Abstract
Attenuated activity in performance-monitoring brain regions following erroneous actions may contribute to the repetition of maladaptive behaviors such as continued drug use. Externalizing is a broad personality construct characterized by deficient impulse control, vulnerability to addiction, and reduced neurobiological indices of error processing. The insula and dorsal anterior cingulate cortex (dACC) are regions critically linked with error processing as well as the perpetuation of cigarette smoking. As such, we examined the interrelations between externalizing tendencies, erroneous task performance, and error-related insula and dACC activity in overnight-deprived smokers (n=24) and nonsmokers (n=20). Participants completed a self-report measure assessing externalizing tendencies (Externalizing Spectrum Inventory) and a speeded Flanker task during fMRI scanning. We observed that higher externalizing tendencies correlated with the occurrence of more performance errors among smokers but not nonsmokers. Suggesting a neurobiological contribution to such sub-optimal performance among smokers, higher externalizing also predicted less recruitment of the right insula and dACC following error commission. Critically, this error-related activity fully mediated the relationship between externalizing traits and error rates. That is, higher externalizing scores predicted less error-related right insula and dACC activity and, in turn, less error-related activity predicted more errors. Relating such regional activity with a clinically-relevant construct, less error-related right insula and dACC responses correlated with higher tobacco craving during abstinence. Given that inadequate error-related neuronal responses may contribute to continued drug use despite negative consequences, these results suggest that externalizing tendencies and/or compromised error processing among subsets of smokers may be relevant factors for smoking cessation success.
Keywords: anterior cingulate cortex, errors, functional magnetic resonance imaging (fMRI) impulsivity, insula, nicotine abstinence
INTRODUCTION
Online monitoring of actions and their outcomes is necessary for adapting to dynamic environments and optimizing goal-directed behavior. A diminished capacity for performance monitoring has been described in a number of neuropsychiatric conditions (Melcher et al., 2008; Ullsperger, 2006) including drug abuse (Garavan and Stout, 2005). The bilateral insulae and dorsal anterior cingulate cortex (dACC) are critical nodes in the brain’s performance-monitoring network showing increased activity in situations requiring behavioral adaptation, particularly following errors (Dosenbach et al., 2006; Seeley et al., 2007; Ullsperger et al., 2010). Whereas dACC activity is conceptualized as signaling the need for behavioral adjustments (Ridderinkhof et al., 2004), insula activity is associated with the subjective awareness, autonomic arousal, and/or motivational significance accompanying erroneous actions (Hester et al., 2005; Klein et al., 2013). Diminished error-related activity in these regions is a common characteristic of drug abuse having been observed when considering chronic cocaine (Kaufman et al., 2003), methamphetamine (London et al., 2005), opiate (Forman et al., 2004), cannabis (Hester et al., 2009), and nicotine users (Luijten et al., 2011). Critically, compromised insula and/or dACC function may contribute to the persistence of sub-optimal responses, maladaptive behaviors, and/or impulsive actions despite knowledge of negative consequences to self or others (Goldstein et al., 2009; Goldstein and Volkow, 2002).
Diminished error-related brain activity is also linked with such personality characteristics as impulsivity (Hoffmann et al., 2012; Luijten et al., 2011) risk-taking (Santesso and Segalowitz, 2009), and antisocial tendencies (Chang et al., 2010). These personality dimensions have been conceptualized as facets of a broader construct labeled externalizing (Krueger et al., 2007; Patrick et al., 2012). The externalizing construct generally reflects an increased proneness to impulsive behavioral tendencies, has a strong genetic basis, lies on a continuum, and is regarded as a dispositional factor for several neuropsychiatric conditions including attention deficit hyperactivity disorder, antisocial personality disorder, and addiction (Krueger et al., 2002). High-externalizing individuals show reduced amplitudes in the error-related negativity (ERN), an electrophysiological index of dACC-related error processing (Hall et al., 2007; Nelson et al., 2011; Olvet and Hajcak, 2008). Thus, personality traits in general and externalizing tendencies in particular may account for individual variation in error processing and, in turn, behavioral performance. Elucidating such individual variation could aid in the fractionation of the smoker phenotype and contribute to the implementation of tailored strategies for smoking cessation.
Regarding smoking behaviors, greater externalizing tendencies are linked with an earlier age of smoking initiation, higher overall cigarette consumption, and reduced cessation success in adolescents (Fischer et al., 2012; Leff et al., 2003; Moolchan et al., 2007). Similarly, higher trait-impulsivity correlates with elevated cigarette-cue reactivity and tobacco craving (Doran et al., 2008; VanderVeen et al., 2008). The insula and ACC, in addition to other brain regions, show increased activity following drug-cue presentation which often correlates with smokers’ subjective tobacco cravings (Chase et al., 2011; Engelmann et al., 2012; Garavan, 2010; Wang et al., 2007). Accordingly, several perspectives have emerged relating dysregulated insula and ACC function with subjective drug urges, impaired behavioral monitoring, and maladaptive decision making (Garavan, 2010; Goldstein et al., 2009; Naqvi and Bechara, 2009; Paulus, 2007). During early smoking abstinence, the insula, serving an interoceptive monitoring role (Craig, 2009), is thought to track homeostatically-relevant body sensations and modulate affective, motivational, and attentional processes accordingly (Naqvi and Bechara, 2010; Sutherland et al., 2013a). Increased insula and dACC activity subserving interoceptive- and/or craving-related processes may be accompanied by ruminative thoughts and/or planning for future drug use which could then limit cognitive resources available for exogenous information processing and endogenous performance monitoring (Sutherland et al., 2012b). Given that most smoking cessation attempts fail within the first week (Hughes et al., 2004), assessment of individual differences in insula and dACC functioning during acute abstinence may provide insight into the neurobiological mechanisms contributing to the persistence of cigarette smoking.
Towards this end, we investigated the interrelations between externalizing personality traits, behavioral task performance, and error-related insula and dACC activity in overnight-deprived smokers and nonsmokers. Participants completed a self-report questionnaire assessing externalizing tendencies (Krueger et al., 2007; Venables and Patrick, 2012) and a speeded Flanker task during fMRI scanning allowing for assessment of regional error-related activity. All participants were scanned under two general conditions: (1) in the absence of pharmacological manipulations (i.e., during nicotine withdrawal in smokers), and (2) following administration of varenicline and/or nicotine, two modestly efficacious smoking cessation aids. Assessment of smokers in both the absence and presence of these pharmacological manipulations allowed us to determine if the relations between externalizing traits, task performance, and error-related brain activity were specific to the acutely withdrawn state. We hypothesized that: (1) abstinent smokers relative to nonsmokers (between-groups assessment) would show less error-related activity in the insula and dACC in line with findings from other drugs of abuse, (2) higher externalizing tendencies in abstinent smokers and/or nonsmokers (within-groups assessment) would be predictive of sub-optimal task performance (i.e., higher error rates) and less engagement of the insula and dACC following errors, and (3) less error-related insula and dACC activity would correlate with higher tobacco craving in smokers.
METHODS
Participants
A total of 24 cigarette smokers (12 females) and 20 nonsmokers (10 females) completed the study. Two participants, one male smoker and one male nonsmoker, were excluded from analyses due to poor behavioral performance and excessive head motion during scanning, respectively. Participants were right-handed, 18–55 years of age, and reported no history of drug dependence (other than nicotine in smokers), neurologic or psychiatric disorders, or contraindications for MRI scanning. We recruited non-treatment seeking smokers who reported smoking 10 or more cigarettes per day for a minimum of two years. Smokers were 35±10 years of age (mean±SD), smoked 18±8 cigarettes per day, reported daily cigarette use for 18±11 years, and were moderately nicotine dependent (Fagerström scores: 5±2; see Supplemental Table S1 for details). We recruited nonsmokers who reported no history of daily nicotine use and no smoking within the preceding two years. Smokers and nonsmokers were matched for sex, age, and race/ethnicity. We obtained written informed consent in accordance with the NIDA-IRP Institutional Review Board.
Procedures
As part of a larger study, both smokers and nonsmokers completed 6 fMRI sessions on different days in a two-drug, double-blind, placebo-controlled study (Sutherland et al., 2012a, 2013a; 2013b). At three points during a varenicline administration regime (i.e., pre-pill, varenicline pill, placebo pill), participants were scanned twice, once each wearing a nicotine or placebo patch. After the two initial pre-pill sessions, participants were administered varenicline and placebo pills for ~2 weeks each (randomized order) and again completed nicotine and placebo patch scans towards the end of each pill interval (Supplemental Figure S1). In other words, participants were scanned both in the absence (pre-pill/placebo-patch and placebo-pill/placebo-patch) and presence of pharmacological interventions (pre-pill/nicotine-patch, placebo-pill/nicotine-patch, varenicline-pill/nicotine-patch, and varenicline-pill/placebo-patch). Examination of smokers in these two general states allowed us to assess the state/condition specificity (e.g., only present during withdrawal) of any relations between externalizing, task performance, and error-related brain activity. Below we focus on the pre-pill/placebo-patch session (i.e., smokers’ first “full-withdrawal session”).
We instructed smokers to have their last cigarette 12 h before their scheduled arrivals. Before data collection, participants were tested for recent drug and alcohol use and for expired carbon monoxide (CO) levels. As CO half-life during sleep can be up to 4–8 h (SRNT-Subcommittee, 2002), we used a guideline of ≤15 parts per million (ppm) to verify overnight abstinence. Indicative of compliance, smokers’ CO levels were lower on scan days (6.7±3.2 ppm) relative to the initial orientation/consent visit which did not require abstinence (18.1±9.0 ppm), t(22)=−7.2, p<0.001. MRI scanning occurred ~2 h after CO measurements.
Self-report questionnaires
We measured trait-level externalizing tendencies and state-level tobacco craving using previously validated self-report instruments. We employed the Externalizing Spectrum Inventory (ESI, 159-item version; Krueger et al., 2007; Venables and Patrick, 2012), which queries participants regarding personality-trait and past-behavioral indicators to quantify externalizing tendencies. The ESI assesses the higher-order construct of externalizing across lower-order facets such as behavioral disinhibition, impulsivity, aggression, irresponsibility, boredom proneness, and illicit drug use. Participants completed the ESI once during this study, generally at the third visit under their randomized drug condition for that day, since externalizing is a trait measure that would not be expected to vary by drug condition. Participants rated items (e.g., “If I could control my impulses, my life would be much better”) on a four-point scale as being false, mostly false, mostly true, and true. A total ESI score was calculated and expressed as a proportion (range: 0.0–1.0) such that higher scores reflect greater externalizing tendencies. As ESI total scores index a general lack of inhibitory control (Patrick et al., 2012) and higher scores have been linked with reduced ERN amplitude (Hall et al., 2007; Nelson et al., 2011), we focused specifically on this total score in our analyses.
Smokers’ tobacco cravings were assessed each session ~2.5 h after the Flanker task with the 12-item, short form of the Tobacco Craving Questionnaire (TCQ: Heishman et al., 2008). The TCQ consists of four factors: emotionality, expectancy, compulsivity, and purposefulness. We examined the putative links between error-related brain activity and TCQ total and subscale scores with emphasis on the purposefulness factor. The purposefulness subscale (range 3–21: higher scores reflect more craving) is thought to assess craving aspects related to intentions and planning to smoke (Heishman et al., 2003). This subscale contains three items: (1) “If I had a lit cigarette in my hand, I probably would smoke it,” (2) “It would be hard to pass up the chance to smoke,” and (3) “I could not easily limit how much I smoked right now.”
Flanker Task
Participants performed a modified, speeded Flanker task known to reliably induce interference effects and error-related brain activity. On each trial, participants were shown a five letter stimulus array consisting of four flanker items for 150ms (HH_HH or SS_SS). The center target letter (H or S) was presented 100ms after onset of the flanking stimuli and remained on the screen for 50ms. Thus, four types of stimulus arrays were presented with equal frequency across the entire task, two congruent (n=260; HHHHH or SSSSS) and two incongruent arrays (n=260; HHSHH or SSHSS). We instructed participants to identify the target letter with a button press using the left or right index finger (counterbalanced across participants) as quickly and accurately as possible. To adjust task difficulty and ensure an adequate number of errors, we generated an individualized response deadline from a practice run (130 trials) performed in a mock scanner. The mean reaction time plus one standard deviation from all correct-response trials during this practice run was used as the deadline during fMRI data collection. When a participant failed to meet this deadline, feedback appeared to indicate a missed response and encourage faster responding on subsequent trials (no feedback was presented following either correct or error trials). Between trials, participants viewed a central fixation cross with a variable inter-trial interval (2–6 sec). Task performance measures were reaction times (RTs), error rates (errors of commission) and missed response rates (errors of omission). Participants completed the task in four, 9-min runs with short rest periods between each.
MRI data collection and analysis
Whole-brain blood oxygenation level-dependent (BOLD) echo-planar imaging (EPI) data were acquired with a Siemens 3T Magnetom Allegra scanner (Erlangen, Germany). Thirty-three 5-mm thick slices were acquired in the sagittal plane (272 volumes/run, repetition time=2,000ms, echo time=27ms, flip angle=80°, field of view=220mm in a 64×64 matrix). Imaging data were collected with a delay (332ms) between volume collections to aid the processing of simultaneously recorded EEG data (not discussed further). Structural images were acquired using a magnetization prepared rapid gradient-echo sequence (MPRAGE: TR=2,500ms; TE=4.38ms; FA=8°; voxel size=1mm3).
We processed and analyzed imaging data with AFNI (Cox, 1996). Functional images were slice-time and motion corrected and aligned to anatomical images. Functional time series were normalized to percent signal change and submitted to voxel-wise multiple regression. For each participant, we modeled six task-related regressors (error, correct, and missed responses for congruent and incongruent trials) as impulse functions time-locked to stimulus-array onset and convolved with a model hemodynamic response (gamma) function and its temporal derivative. Six motion-correction parameters also were included to account for residual head motion. To assess error-related brain activity, we calculated subject-level contrast images comparing hemodynamic responses to error versus correct responses for incongruent trials. Incongruent trials were used to estimate error-related brain activity for two reasons: (1) to hold constant the influence of interference effects (i.e., incongruent vs. congruent trials), and (2) the majority of errors occurred on incongruent trials, which therefore provided a greater number of occurrences for assessment. These contrast images were normalized into Talairach space with re-sampled 3mm isotropic voxels and spatially blurred using a 3mm Gaussian kernel.
To identify regions of interest (ROIs) showing increased error-related activity (errors>correct), we performed a whole-brain group-level, one-sample t-test. We applied a voxel-wise threshold of p<10−5 to the resulting statistical map with a minimum cluster size of 10 voxels (pcorrected<0.001). To characterize the relationship between ESI scores and error-related brain activity, we extracted percent signal change values from group-level ROIs by averaging across all voxels within a ROI.
Statistical Analyses
We examined the influence of self-reported externalizing tendencies on behavioral and brain measures by conducting ANCOVAs to determine if ESI scores correlated with task performance and error-related ROI activity among smokers and/or nonsmokers (GROUP). A significant ESI x GROUP interaction in these ANCOVAs indicated that the correlations between ESI scores and the dependent variable significantly differed between smokers and nonsmokers. We used a Bonferroni adjustment to control for α-inflation when characterizing multiple correlations within the smoker group.
We subsequently conducted mediation analyses testing whether the relationship between ESI scores (X) and performance measures (Y) was explained by regionally specific error-related brain activity (M), model: ESI scores → ROI activity → performance. By convention, ‘path c’ in these mediation models refers to the total effect of ESI scores on performance, ‘path a’ refers to the impact of ESI scores on ROI activity, and ‘path b’ refers to the effect of ROI activity on performance (controlling for ESI). The mediation analyses decomposed the total effect of ESI on performance (path c) into direct (path c′) and indirect effects (path ab; i.e., c = c′ + ab). Error-related ROI activity was considered to completely mediate the relationship between ESI and performance if the coefficients from paths a, b, and ab were significant and thus path c′ differed from path c. Mediation results are reported as unstandardized path coefficients (β) and standard errors (SE). The ab indirect path was considered significant if the bootstrapped 95% confidence interval (CI95%) (Preacher and Hayes, 2008) did not encompass zero. Lastly, we performed correlation analyses to explore the association between error-related ROI activity and tobacco craving and/or nicotine dependence.
RESULTS
Task performance
As expected, Flanker interference effects were observed on task performance measures (error rates, RT, and miss rates) in smokers and nonsmokers (Table 1). We assessed error rates in a TRIAL type (incongruent vs. congruent) x GROUP (smoker vs. nonsmoker) mixed-effects ANOVA. Both smokers and nonsmokers showed higher error rates on incongruent versus congruent trials (F[1,40]=109.7, p<0.001) in the absence of a TRIAL x GROUP interaction (p=0.5) or GROUP main effect (p=0.4).
Table 1.
Flanker task performance measures among smokers, nonsmokers, and all participants.
| GROUP | TRIAL | Error rate (%) | RT (ms)
|
Missed (%) | |
|---|---|---|---|---|---|
| correct trials | error trials | ||||
| Smokers (n = 23) | |||||
| Incongruent | 22.1 (2.5)† | 482 (11)† | 388 (18)† | 20.0 (2.5)† ‡ | |
| Congruent | 5.9 (1.0)† | 434 (8)† | 429 (12)† | 12.3 (2.0)† ‡ | |
| Overall | 14.0 (1.6) | 443 (10) | 16.1 (2.0)‡ | ||
| Nonsmokers (n = 19) | |||||
| Incongruent | 19.5 (2.5)† | 485 (9)† | 417 (10)† | 11.5 (1.1)† ‡ | |
| Congruent | 5.1 (0.8)† | 442 (10)† | 453 (11)† | 7.2 (1.0)† ‡ | |
| Overall | 12.3 (1.6) | 456 (10) | 9.4 (0.9)‡ | ||
| All (N = 42) | |||||
| Incongruent | 20.9 (1.8)† | 483 (7)† | 401 (11)† | 16.2 (1.6)† | |
| Congruent | 5.5 (0.6)† | 438 (6)† | 440 (8)† | 10.0 (1.2)† | |
| Overall | 13.2 (1.1) | 449 (7) | 13.1 (1.3) | ||
Note. Data are expressed as mean (standard error of the mean).
Significant trial-type difference (i.e., incongruent vs. congruent).
Significant group-wise difference (i.e., smoker versus nonsmoker). See also Supplemental Figure S2. Post-error slowing data can be found in Supplemental Table S2.
We then assessed RT in a RESPONSE outcome (correct vs. error) x TRIAL type x GROUP mixed-effects ANOVA. RT was modulated by response outcome and trial type similarly in both smokers and nonsmokers as indicated by a non-significant RESPONSE x TRIAL x GROUP interaction, F(1,40)=0.1, p=0.7. Specifically, we observed a RESPONSE x TRIAL interaction (F[1,40]=54.4, p<0.001: Supplemental Figure S2) such that incongruent (vs. congruent) RT was slower on correct-trials (t[41]=−10.8, pcorrected1<0.001), but faster on incorrect-trials (t[41]=3.5, pcorrected=0.002). These error rate and RT outcomes indicated that more impulsive/disinhibited responding (i.e., faster RT), executed before the full processing of incongruent stimuli, was associated with increased error rates. Data regarding post-error slowing can be found in Supplemental Table S2.
We also assessed miss rates in a TRIAL x GROUP mixed-effects ANOVA. Both smokers and nonsmokers showed higher miss rates on incongruent versus congruent trials (F[1,40]=24.1, p<0.001) consistent with incongruent-stimulus processing necessitating additional cognitive resources. In addition, abstinent smokers showed higher miss rates than nonsmokers (F[1,40]=8.4, p=0.006) in the absence of a significant TRIAL x GROUP interaction (p=0.2). These outcomes indicated that acutely abstinent smokers experienced more lapses in attention during task performance relative to nonsmokers.
Error-related brain activity
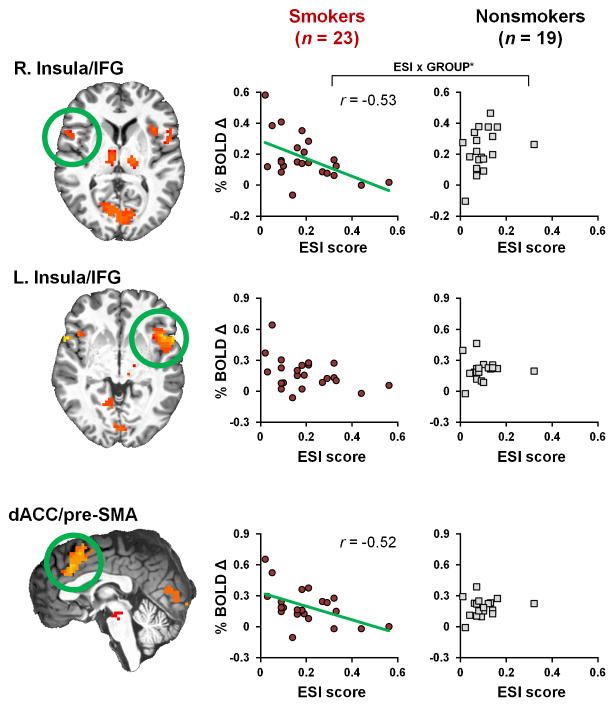
Among all participants, errors produced increased activation in a network of regions including the bilateral insulae extending into the inferior frontal gyri (IFG) and dACC extending into the pre-supplemental motor area (pre-SMA) (Figure 1). In addition, errors produced increased activation in bilateral inferior parietal lobe, bilateral thalamus, left inferior frontal gyrus, cuneus, and brainstem (Table 2). These regions are routinely observed during performance monitoring in general and error processing in particular (King et al., 2010; Klein et al., 2007; Ullsperger et al., 2010). No differences in activity were detected between smokers and nonsmokers at the whole-brain or ROI levels (p’s>0.2).
Figure 1.
Greater externalizing tendencies predicted less error-related brain activity in acutely abstinent smokers. (left) Error commission was associated with increased activation notably in the right and left insula and dACC. (middle and right) Correlations between self-reported externalizing tendencies (ESI score) and regional error-related activity (% BOLD Δ) among smokers (middle column) and nonsmokers (right column). Higher ESI scores indicated less right insula recruitment following error commission among smokers, but not nonsmokers as indicated by a significant ESI x GROUP interaction. A similar although non-significant outcome was observed when considering left insula activity. Higher ESI scores also indicated less dACC recruitment following errors among smokers. See Table 2 for ROI coordinates. * p < 0.05.
Table 2.
Error-related brain activity.
| Region | Center Coordinates (Talairach)
|
Cluster size
|
||
|---|---|---|---|---|
| x | y | z | (voxels) | |
| insula/IFG (R) | 55 | 15 | 3 | 64 |
| insula/IFG (L) | −46 | 14 | 1 | 174 |
| dACC/pre-SMA (B) | 1 | 18 | 42 | 344 |
| inferior parietal lobe (R) | 59 | −45 | 37 | 36 |
| inferior parietal lobe (L) | −42 | −47 | 38 | 36 |
| thalamus (R) | 9 | −13 | 9 | 22 |
| thalamus (L) | −12 | −19 | 6 | 45 |
| inferior frontal gyrus (L) | −52 | 6 | 27 | 10 |
| cueneus (B) | 3 | −74 | 6 | 156 |
| brainstem (B) | −1 | −24 | 27 | 10 |
Note. Error-related activity was assessed at the whole-brain level (errors > correct; threshold pvoxel-wise< 10−5; cluster extent: 10 voxels). Voxel size: 3 × 3 × 3 mm3 (one voxel = 27 μL). Talairach coordinates X: Left (−), Right (+); Y: Posterior (−), Anterior (+); Z: Inferior (−), Superior (+). B: bilateral; L: left; R: right. Of particular interest, errors produced increased activation in clusters within the bilateral insulae that extended into the neighboring inferior frontal gyri (labeled as insula/IFG) and within the dorsal ACC extending into the pre-supplemental motor area (labeled as dACC/pre-SMA).
Externalizing traits predicted task performance measures in smokers
When considering between-group differences, ESI scores were higher among smokers (0.20±0.13) relative to nonsmokers (0.10±0.07), t(40)=2.9, p=0.006. We then assessed the influence of ESI scores on performance measures within-groups by performing ANCOVAs (Supplemental Table S3, Figure S3). With respect to overall error rates, higher ESI scores were associated with the commission of more errors among smokers (r[21]=0.50, pcorrected=0.045), but not nonsmokers (r[17]=0.07, p=0.7), ESI x GROUP: F(2,39)=3.6, p=0.04. Similarly, higher ESI scores were associated with faster overall RT among smokers (r[21]=−0.64, pcorrected=0.003), but not nonsmokers (r[17]=−0.29, p=0.2), ESI x GROUP: F(2,39)=7.7, p=0.002. ESI scores also were positively correlated with miss rates among smokers, although this association failed to reach significance when corrected for multiple comparisons (r[21]=0.44, pcorrected=0.09). As such, miss rates are not discussed further.
Externalizing traits predicted less error-related brain activity in smokers
When considering error-related ROI activity, higher ESI scores were associated with less right insula/IFG activity among smokers (r[21]=−0.53, pcorrected2=0.03), but not nonsmokers (r[17]=0.33, p=0.2), ESI x GROUP: F(2,39)=4.1, p=0.02 (Figure 1). We observed similar, albeit non-significant, outcomes when considering the relation between ESI scores and left insula/IFG activity (smokers: r[21]=−0.4, pcorrected>0.05; nonsmokers: r[17]=0.02, p=0.9; ESI x GROUP: F[2,39]=2.5, p=0.09). Additionally, higher ESI scores were also associated with less error-related dACC/preSMA activity among smokers (r[21]=−0.52, pcorrected=0.04), but not nonsmokers (r[17]=0.18, p=0.5), although the ESI x GROUP interaction failed to reach significance, F(2,39)=2.5, p=0.1. Correlations between ESI scores and all ROIs showing error-related activity are presented in Supplemental Table S4.
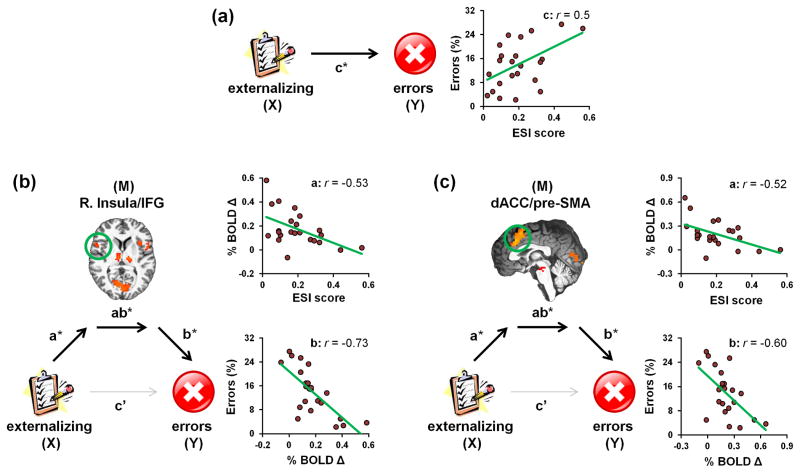
Error-related activity mediated externalizing’s influence on smokers’ performance
As externalizing traits correlated with both behavioral and brain measures during acute nicotine withdrawal, we subsequently conducted mediation analyses testing the hypothesis that the impact of ESI scores (X) on error rates (Y) was mediated by right insula and dACC activity (M) (Figure 2, Table 3). Error-related right insula activity fully mediated the relation between ESI scores and error rates (Figure 2b). Specifically, when including the insula mediator in the model, ESI’s direct effect on error rates failed to reach significance (c′ path: β=9.6, SE=10.3, p=0.4), whereas the indirect effect was significant (ab path: CI95%: 7.0, 36.4). We observed a similar outcome when employing error-related dACC activity as the sole mediator in a separate model (c′ path: β=15.2, SE=11.7, p=0.2; ab path: CI95%: 2.7, 30.3; Figure 2c). Indicative of state/condition specificity, these insula and dACC meditational relations appeared critically linked with acute nicotine withdrawal as such effects were not detected following nicotinic receptor stimulation by varenicline and/or nicotine (Supplemental Table S5, Figure S4). These outcomes suggest that higher ESI scores predicted less error-related right insula and dACC activity and, in turn, less error-related activity predicted more performance errors only during nicotine withdrawal.
Figure 2.
Mediation models of the association between externalizing tendencies, error rates, and error-related brain activity among acutely abstinent smokers. (a) ESI scores were positively correlated with error rates (i.e., the total effect of X on Y was significant, c path). (b) Error-related right insula activity (M) fully mediated the effect of ESI scores (X) on error rates (Y) as: [1] ESI scores accounted for significant variance in insula activity (a path), [2] insula activity accounted for unique variance in error rates when controlling for ESI scores (b path), [3] the indirect mediation effect was significant (ab path), and [4] ESI’s direct effect on errors was no longer significant when the insula mediator was included in the model (c′ path). (c) Similarly, error-related dACC activity (M) mediated the effect of ESI scores (X) on error rates (Y) when included as the sole mediator in a separate model. See Table 3 for path coefficients. * p < 0.05.
Table 3.
Path coefficients from separate mediation analyses characterizing the relations between externalizing tendencies (X), error rates (Y), and error-related brain activity (M).
| region | PATH | ||||
|---|---|---|---|---|---|
|
| |||||
| a X-M |
b M-Y |
c X-Y (total) |
c′ direct |
ab indirect |
|
| insula/IFG (R)a, b | −0.6 (0.2)** | −34.0 (9.4)** | 29.2 (11.0)* | 9.6 (10.3) | 7.0, 36.4 |
| dACC/pre-SMA (B)a, b | −0.7 (0.2)* | −21.1 (9.1)* | 29.2 (11.0)* | 15.2 (11.7) | 2.7, 30.3 |
| inferior Parietal Lobe (R)† | −0.6 (0.2)* | −17.5(10.9) | 29.2(11.0)* | 19.25 (12.2) | −2.0, 28.9 |
Note. For paths a, b, c, and c′, values are reported as unstandardized path coefficients (standard error). For the ab path values reported are the upper and lower bounds of the bootstrapped 95% confidence interval (CI95%). See Figure 2 for a schematic representation of these mediation analyses.
p < 0.05,
p < 0.01.
We subsequently conducted additional mediation analyses to provide support for the regional and domain specificity of the right insula and dACC mediational effects. Indicative of regional specificity and thus providing a negative control, error-related right inferior parietal lobe activity (activity that was negatively correlated with ESI scores in smokers, Supplemental Table S4) did not mediate the relation between ESI scores and error rates (Supplemental Table S6). Indicative of domain specificity, neither error-related right insula nor dACC activity mediated the relation between ESI scores and RT (Supplemental Table S7).
Less error-related right insula and dACC activity indicated more tobacco craving
Finally, we conducted exploratory correlation analyses between TCQ ratings and error-related right insula and dACC activity (Figure 3). Focusing on the TCQ-purposefulness subscale, higher levels of craving in acutely abstinent smokers were correlated with less error-related right insula (r[21]=−0.49, p=0.02) and dACC activity (r[21]=−0.45, p=0.03). No significant correlations were detected between error-related activity and other TCQ-subscale scores (right insula: p’s>0.14; dACC: p’s>0.08), TCQ total scores (right insula: r[21]=−0.37, p=0.08; dACC: r[21]=−0.35, p=0.1), or Fagerström ratings (right insula: p=0.12; dACC: p=0.2).
Figure 3.
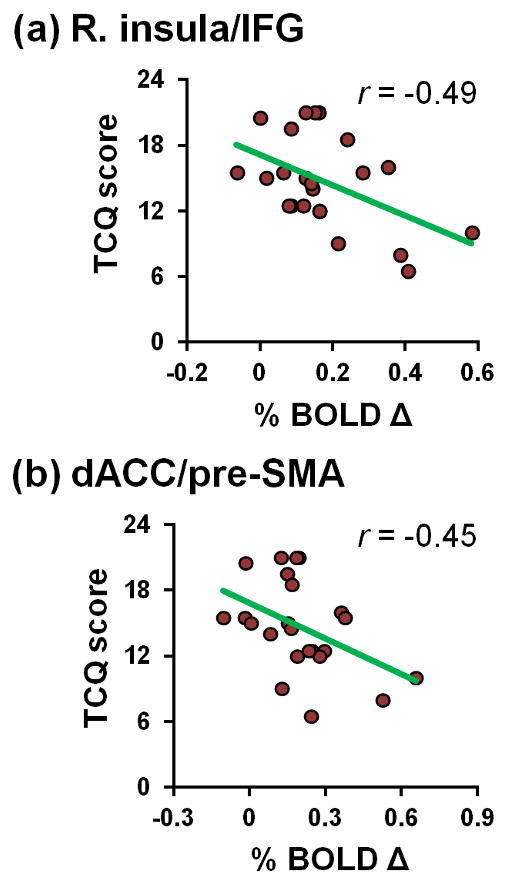
Error-related activity and tobacco craving among acutely abstinent smokers. Less error-related right insula (a) and dACC (b) activity correlated with higher scores on the TCQ-purposefulness subscale.
DISCUSSION
Externalizing is a broad personality construct generally reflecting increased proneness to impulsive behaviors and linked with reduced electrophysiological correlates of error processing (Hall et al., 2007; Nelson et al., 2011; Patrick et al., 2012). Given that reduced activity in canonical error-processing brain regions has been conceptualized as a contributing factor to continued drug use (Garavan and Stout, 2005; Goldstein et al., 2009; Kaufman et al., 2003), we investigated the interrelations between externalizing tendencies, task performance, and error-related brain activity in overnight-deprived cigarette smokers and nonsmokers. We observed that higher externalizing tendencies among smokers were associated with a less optimal (increased error rates) and more impulsive task-performance style (faster RTs). Indicative of a neurobiological contribution to this sub-optimal performance style, higher externalizing tendencies also were associated with less recruitment of the right insula and dACC following error commission. Critically, this error-related brain activity fully mediated the externalizing-performance relation as higher ESI scores indicated less right insula and dACC activity, and in turn, less error-related activity indicated the occurrence of more errors. As deficits in monitoring ongoing behaviors may contribute to the repetition of maladaptive responses and disadvantageous decisions, our results suggest that externalizing traits and/or error-related right insula/dACC activity may be relevant factors for smoking cessation success.
Behaviorally, smokers self-reporting higher degrees of externalizing committed more errors while also executing their responses more quickly. Such behavioral outcomes may have treatment implications as similar performance indices assessed during early abstinence have been used to predict smoking relapse (Patterson et al., 2010). Furthermore, Bold and colleagues (2013) noted that abstinent smokers who committed more errors in a sustained attention task showed greater difficulties inhibiting drug use impulses as indicated by quicker times to smoke when given the opportunity. Elevated externalizing tendencies themselves also predict subsequent tobacco use and have been associated with earlier age of smoking initiation as well as reduced cessation success in adolescents (Fischer et al., 2012; Leff et al., 2003; Moolchan et al., 2007). In parallel, trait-impulsivity, a lower-order facet of the broad externalizing construct, has been linked with elevated cigarette-cue reactivity (Doran et al., 2008) and increased abstinence-induced tobacco craving (VanderVeen et al., 2008). The affective and cognitive consequences of smoking abstinence appear to serve as more potent negative reinforcers in smokers with elevated impulsivity (Doran et al., 2006), which may account for greater difficulties in abstinence maintenance among such individuals (Doran et al., 2004). Despite accumulating evidence linking behavioral and personality indicators of externalizing tendencies with smoking behaviors, little research has examined the neurobiological mechanisms contributing to such a relation.
To this end, mechanistically, we observed that smokers higher in externalizing showed reduced recruitment of the right insula and dACC following error commission. Furthermore, we observed a pattern of correlations consistent with the hypothesis that the effect of externalizing on error rates was mediated by error-related activity in the right insula and dACC. Critically and indicative of state/conditional specificity, we observed these mediation effects only during nicotine withdrawal but not following drug administration (i.e., varenicline and nicotine). Whereas previous studies have noted between-group differences in error-related brain activity when comparing non-drug using controls with cocaine (Kaufman et al., 2003), methamphetamine (London et al., 2005), opiate (Forman et al., 2004), cannabis (Hester et al., 2009), and nicotine abusers (Luijten et al., 2011), our results suggest that within-group variations in externalizing traits account for additional variability in brain measures. Elucidating such individual variations may prove beneficial for facilitating the implementation of individualized treatment regimens.
The bilateral insulae and dACC are primary constituents of the so-called salience network and often co-activate in situations necessitating behavioral change (Dosenbach et al., 2006; Seeley et al., 2007; Ullsperger et al., 2010). The right insula appears to play a central role in the response of this network to errors, as input to other nodes has been suggested to propagate through this critical outflow hub (Ham et al., 2013). The bilateral insulae are further implicated in the conscious awareness of errors (Hester et al., 2005; Klein et al., 2013). With respect to drug abuse, right insula activity is lower in cannabis users performing an error awareness task and such activity is negatively correlated with amount of recent drug use (Hester et al., 2009). Further, the dACC is thought to signal the need for behavioral adjustments following salient events via increased interactions with such regions as the lateral prefrontal cortex (Ham et al., 2013; Ridderinkhof et al., 2004). Using a modified Flanker task incorporating smoking cues to examine error processing in minimally-deprived smokers, Luijten and coworkers (2011) detected reduced electrophysiological correlates of initial error processing (i.e., ERN amplitude) as well as reductions in a second event-related potential thought to index the motivational significance of an error (i.e., the error positivity, Pe). Our results demonstrating less error-related insula and dACC activity associated with higher externalizing are consistent with the findings of Luijten et al. (2011) who further observed smaller ERN amplitudes associated with greater trait-impulsivity.
Taken together, one interpretation of our results is that insufficient recruitment of the right insula and dACC following error commission underlies decreased error awareness and/or a motivational insensitivity to errors in higher-externalizing smokers during early smoking abstinence. This attenuated error response appears accompanied by the continued occurrence of sub-optimal behaviors. These observations are consistent with the hypothesis that inadequate brain responses to errors contributes to continued drug use despite negative consequences (Goldstein et al., 2009; Goldstein and Volkow, 2002) in some individuals.
Relating individual variation in brain activity with a clinically-relevant construct, we further observed that less error-related right insula and dACC activity conferred increased liability for tobacco craving during early smoking abstinence. Specifically, less error-related activity was associated with higher scores on the TCQ-purposefulness subscale. As craving is a multifaceted psychological construct, the TCQ was developed as a multidimensional instrument (Heishman et al., 2003; Heishman et al., 2008). The dimensions of craving assessed by the TCQ are: emotionality (anticipation of withdrawal relief by smoking), expectancy (anticipation of positive outcomes by smoking), compulsivity (inability to control smoking), and purposefulness (intentions and planning to smoke). Our finding that error-related insula and dACC activity significantly correlated with only the TCQ-purposefulness subscale supports the notion that distinct neural circuits contribute to these various facets of tobacco craving. We have previously shown that insula and ventromedial prefrontal circuitry (regions implicated in emotional processing and regulation, e.g., Myers-Schulz and Koenigs, 2012) selectively relate to the TCQ-emotionality subscale (Sutherland et al., 2013b). In light of the involvement of insula/dACC circuitry in goal-directed behaviors, it is not surprising that the error-related ROI activity described herein selectively correlated with the TCQ-purposefulness subscale.
Recent theorizing has related the insula’s role in interoception with multiple stages of the addiction cycle (Craig, 2009; Naqvi and Bechara, 2010; Paulus, 2007; Sutherland et al., 2013a). These views posit that insular reactivity and subsequent network dysregulation contribute to alterations in interoceptive functions and the phenomenological experience of drug craving that then limit cognitive resources available for behavioral monitoring and optimal decision-making (Sutherland et al., 2012b). Systems-level perspectives highlight three large-scale brain networks and their roles in these psychological processes: the salience network (SN: Seeley et al., 2007), the default-mode network (DMN: Raichle et al., 2001), and the executive control network (ECN: Fox et al., 2005). The insulae and ACC are critical nodes of the SN, which plays a role in directing attention towards either internal or external stimuli by toggling dynamic activity between the typically anticorrelated DMN and ECN (Fox et al., 2005; Hamilton et al., 2011; Sridharan et al., 2008). As the DMN is generally associated with endogenous information processing and the ECN with exogenous information processing, intermittent failures to adequately suppress the DMN (Sonuga-Barke and Castellanos, 2007) and/or maladaptive interactions between components of these two networks (Kelly et al., 2008; Weissman et al., 2006) represent systems-level mechanisms contributing to sub-optimal goal-directed behavior in various neuropsychiatric conditions (Menon, 2011) and during nicotine withdrawal (Sutherland et al., 2012b). In other words, the neurobiological mechanisms associated with the processing of endogenous, homeostatically-relevant bodily states (i.e., abstinence-induced processes) may impede exogenous information processing and behavioral self-monitoring. This line of reasoning leads to the speculation that certain pharmacological agents such as methylphenidate, which augments error processing and associated brain activity in healthy volunteers (Hester et al., 2012), may restore optimal network dynamics and provide smoking cessation benefits as an adjunct intervention for high-externalizing smokers.
Our findings should be considered in light of several issues. First, we failed to detect group differences in error-related brain activity when comparing acutely abstinent smokers and nonsmokers. However, this outcome is in line with observations from electrophysiological studies that similarly did not detect smoker versus nonsmoker differences in ERN amplitude when employing a Flanker task comparable to that used in the current study (Franken et al., 2010), but did detect such differences when incorporating cigarette cues into the task (Luijten et al., 2011). Such observations are consistent with the notion that presentation of cigarette cues and associated craving induction limits cognitive resources. Second, the range of ESI scores observed in our nonsmokers was rather limited relative to that from the smoker cohort as well as the full dynamic range of the instrument. This limited range may account for an inability to detect significant associations between externalizing tendencies and error-related brain activity in the nonsmoker group. Third, while the ESI assesses externalizing tendencies via personality-trait and past-behavioral indicators, we cannot discount the possibility that state-like variations (e.g., smoking abstinence) influenced ESI scores. Fourth, regarding post-error slowing, we observed that RT among smokers was modestly, but significantly slower on trials following an error relative to trials following a correct response (Supplemental Table S2). While the current task implementation was not designed or optimized to assess post-error slowing, we did not observe a post-error slowing effect among nonsmokers. Fifth, given the correlational nature of the present study, an alternative explanation of the relationship between error-related right insula/dACC activity and error rates is that such ROI activity may ‘habituate’ as individuals commit more errors. Relatedly, while we used ESI scores as the predictor variable (X) and task performance or error-related brain activity as dependent variables (Y) in our analyses, the opposite approach is equally valid. Nonetheless, our results provide evidence for a relationship between the externalizing construct, error-related insula and dACC activity, and task performance indices. Finally, while reduced ERN amplitude has been used to predict treatment outcomes in cocaine abusers (Marhe et al., 2013), the ability of error-related right insula or dACC activity to predict smoking cessation outcomes remains indeterminate.
In conclusion, our results highlight the utility of examining individual variation in personality traits, task performance, and regional brain activity to elucidate neurobiological mechanisms potentially contributing to the persistence of drug use in general and cigarette smoking in particular. Specifically, we observed that higher externalizing tendencies in acutely abstinent smokers were associated with less recruitment of the right insula and dACC following error commission and that such diminished regional activity was associated with the occurrence of more errors during task performance. Additionally, diminished error-related right insula and dACC activity correlated with higher degrees of tobacco craving. Externalizing tendencies and/or compromised error processing in subsets of smokers during early abstinence may be relevant targets for smoking cessation treatments in the service of curtailing maladaptive behaviors and disadvantageous decisions precipitating relapse.
Supplementary Material
Acknowledgments
This work was supported by the National Institute on Drug Abuse Intramural Research Program, National Institutes of Health, Department of Health and Human Services (NIDA-IRP/NIH/DHHS). We thank Eliscia Smith, Angela Neal, Kimberly Slater, Loretta Spurgeon, and the NIDA-IRP nurses and recruitment staff for assistance with data collection.
Footnotes
Bonferroni corrected for 2 comparisons (α=0.05/2=0.025).
Bonferroni corrected for 3 comparisons (left, right insula and dACC; α=0.05/3).
FINANCIAL DISCLOSURE: None reported.
AUTHORS CONTRIBUTION: MTS, BJS, TJR, and EAS were responsible for the study concept and design. AJC, MTS, BJS and TJR contributed to data acquisition. MTS, AJC, and TJR assisted with data analysis. MTS and AJC wrote the manuscript. BJS, TJR and EAS provided revision of the manuscript for intellectual content. All authors critically reviewed the content and approved the final manuscript.
References
- Bold KW, Yoon H, Chapman GB, McCarthy DE. Factors predicting smoking in a laboratory-based smoking-choice task. Exp Clin Psychopharmacol. 2013;21:133–143. doi: 10.1037/a0031559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WP, Davies PL, Gavin WJ. Individual differences in error monitoring in healthy adults: psychological symptoms and antisocial personality characteristics. European Journal of Neuroscience. 2010;32:1388–1396. doi: 10.1111/j.1460-9568.2010.07384.x. [DOI] [PubMed] [Google Scholar]
- Chase HW, Eickhoff SB, Laird AR, Hogarth L. The Neural Basis of Drug Stimulus Processing and Craving: An Activation Likelihood Estimation Meta-Analysis. Biological Psychiatry. 2011;70:785–793. doi: 10.1016/j.biopsych.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel - now? The anterior insula and human awareness. Nature Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Doran N, McChargue D, Spring B. Effect of impulsivity on cardiovascular and subjective reactivity to smoking cues. Addict Behav. 2008;33:167–172. doi: 10.1016/j.addbeh.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Doran N, McChargue D, Spring B, VanderVeen J, Cook JW, Richmond M. Effect of nicotine on negative affect among more impulsive smokers. Exp Clin Psychopharmacol. 2006;14:287–295. doi: 10.1037/1064-1297.14.3.287. [DOI] [PubMed] [Google Scholar]
- Doran N, Spring B, McChargue D, Pergadia M, Richmond M. Impulsivity and smoking relapse. Nicotine & Tobacco Research. 2004;6:641–647. doi: 10.1080/14622200410001727939. [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HSC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, Brown VL, Cinciripini PM. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage. 2012;60:252–262. doi: 10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer JA, Najman JM, Williams GM, Clavarino AM. Childhood and adolescent psychopathology and subsequent tobacco smoking in young adults: findings from an Australian birth cohort. Addiction. 2012;107:1669–1676. doi: 10.1111/j.1360-0443.2012.03846.x. [DOI] [PubMed] [Google Scholar]
- Forman SD, Dougherty GG, Casey BJ, Siegle GJ, Braver TS, Barch DM, Stenger VA, Wick-Hull C, Pisarov LA, Lorensen E. Opiate addicts lack error-dependent activation of rostral anterior cingulate. Biol Psychiatry. 2004;55:531–537. doi: 10.1016/j.biopsych.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. PNAS. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken IH, van Strien JW, Kuijpers I. Evidence for a deficit in the salience attribution to errors in smokers. Drug Alcohol Depend. 2010;106:181–185. doi: 10.1016/j.drugalcdep.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Garavan H. Insula and drug cravings. Brain Struct Funct. 2010;214:593–601. doi: 10.1007/s00429-010-0259-8. [DOI] [PubMed] [Google Scholar]
- Garavan H, Stout JC. Neurocognitive insights into substance abuse. Trends Cogn Sci. 2005;9:195–201. doi: 10.1016/j.tics.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Craig AD, Bechara A, Garavan H, Childress AR, Paulus MP, Volkow ND. The neurocircuitry of impaired insight in drug addiction. Trends Cogn Sci. 2009;13:372–380. doi: 10.1016/j.tics.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiat. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JR, Bernat EM, Patrick CJ. Externalizing psychopathology and the error-related negativity. Psychol Sci. 2007;18:326–333. doi: 10.1111/j.1467-9280.2007.01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham T, Leff A, de Boissezon X, Joffe A, Sharp DJ. Cognitive Control and the Salience Network: An Investigation of Error Processing and Effective Connectivity. J Neurosci. 2013;33:7091–7098. doi: 10.1523/JNEUROSCI.4692-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-Mode and Task-Positive Network Activity in Major Depressive Disorder: Implications for Adaptive and Maladaptive Rumination. Biol Psychiatry. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG, Moolchan ET. Tobacco Craving Questionnaire: Reliability and validity of a new multifactorial instrument. Nic Tob Res. 2003;5:645–654. doi: 10.1080/1462220031000158681. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Singleton EG, Pickworth WB. Reliability and validity of a short form of the tobacco craving questionnaire. Nicotine & Tobacco Research. 2008;10:643–651. doi: 10.1080/14622200801908174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Foxe JJ, Molholm S, Shpaner M, Garavan H. Neural mechanisms involved in error processing: a comparison of errors made with and without awareness. Neuroimage. 2005;27:602–608. doi: 10.1016/j.neuroimage.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Hester R, Nandam LS, O’Connell RG, Wagner J, Strudwick M, Nathan PJ, Mattingley JB, Bellgrove MA. Neurochemical enhancement of conscious error awareness. J Neurosci. 2012;32:2619–2627. doi: 10.1523/JNEUROSCI.4052-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology. 2009;34:2450–2458. doi: 10.1038/npp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann S, Wascher E, Falkenstein M. Personality and error monitoring: an update. Frontiers in human neuroscience. 2012;6 doi: 10.3389/fnhum.2012.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. Journal of Neuroscience. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuro Image. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- King JA, Korb FM, von Cramon DY, Ullsperger M. Post-error behavioral adjustments are facilitated by activation and suppression of task-relevant and task-irrelevant information processing. J Neurosci. 2010;30:12759–12769. doi: 10.1523/JNEUROSCI.3274-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TA, Endrass T, Kathmann N, Neumann J, von Cramon DY, Ullsperger M. Neural correlates of error awareness. Neuroimage. 2007;34:1774–1781. doi: 10.1016/j.neuroimage.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Klein TA, Ullsperger M, Danielmeier C. Error awareness and the insula: links to neurological and psychiatric diseases. Frontiers in human neuroscience. 2013;7:14. doi: 10.3389/fnhum.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. J Abnorm Psychol. 2002;111:411–424. [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: an integrative quantitative model of the adult externalizing spectrum. J Abnorm Psychol. 2007;116:645–666. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff MK, Moolchan ET, Cookus BA, Spurgeon L, Evans LA, London ED, Kimes A, Schroeder JR, Ernst M. Predictors of smoking initiation among at risk youth: A controlled study. J Child Adolesc Subst Abus. 2003;13:59–75. [Google Scholar]
- London ED, Berman SM, Voytek B, Simon SL, Mandelkern MA, Monterosso J, Thompson PM, Brody AL, Geaga JA, Hong MS, Hayashi KM, Rawson RA, Ling W. Cerebral metabolic dysfunction and impaired vigilance in recently abstinent methamphetamine abusers. Biol Psychiatry. 2005;58:770–778. doi: 10.1016/j.biopsych.2005.04.039. [DOI] [PubMed] [Google Scholar]
- Luijten M, van Meel CS, Franken IH. Diminished error processing in smokers during smoking cue exposure. Pharmacol Biochem Behav. 2011;97:514–520. doi: 10.1016/j.pbb.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Marhe R, van de Wetering BJM, Franken IHA. Error-Related Brain Activity Predicts Cocaine Use After Treatment at 3-Month Follow-up. Biological Psychiatry. 2013;73:782–788. doi: 10.1016/j.biopsych.2012.12.016. [DOI] [PubMed] [Google Scholar]
- Melcher T, Falkai P, Gruber O. Functional brain abnormalities in psychiatric disorders: neural mechanisms to detect and resolve cognitive conflict and interference. Brain Res Rev. 2008;59:96–124. doi: 10.1016/j.brainresrev.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cog Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Moolchan ET, Frazier M, Franken FH, Ernst M. Adolescents in smoking cessation treatment: Relationship between externalizing symptoms, smoking history and outcome. Psychiatry Research. 2007;152:281–285. doi: 10.1016/j.psychres.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Myers-Schulz B, Koenigs M. Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders. Molecular Psychiatry. 2012;17:132–141. doi: 10.1038/mp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: The insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The insula and drug addiction: An interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214:435–450. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LD, Patrick CJ, Bernat EM. Operationalizing proneness to externalizing psychopathology as a multivariate psychophysiological phenotype. Psychophysiology. 2011;48:64–72. doi: 10.1111/j.1469-8986.2010.01047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The error-related negativity (ERN) and psychopathology: toward an endophenotype. Clin Psychol Rev. 2008;28:1343–1354. doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Durbin CE, Moser JS. Reconceptualizing antisocial deviance in neurobehavioral terms. Development and psychopathology. 2012;24:1047–1071. doi: 10.1017/S0954579412000533. [DOI] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, Frey J, Gur R, Lerman C. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug and Alcohol Dependence. 2010;106:61–64. doi: 10.1016/j.drugalcdep.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP. Decision-making dysfunctions in psychiatry - Altered homeostatic processing? Science. 2007;318:602–606. doi: 10.1126/science.1142997. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. PNAS. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuiss S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Segalowitz SJ. The error-related negativity is related to risk taking and empathy in young men. Psychophysiology. 2009;46:143–152. doi: 10.1111/j.1469-8986.2008.00714.x. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. PNAS. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRNT-Subcommittee. Biochemical verification of tobacco use and cessation. Nic Tob Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Hong LE, Stein EA. Individual differences in amygdala reactivity following nicotinic receptor stimulation in abstinent smokers. Neuroimage. 2012a;66:585–593. doi: 10.1016/j.neuroimage.2012.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage. 2012b;62:2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Hong LE, Stein EA. Down-regulation of amygdala and insula functional circuits by varenicline and nicotine in abstinent cigarette smokers. Biological Psychiatry. 2013a;74:538–546. doi: 10.1016/j.biopsych.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Stein EA. Insula’s functional connectivity with ventromedial prefrontal cortex mediates the impact of trait alexithymia on state tobacco craving. Psychopharmacology. 2013b;228:143–155. doi: 10.1007/s00213-013-3018-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M. Performance monitoring in neurological and psychiatric patients. Int J Psychophysiol. 2006;59:59–69. doi: 10.1016/j.ijpsycho.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, Harsay HA, Wessel JR, Ridderinkhof KR. Conscious perception of errors and its relation to the anterior insula. Brain Struct Funct. 2010;214:629–643. doi: 10.1007/s00429-010-0261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderVeen JW, Cohen LM, Cukrowicz KC, Trotter DR. The role of impulsivity on smoking maintenance. Nicotine Tob Res. 2008;10:1397–1404. doi: 10.1080/14622200802239330. [DOI] [PubMed] [Google Scholar]
- Venables NC, Patrick CJ. Validity of the Externalizing Spectrum Inventory in a criminal offender sample: relations with disinhibitory psychopathology, personality, and psychopathic features. Psychol Assess. 2012;24:88–100. doi: 10.1037/a0024703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto EP, Detre JA, Lerman C. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J Neurosci. 2007;27:14035–14040. doi: 10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.