DOI] [PubMed] [Google Scholar]
- 40.Polderman KH, Stehouwer CD, van Kamp GJ, Dekker GA, Verheugt FW, Gooren LJ. Influence of sex hormones on plasma endothelin levels. Ann Intern Med. 1993;118:429–432. doi: 10.7326/0003-4819-118-6-199303150-00006. [DOI] [PubMed] [Google Scholar]
- 41.Morris AA, Patel RS, Binongo JN, Poole J, Mheid IA, Ahmed Y, Stoyanova N, Vaccarino V, Din-Dzietham R, Gibbons GH, Quyyumi A. Racial differences in arterial stiffness and microcirculatory function between Black and White Americans. J Am Heart Assoc. 2013;2:e002154. doi: 10.1161/JAHA.112.002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snieder H, Harshfield GA, Treiber FA. Heritability of blood pressure and hemodynamics in African- and European-American youth. Hypertension. 2003;41:1196–1201. doi: 10.1161/01.HYP.0000072269.19820.0D. [DOI] [PubMed] [Google Scholar]
- 44.Bentley T, Widom CS. A 30-year follow-up of the effects of child abuse and neglect on obesity in adulthood. Obesity (Silver Spring) 2009;17:1900–1905. doi: 10.1038/oby.2009.160. [DOI] [PubMed] [Google Scholar]
- 45.Galobardes B, Lynch JW, Smith GD. Is the association between childhood socioeconomic circumstances and cause-specific mortality established? Update of a systematic review. J Epidemiol Community Health. 2008;62:387–390. doi: 10.1136/jech.2007.065508. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.