Abstract
Opioids represent effective drugs for the relief of pain, yet chronic opioid use often leads to a state of increased sensitivity to pain that is exacerbated during withdrawal. A sensitization of pain-related negative affect has been hypothesized to closely interact with addiction mechanisms. Neuroadaptive changes occur as a consequence of excessive opioid exposure, including a recruitment of corticotropin-releasing factor (CRF) and norepinephrine (NE) brain stress systems. To better understand the mechanisms underlying the transition to dependence, we determined the effects of functional antagonism within these two systems on hyperalgesia-like behavior during heroin withdrawal utilizing models of both acute and chronic dependence. We found that passive or self-administered heroin produced a significant mechanical hypersensitivity. During acute opioid dependence, systemic administration of the CRF1 receptor antagonist MPZP (20 mg/kg) alleviated withdrawal-induced mechanical hypersensitivity. In contrast, several functional adrenergic system antagonists (clonidine, prazosin, propranolol) failed to alter mechanical hypersensitivity in this state. We then determined the effects of chronic MPZP or clonidine treatment on extended access heroin self-administration and found that MPZP, but not clonidine, attenuated escalation of heroin intake, whereas both drugs alleviated chronic dependence-associated hyperalgesia. These findings suggest that an early potentiation of CRF signaling occurs following opioid exposure that begins to drive both opioid-induced hyperalgesia and eventually intake escalation.
Keywords: acute dependence, addiction, adrenergic system, CRF, heroin self-administration, opioid-induced hyperalgesia
Introduction
Drug addiction is a chronically relapsing disorder characterized by drug intake escalation (Edwards and Koob, 2013) and the emergence of negative emotional states during abstinence (Edwards and Koob, 2010). A negative emotional state is defined as a dysphoric-like state accompanied by depression- and anxiety-like symptoms and is thought to contribute to the compulsivity associated with dependence via the process of negative reinforcement (Koob, 2008). A sensitization of negative affect associated with pain systems (Ji et al., 2007) has also been hypothesized to be mediated by central reinforcement circuitry and to closely interact with addiction mechanisms (Shurman et al., 2010; Egli et al., 2012).
Approximately one third of the adult population in the United States suffers from chronic pain (Johannes et al., 2010). Many different formulations of opioids have been proven to be effective in the treatment of a variety of chronic pain conditions, and opioids are one of the best resources for short-term analgesia and improvement in quality of life (Fields, 2011). Although only a small percentage of chronic pain patients using opioids become addicted (Fishbain et al., 2008), abuse of and addiction to opioids in vulnerable populations continue to be a major health concern. Chronic opioid exposure for the purpose of alleviating pain often renders individuals more sensitive to nociception, a condition known as hyperalgesia (Angst and Clark, 2006), which may be associated with the transition to dependence in patients with abuse histories. Hyperalgesia is prevalent in former opioid addicts who are maintained on methadone as a treatment of opioid dependence, suggesting that this condition emerges with extended opioid use (Compton et al., 2001). Opioid exposure in rodents leads to a spontaneous reduction in mechanical nociceptive thresholds (Laulin et al., 1998) that is exacerbated after chronic treatment (Simonnet and Rivat, 2003), suggesting a recruitment or sensitization of pro-nociceptive systems (Celerier et al., 2001). Given that chronic pain is well known to cause emotional distress and produce a sustained negative emotional state (King et al., 2009), opioid-induced hyperalgesia may constitute a condition intimately associated with the progressive transition to drug dependence by facilitating negative reinforcement processes. Characterizing pain responsiveness during both acute and chronic opioid administration may extend our knowledge of the early processes promoting negative reinforcement mechanisms associated with the gradual transition to addiction.
Opioid addiction has been linked to neuroadaptation and dysregulation of brain stress systems (Edwards et al., 2009; Koob, 2008), including those regulated by corticotropin-releasing factor (CRF) and norepinephrine (NE) within the extended amygdala (Heinrichs et al., 1995; Koob and Le Moal, 2008). Extracellular CRF in the extended amygdala is increased during acute withdrawal from opioids, and CRF receptor antagonists block excessive heroin intake associated with dependence (Greenwell et al., 2009; Weiss et al., 2001). Importantly, CRF1 receptor antagonism dose-dependently alleviates mechanical hypersensitivity exhibited by opioid-dependent rats (Edwards et al., 2012; McNally and Akil, 2002). Similarly, NE in the extended amygdala and locus coeruleus increases during acute withdrawal from drugs of abuse, and hyperactivity of brain NE accompanied by increased CRF signaling has been implicated in mechanisms of opioid withdrawal in the extended amygdala (Aston-Jones et al., 1999; Maldonado, 1997; Smith and Aston-Jones, 2008). Interestingly, intrathecal clonidine (a presynaptic alpha-2-adrenoceptor agonist) has been shown to reverse mechanical hyperalgesia and reduce heroin intake only in spinal nerve-injured rats (Martin et al., 2007). To further investigate the interactive role of nociception and brain stress systems during opioid withdrawal (Shurman et al., 2010), the current study examined the effects of CRF and adrenergic signaling on pain- and addiction-like behaviors utilizing models of acute and chronic opioid dependence. We report here that a potentiation of CRF signaling resulting from heroin exposure comes to drive both hyperalgesia and compulsive heroin intake.
Materials and Methods
Animals
Male Wistar rats (n = 79) weighing between 200 – 300 g were housed in groups of two to three per cage, and maintained on a 12-h light/dark cycle (lights on at 0800) with free access to food and water. The animals were allowed to acclimate to these conditions for at least 7 days in our animal facilities before behavioral testing. Animals were regularly handled for one week prior to surgery or any behavioral testing. All procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Mechanical Sensitivity Testing
This test was conducted as previously reported (Edwards et al., 2012). Up to eight rats were placed in individual plastic compartments (26 × 11 × 20 cm) with stainless steel mesh floors for 30 minutes until the rats’ grooming and exploratory behaviors ceased. To assess the presence of mechanical hypersensitivity, the mid-plantar area of each hind paw was perpendicularly stimulated with calibrated nylon von Frey filaments (Weinstein-Semmes algesiometer forces) for 5 seconds using the up-down method starting with the 28.84 g force. A brisk withdrawal of the paw (often followed by a sustained retraction and/or licking, possibly indicative of supraspinal organization) is considered a positive response, but paw withdrawals due to locomotion or weight shifting were not counted. For quantitative assessment, the 50% probability withdrawal threshold, or paw withdrawal threshold (PWT), was calculated as previously described (Chaplan et al., 1994). Baseline mechanical nociceptive thresholds were similar to those reported for the ages of rats employed in this study (Ririe and Eisenach, 2006). Paw withdrawal thresholds were measured 10–12 h following the previous heroin self-administration session (i.e., just prior to subsequent sessions). For the prophylactic drug regimen study (eight-hour self-administration sessions), this corresponded to approximately 18–20 h after the final prophylactic drug treatment.
Drugs
Heroin (3,6-diacetylmorphine) was provided by the National Institute on Drug Abuse and was dissolved in 0.9% sterile saline and injected subcutaneously (SC). Clonidine hydrochloride (presynaptic alpha-2-adrenoceptor agonist) was purchased from Sigma-Aldrich and dissolved in 0.9% saline and injected SC in a volume of 1 ml/kg body weight. Prazosin hydrochloride (alpha-1-adrenoceptor antagonist) and propranolol hydrochloride were purchased from Sigma-Aldrich and dissolved in 0.9% saline and injected intraperitoneally (IP) in a volume of 1 ml/kg body weight. The CRF 1 receptor antagonist MPZP was prepared for systemic administration as described (Richardson et al., 2008). Animals were administered MPZP in a volume of 2 ml/kg 20% HBC (hydroxypropyl-beta-cyclodextrin, SC). For the chronic prophylactic administration studies, the vehicle-treated rats were given repeated SC injections of 2 ml 20% HBC vehicle/kg body weight.
Acute Heroin Dependence Model
Acute opioid dependence models are designed to reveal early behavioral neuroadaptations associated with the initiation and progression of dependence symptomatology (Azar et al., 2003; Liu and Schulteis, 2004; Schulteis et al., 1999; Zhang and Schulteis, 2008). To model acute heroin dependence, animals were injected (SC) daily with 1.25 mg/kg heroin. This dose was previously shown to induce mechanical hyperalgesia during heroin withdrawal (Laulin et al., 1998) that progressively increases after repeated, intermittent heroin injections (Celerier et al., 2001). Control animals received repeated injections of saline on equivalent schedules.
Heroin Self-Administration
The surgery and self-administration procedures have been reported in detail previously (Vendruscolo et al., 2011). Briefly, rats were anesthetized with isoflurane (2%) and chronic intravenous catheters were placed in the jugular vein. Rats were allowed to recover for 7 days before behavioral testing. Rats were trained to lever press for heroin (60 μg/kg/infusion) 1 h per day, on a fixed-ratio (FR) 1 schedule, 5 days per week. Drug infusion was paired with a cue light (above the active lever) for 20 s signaling a time-out period. Presses during the time-out period were recorded but no drug was delivered. Once stable lever press responding was achieved, the rats were split in two groups matched for responding (baseline): short-access (1 h session: ShA) or long-access (12 h session: LgA), to test mechanical sensitivity and intake. For the prophylactic drug regimen study (Figure 4), rats were split into three pretreatment groups with 8 h long-access sessions. During all self-administration sessions, rats were allowed to nose-poke for food (45 mg pellets, Bio-Serve) on an FR 3 schedule, and water under an FR 1 schedule.
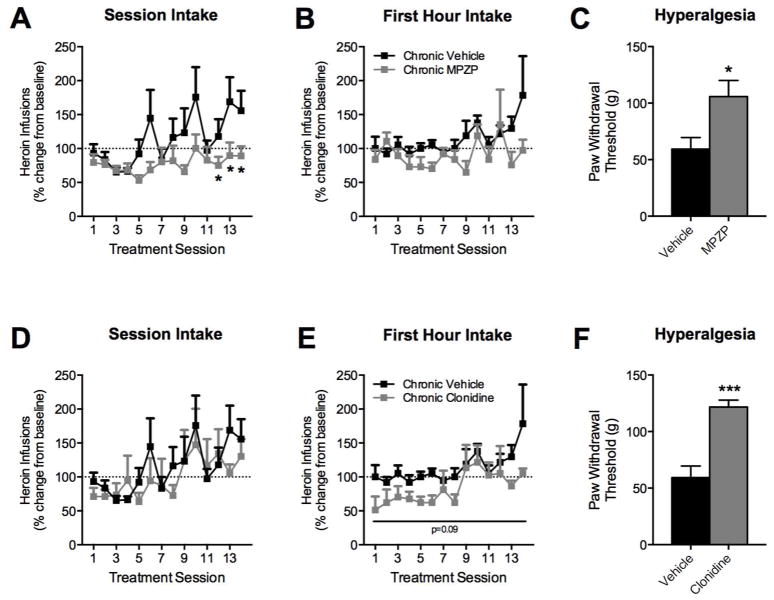
Figure 4.
Chronic CRF1R antagonism prevents escalation of heroin self-administration and resultant hyperalgesia. Rats were trained to lever press for heroin and split into three groups (n = 6 each) receiving repeated treatments of vehicle, MPZP, or clonidine prior to each eight-hour self-administration session. Heroin intake is expressed as percent change from chronic vehicle-treated animals on treatment day one. Paw withdrawal thresholds were measured 10–12 h following the last heroin self-administration session (approximately 18–20 h after the last drug treatment). Over 14 treatment sessions, vehicle-treated rats displayed a gradual increase in heroin intake over the entire session that significantly diverged from the chronic MPZP-treated rats by the end of the training (A–B). A significant difference between vehicle and MPZP groups was present on days 12, 13, and 14. (* p < 0.05). Animals treated chronically with MPZP also displayed higher paw withdrawal thresholds, reflecting an absence of heroin-induced hyperalgesia (C). In comparison, heroin self-administering animals treated chronically with clonidine did not display a significant difference in intake from the vehicle group across the entire eight-hour session (D) but did exhibit a trend (p = 0.09) for decreased heroin intake over the first hour of self-administration (E). Despite similar heroin intake levels between groups, clonidine-treated animals exhibited higher paw withdrawal thresholds (F), suggesting a reduction of heroin-induced hyperalgesia in this group relative to animals receiving chronic vehicle treatments.
Pharmacological Testing
All pretreatment times and doses were derived from published studies (see discussion). For the acute dependence studies, rats were injected with heroin or saline 24 h prior to testing, and then pretreated with drugs or vehicles at different time points. Clonidine (10, 50 μg/kg) or MPZP (20 mg/kg) was given 60 min prior to paw withdrawal testing. Prazosin (2 mg/kg) or propranolol (10 mg/kg) was given 30 min prior to paw withdrawal testing. For the self-administration study, vehicle (20% HBC), clonidine (10 μg/kg), or MPZP (20 mg/kg) was injected SC immediately prior to each self-administration session.
Statistical Analysis
All data are expressed as means and standard errors of the mean (SEM). Data were analyzed using a one-way analysis of variance (ANOVA) with group (repeated saline, repeated heroin) as a between-subjects factor or using a repeated-measure two-way ANOVA with group as a between-subjects factor and treatment (clonidine, MPZP, prazosin, or propranolol vs. vehicle) or time as within-subjects factors. Student’s t-tests were used to analyze paw withdrawal thresholds in heroin self-administering animals following chronic vehicle vs. drug (clonidine or MPZP) treatment. Fisher’s Least Significant Difference (LSD) tests or Dunnett’s tests were used for post hoc comparisons when appropriate. All statistical analyses were performed with Prism 6.0 or Statistica 10, and p-values less than 0.05 were considered statistically significant.
Results
Acute heroin dependence induces mechanical hypersensitivity
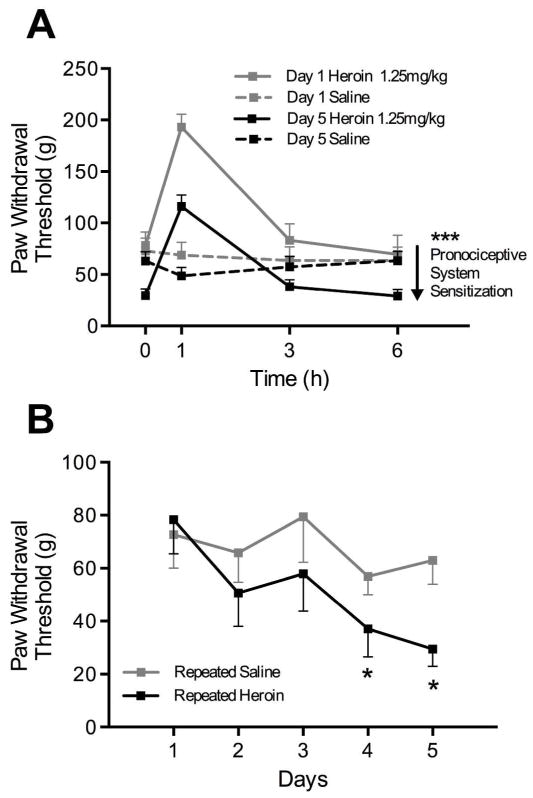
Figure 1A shows the development of mechanical hypersensitivity following a series of intermittent heroin injections (1.25 mg/kg, SC). Rats were tested 1, 3, and 6 h after heroin injections on day 1 and day 5, in addition to 24 h after daily heroin administration for five days. Comparing the heroin-treated rats between day 1 and day 5, the ANOVA revealed an overall group effect (F(1, 14) = 19.09, p < 0.001) as well as a time effect (F(3, 42) = 51.2, p < 0.0001). Thus, heroin produced a significant modification of paw withdrawal thresholds that shifted downward over days, indicative of a sensitization of pronociceptive systems (Celerier et al., 2001). Figure 1B depicts the lowering of paw withdrawal thresholds over several days of repeated heroin administration. One-way ANOVA revealed a day effect for heroin (F(4, 24) = 5.03, p < 0.005). Post hoc comparisons showed a significant difference on day 4 and 5 compared to day 1 (p < 0.05). No effect was seen for the vehicle group (F (4, 28) = 1.15, p = 0.35).
Figure 1.
(A) Mechanical sensitivity responsiveness following single vs. repeated heroin exposure. Rats were administered heroin (1.25 mg/kg, SC, n = 8) for five days and tested for paw withdrawal thresholds 1, 3, and 6 h post-heroin injection on day 1 and day 5. The magnitude of the increase in threshold at 1 h is lessened on day 5, while the thresholds at 0 h and 6 h are below day 1, indicating hyperalgesia. These neuroadaptations are hypothesized by Simonnet and colleagues to reflect a sensitization of pronociceptive systems (Celerier et al., 2001). Asterisks indicate a significant group effect (*** p < 001) between acute vs. chronic heroin-treated groups. (B) Paw withdrawal thresholds of heroin-treated rats continue to decrease over five days. On days 4 and 5, thresholds are significantly lower compared to baseline (* p < 0.05).
Paw withdrawal thresholds decrease as heroin intake escalates
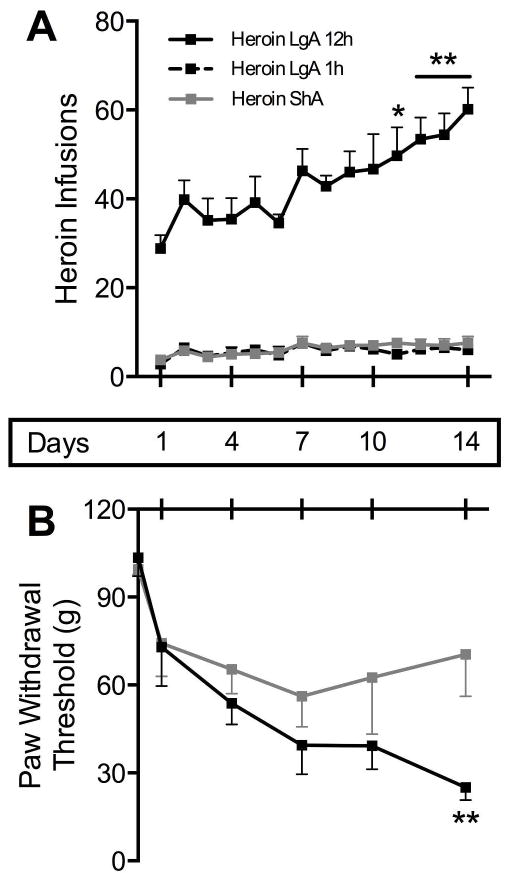
Figure 2 represents the development of hyperalgesia during heroin self-administration. Heroin intake data (A) show that LgA rats increase the number of heroin infusions while the ShA rats maintain a low, stable number of infusions. Average daily intake in LgA rats over the fourteen sessions was 2.62 mg/kg/day versus 0.37 mg/kg/day in ShA rats. The ANOVA revealed a group × session interaction: F (13, 130) = 2.12, p < 0.05). Dunnett’s post-hoc analysis showed a significant escalation of intake in the LgA group over the entire session starting on day 11 (p < 0.05) until day 14 (p < 0.01) compared to day 1. LgA animals did not escalate intake in the first hour of the session as previously described (Ahmed, Walker, and Koob, 2000). One potential explanation for this discrepancy is that food reinforcement was concurrently available in our operant protocol, which may attenuate intake escalation (Lenoir and Ahmed, 2008; Ahmed et al., 2013). We tracked mechanical sensitivity in parallel with heroin intake escalation (B). Paw withdrawal thresholds were measured 10–12 h following the previous heroin self-administration session (i.e., just prior to subsequent sessions). The ANOVA revealed an overall group effect between ShA and LgA (F(1, 11) = 4.86, p < 0.05) and a day effect (F(5, 55) = 7.55, p < 0.0001). On day 1, both ShA and LgA rats tended to show a decrease in paw withdrawal thresholds. After day 1, however, thresholds for ShA rats become stable, whereas those for LgA rats continue to decrease. By day 14, the thresholds for ShA rats significantly differ from those of LgA rats (p < 0.005).
Figure 2.
Gradual development of mechanical hyperalgesia across heroin self-administration history. Paw withdrawal thresholds were measured in heroin self-administering rats over the escalation period just prior to subsequent self-administration sessions. As long-access (Heroin LgA 12h, n = 8) rats increase their heroin intake across subsequent twelve-hour sessions (A), thresholds continue to decrease over time (B). Heroin intake for LgA rats increased significantly over the entire session (but not in the first hour, Heroin LgA 1h) compared to baseline on days 11–14 (* p < 0.05, ** p < 0.01), and paw withdrawal thresholds were different from short-access (Heroin ShA, n = 5) rats on day 14 (** p < 0.01). Paw withdrawal thresholds of ShA rats do not decrease significantly compared to baseline, and their heroin intake remains relatively constant (averaging 0.37 mg/kg/day).
MPZP reverses acute heroin dependence-induced mechanical hypersensitivity
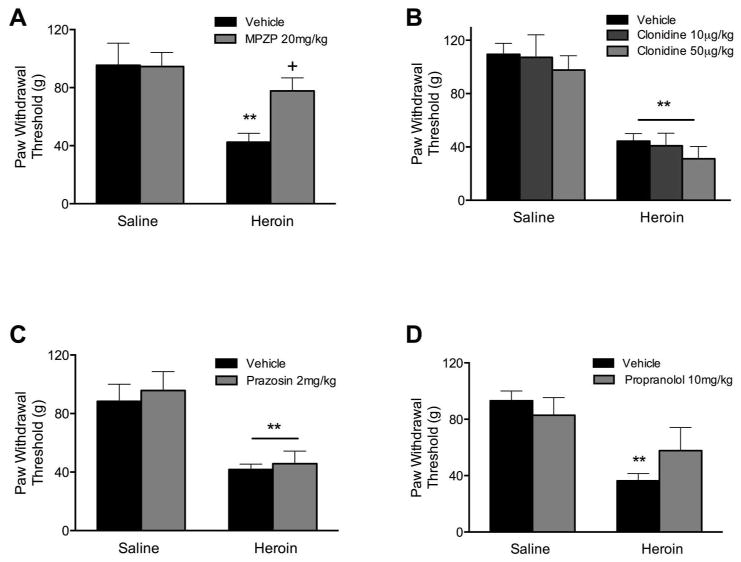
Figure 3A represents the effects of MPZP on heroin-induced decreases in paw withdrawal thresholds. MPZP significantly alleviated the hyperalgesia during acute dependence. The ANOVA revealed a treatment ×group interaction (F(1, 14) = 6.13, p < 0.05). Post-hoc analysis revealed that the heroin-vehicle group differed from the heroin-MPZP group (p < 0.05) and that the heroin-vehicle group was different from the saline-vehicle group (p < 0.0001). Figure 3B shows the effect of clonidine at two different doses on paw withdrawal thresholds during acute heroin dependence. The ANOVA revealed only a main heroin effect in the context of clonidine at doses of 10 μg (F(1, 14) = 16.82, p < 0.005) or 50 μg (F(1, 14) = 42.00, p < 0.0001). Figure 3C shows that prazosin also did not reverse the lowered paw withdrawal thresholds during acute heroin dependence (main effect of heroin: F(1, 14) = 13.89, p < 0.005). Finally, Figure 3D shows the effect of propranolol on paw withdrawal thresholds during acute dependence. Although there seems to be a slight reversal of the heroin-lowered thresholds, the ANOVA revealed that only the factor of heroin exposure significantly lowered thresholds (F(1, 12) = 17.65, p < 0.005).
Figure 3.
CRF1R antagonism, but not functional adrenergic receptor antagonism, reverses heroin withdrawal-induced mechanical hypersensitivity during acute dependence. For all tests, heroin-treated rats (n = 7–8/group) showed significantly lower paw withdrawal thresholds compared to saline-treated rats (** p < 0.005). (A) MPZP treatment significantly raised thresholds, indicating an alleviation of mechanical hypersensitivity (+ p < 0.05). (B–D) Neither clonidine (10 μg/kg, 50 μg/kg, SC), prazosin (2mg/kg, IP), nor propranolol (10 mg/kg, IP) alleviated the acute heroin dependence-induced mechanical hypersensitivity.
MPZP attenuates escalation of heroin self-administration and hyperalgesia
Rats were trained to lever press for heroin and split into three groups (n = 6) according to the first long access (eight-hour) session intake (baseline session). A pretreatment of vehicle, MPZP, or clonidine was given prior to each self-administration treatment session for a total of 14 treatment sessions. Paw withdrawal thresholds were measured 10–12 h following the last heroin self-administration session (approximately 18–20 h after the last drug treatment). Figure 4A represents the percent change in heroin intake for rats treated with MPZP or vehicle. The ANOVA revealed an interaction between group and day (F(13, 130) = 2.63, p < 0.005). Post-hoc analyses indicated that vehicle-treated rats exhibited a significant increase in heroin intake compared to MPZP-treated rats on days 12, 13, and 14 (p < 0.05). This effect was not present in the first hour of heroin intake (Figure 4B). Rats treated chronically with MPZP also displayed significantly higher paw withdrawal thresholds (t10 = 2.642, p < 0.05; Figure 4C). Figure 4D–E shows the comparison between the same vehicle group and the chronic clonidine group. No significant differences were observed in heroin intake over the entire session, although a trend for decreased heroin intake during the first hour of self-administration was observed (main effect of group, F(1,10) = 3.59, p= 0.09). Despite similar levels of heroin intake, animals treated chronically with clonidine displayed significantly higher paw withdrawal thresholds (t10 = 5.232, p < 0.001; Figure 4F).
Discussion
In accordance with previous reports, we found a significant mechanical hypersensitivity associated with both acute heroin dependence and heroin intake escalation. In contrast, hyperalgesia was not observed in animals self-administering a limited amount of heroin (0.37 mg/kg/day average). The CRF1 receptor antagonist MPZP alleviated mechanical hypersensitivity in the acute dependence model, further confirming the role of this drug class in alleviating hyperalgesia resulting from either injury (Ji and Neugebauer, 2007) or repeated exposure to opioids (Edwards et al., 2012; McNally and Akil, 2002).
In contrast to CRF1R antagonism, a variety of drugs systemically blocking adrenergic neurotransmission did not significantly alter paw withdrawal threshold reductions associated with acute heroin dependence. This represents a significant dissociation from the observed anti-hyperalgesic effects of adrenoceptor blockade (at equivalent doses) across multiple pain models. For example, systemic administration of prazosin (2 mg/kg) alleviates subcutaneous hindpaw bee venom-induced mechanical hyperalgesia (Chen et al., 2010), while even lower doses are effective against cold allodynia in a neuropathic pain model (Kim et al., 2005). Chronic administration of a lower dose (0.3 mg/kg) of prazosin delays the development of both streptozotocin- and vincristine-induced hyperalgesia, models of diabetic and toxic neuropathy, respectively (Bujalska et al., 2008). Similarly, doses of propranolol identical to or lower than that employed here (i.e., 1–10 mg/kg) alleviate pain hypersensitivity following catechol-O-methyltransferase inhibition (Nackley et al., 2007) or intraplantar endotoxin administration (Safieh-Garabedian et al., 2002). Our data showed a trend for a reversal of acute heroin-induced mechanical hypersensitivity following administration of 10 mg/kg propranolol. Possible effects with higher doses might be due to unspecific binding and side effects rather than specific blockade of beta-receptors. The expression of chronic opioid-induced hyperalgesia has been tied to beta-2-adrenergic receptor activity (Liang et al., 2006), and this receptor has also been linked to opioid tolerance and chronic dependence (Liang et al., 2007), suggesting that potentiation of this system may occur in response to repeated opioid exposure. In addition, it was recently found that hyperalgesia induced by termination of remifentanil exposure in humans was attenuated by concurrent propranolol exposure (Chu et al., 2012), suggesting that ongoing beta-adrenoceptor blockade during opioid exposure may be more effective in reducing the resultant hyperalgesia.
Alpha-2-adrenergic agonists such as clonidine possess valuable analgesic activity, reduce postoperative opioid consumption (Blaudszun et al., 2012), but also display a narrow therapeutic window and can produce significant side effects (Martin and Eisenach, 2001). Thermal- or capsaicin-induced pain relief occurs following intrathecal, but not intravenous, clonidine administration in humans (Eisenach et al., 1998). Interestingly, in rats, spinal clonidine administration also produces a conditioned place preference (a model of reward) only in animals experiencing pain (Davoody et al., 2011; He et al., 2012; King et al., 2009). For the present study, we administered relatively low clonidine doses below the presumptive analgesic dose range. Indeed, neither the doses of clonidine nor any dose of the other compounds examined increased paw withdrawal thresholds in vehicle-treated animals, which likely represents an absence of analgesic or motor-disruptive effects typically observed following acute administration of opioid analgesics (e.g., Stowe et al., 2011). Instead, clonidine appears to regulate opioid withdrawal-related behaviors at the doses tested (10–50 μg/kg). For example, clonidine (50 μg/kg) reduces naloxone-precipitated physical withdrawal symptoms after chronic morphine exposure (Tierney et al., 1988). Clonidine administered at 10–40 μg/kg also blocks stress-induced reinstatement to heroin-seeking behavior (Shaham et al., 2000). However, this range of dosing failed to alleviate acute heroin dependence-induced hyperalgesia, suggesting that potentiation of noradrenergic signaling may occur only after extensive or excessive opioid exposure to regulate pain-related behaviors (see below).
Previous studies have found that functional noradrenergic receptor blockade reduces both anxiety-like behavior (Park et al., 2013) and heroin intake in dependent rats for the first hour of extended access in animals where escalation of intake had already been established (Greenwell et al., 2009). Both CRF and NE increase in the extended amygdala during drug withdrawal (Maldonado, 1997; Weiss et al., 2001) and activation of both of these brain stress systems is thought to mediate the negative emotional states that manifest during opioid withdrawal. It is thought that a CRF-NE feed forward brain stress system exists where CRF from the extended amygdala activates brainstem NE activity and, in turn, NE activates forebrain CRF activity (Koob, 1999). The effects of CRF during opioid withdrawal seem consistent throughout different behavioral measures as the CRF1R antagonist MPZP blocks withdrawal-potentiated startle (Park et al., 2013). In addition, adrenergic blockers have been shown to decrease acute opioid dependence-potentiated startle in rats (Harris and Gewirtz, 2004; Park et al., 2013; Rothwell et al., 2009). These results suggest a significant dissociation between pain- and anxiety-like behaviors during acute dependence, along with differences in the neuropharmacological mechanisms that mediate these states. Our results also appear to suggest unique roles for adrenergic receptor signaling across different pain models (i.e., injury vs. drug-induced hyperalgesia), and further comparative exploration in these areas is warranted.
Chronic, prophylactic administration of MPZP (20 mg/kg) attenuated escalation of heroin self-administration, indicating a possible link between CRF and early neuroadaptive mechanisms associated with the eventual transition to heroin addiction. For this experiment we employed eight-hour heroin self-administration sessions that were expected to produce a more modest escalation of heroin intake compared to twelve-hour sessions (Vendruscolo et al., 2011). CRF1R antagonist-mediated decreases in heroin intake in LgA rats are thought to occur due to the attenuation of negative reinforcement processes that accompany LgA heroin intake (Chen et al., 2006; Greenwell et al., 2009; Kenny et al., 2006; Koob et al., 2004). In contrast, chronic treatment with clonidine (10 ug/kg) failed to alter heroin intake over the eight-hour sessions, although there was a trend for a reduction in intake during the first hour of self-administration in accord with clonidine’s half-life (Conway and Jarrott, 1980). Future studies will determine the effects of clonidine administration over the entire course of the heroin self-administration session. Not surprisingly, animals treated chronically with MPZP (who self-administered less heroin) exhibited higher paw withdrawal thresholds, indicative of an absence of hyperalgesia. These data confirm and extend previous findings from our lab and others demonstrating that CRF1R blockade reduces opioid withdrawal-induced hyperalgesia and attenuates heroin intake post-escalation (Edwards et al., 2012; Greenwell et al., 2009; McNally and Akil, 2002). In contrast, CRF1R antagonism does not affect food or water intake (Greenwell et al., 2009). It is noteworthy that animals receiving chronic clonidine treatments fail to display hyperalgesia (Figure 4F) despite self-administering similar amounts of heroin as chronic vehicle-treated animals over the entire session (Figure 4D). These latter results suggest that a recruitment of noradrenergic signaling evolves with extensive heroin exposure to drive hyperalgesia, possibly via synergistic interactions with potentiated CRF signaling in the CeA (Ji and Neugebauer, 2007; Koob, 1999).
The observed effects of clonidine also accord with a multi-system view of opioid addiction whereby altered central negative reinforcement mechanisms may continue to drive compulsive drug seeking despite an apparent alleviation of physical withdrawal symptoms (Schulteis and Koob, 1996; Self and Nestler, 1998). Moreover, as a pathological extension of hyperalgesia along the neuraxis, a greater interaction of supraspinal pain and reinforcement circuitry to produce pain-induced emotional dysregulation (termed hyperkatifeia) may be necessary to drive opioid intake escalation (Ji et al., 2007; Shurman et al., 2010). In this regard, our data suggest that blockade of CRF1R signaling would be an effective and comprehensive therapeutic strategy aimed at this critical association of pain and motivational processes underlying opioid addiction (Weiss et al., 2001; Edwards and Koob, 2010).
Acknowledgments
This is publication number 24026 from The Scripps Research Institute. Research was financially supported by National Institutes of Health grant DA04043 from the National Institute on Drug Abuse (NIDA) and the Pearson Center for Alcoholism and Addiction Research. Training for PEP was provided by NIDA grant T32DA007315. Training for JES was provided by NIAAA grant T32AA007456. Salary support for GS was partially provided by a Research Career Scientist Award from the Biomedical Laboratory Research and Development Program, Veterans Health Administration. The authors thank Dr. Kim Janda (TSRI) for his generous gift of MPZP, Dr. Tony Yaksh (UCSD) for helpful discussions related to this work, and Michael Arends for editorial assistance.
Footnotes
Author Contributions
PEP, JES, and LFV performed the behavioral experiments and analyzed the data. PEP, JES, LFV, GS, SE, and GFK were responsible for the study concept, design, and interpretation of findings. PEP drafted the manuscript. JES, LFV, GS, SE, and GFK provided critical revision of the manuscript. All authors critically reviewed the content and approved the final version for publication.
References
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Lenoir M, Guillem K. Neurobiology of addiction versus drug use driven by lack of choice. Curr Opin Neurobiol. 2013;23:581–587. doi: 10.1016/j.conb.2013.01.028. [DOI] [PubMed] [Google Scholar]
- Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis. A target site for noradrenergic actions in opiate withdrawal. Ann N Y Acad Sci. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- Azar MR, Jones BC, Schulteis G. Conditioned place aversion is a highly sensitive index of acute opioid dependence and withdrawal. Psychopharmacology (Berl) 2003;170:42–50. doi: 10.1007/s00213-003-1514-y. [DOI] [PubMed] [Google Scholar]
- Blaudszun G, Lysakowski C, Elia N, Tramer MR. Effect of perioperative systemic alpha2 agonists on postoperative morphine consumption and pain intensity: systematic review and meta-analysis of randomized controlled trials. Anesthesiology. 2012;116:1312–1322. doi: 10.1097/ALN.0b013e31825681cb. [DOI] [PubMed] [Google Scholar]
- Bujalska M, Arazna M, Makulska-Nowak H, Gumulka SW. Alpha(1) and alpha(2)-adrenoreceptor antagonists in streptozotocin- and vincristine-induced hyperalgesia. Pharmacological reports: PR. 2008;60:499–507. [PubMed] [Google Scholar]
- Celerier E, Laulin JP, Corcuff JB, Le Moal M, Simonnet G. Progressive enhancement of delayed hyperalgesia induced by repeated heroin administration: a sensitization process. J Neurosci. 2001;21:4074–4080. doi: 10.1523/JNEUROSCI.21-11-04074.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chen HS, Qu F, He X, Wang Y, Wen WW. Chemical or surgical sympathectomy prevents mechanical hyperalgesia induced by intraplantar injection of bee venom in rats. Brain Res. 2010;1353:86–93. doi: 10.1016/j.brainres.2010.07.069. [DOI] [PubMed] [Google Scholar]
- Chen SA, O’Dell LE, Hoefer ME, Greenwell TN, Zorrilla EP, Koob GF. Unlimited access to heroin self-administration: independent motivational markers of opiate dependence. Neuropsychopharmacology. 2006;31:2692–2707. doi: 10.1038/sj.npp.1301008. [DOI] [PubMed] [Google Scholar]
- Chu LF, Cun T, Ngai LK, Kim JE, Zamora AK, Young CA, Angst MS, Clark DJ. Modulation of remifentanil-induced postinfusion hyperalgesia by the beta-blocker propranolol in humans. Pain. 2012;153:974–981. doi: 10.1016/j.pain.2012.01.014. [DOI] [PubMed] [Google Scholar]
- Compton P, Charuvastra VC, Ling W. Pain intolerance in opioid-maintained former opiate addicts: effect of long-acting maintenance agent. Drug Alcohol Depend. 2001;63:139–146. doi: 10.1016/s0376-8716(00)00200-3. [DOI] [PubMed] [Google Scholar]
- Conway EL, Jarrott B. Clonidine distribution in the rat: temporal relationship between tissue levels and blood pressure response. Br J Pharmacol. 1980;71:473–478. doi: 10.1111/j.1476-5381.1980.tb10960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoody L, Quiton RL, Lucas JM, Ji Y, Keller A, Masri R. Conditioned place preference reveals tonic pain in an animal model of central pain. J Pain. 2011;12:868–874. doi: 10.1016/j.jpain.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney AJ, Crane JW, Sah P. Noradrenaline modulates transmission at a central synapse by a presynaptic mechanism. Neuron. 2007;56:880–892. doi: 10.1016/j.neuron.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Edwards S, Graham DL, Whisler KN, Self DW. Phosphorylation of GluR1, ERK, and CREB during spontaneous withdrawal from chronic heroin self-administration. Synapse. 2009;63:224–235. doi: 10.1002/syn.20601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Koob GF. Neurobiology of dysregulated motivational systems in drug addiction. Future Neurol. 2010;5:393–401. doi: 10.2217/fnl.10.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Koob GF. Escalation of drug self-administration as a hallmark of persistent addiction liability. Behav Pharmacol. 2013;24:356–362. doi: 10.1097/FBP.0b013e3283644d15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Vendruscolo LF, Schlosburg JE, Misra KK, Wee S, Park PE, Schulteis G, Koob GF. Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: alleviation by CRF(1) receptor antagonism. Neuropharmacology. 2012;62:1142–1151. doi: 10.1016/j.neuropharm.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli M, Koob GF, Edwards S. Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev. 2012;36:2179–2192. doi: 10.1016/j.neubiorev.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach JC, Hood DD, Curry R. Intrathecal, but not intravenous, clonidine reduces experimental thermal or capsaicin-induced pain and hyperalgesia in normal volunteers. Anesthesia and analgesia. 1998;87:591–596. doi: 10.1097/00000539-199809000-00018. [DOI] [PubMed] [Google Scholar]
- Fields HL. The doctor’s dilemma: opiate analgesics and chronic pain. Neuron. 2011;69:591–594. doi: 10.1016/j.neuron.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbain DA, Cole B, Lewis J, Rosomoff HL, Rosomoff RS. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain Med. 2008;9:444–459. doi: 10.1111/j.1526-4637.2007.00370.x. [DOI] [PubMed] [Google Scholar]
- Greenwell TN, Funk CK, Cottone P, Richardson HN, Chen SA, Rice KC, Zorrilla EP, Koob GF. Corticotropin-releasing factor-1 receptor antagonists decrease heroin self-administration in long- but not short-access rats. Addict Biol. 2009;14:130–143. doi: 10.1111/j.1369-1600.2008.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Gewirtz JC. Elevated startle during withdrawal from acute morphine: a model of opiate withdrawal and anxiety. Psychopharmacology (Berl) 2004;171:140–147. doi: 10.1007/s00213-003-1573-0. [DOI] [PubMed] [Google Scholar]
- He Y, Tian X, Hu X, Porreca F, Wang ZJ. Negative reinforcement reveals non-evoked ongoing pain in mice with tissue or nerve injury. J Pain. 2012;13:598–607. doi: 10.1016/j.jpain.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs SC, Menzaghi F, Schulteis G, Koob GF, Stinus L. Suppression of corticotropin-releasing factor in the amygdala attenuates aversive consequences of morphine withdrawal. Behav Pharmacol. 1995;6:74–80. [PubMed] [Google Scholar]
- Ji G, Fu Y, Ruppert KA, Neugebauer V. Pain-related anxiety-like behavior requires CRF1 receptors in the amygdala. Molecular pain. 2007;3:13. doi: 10.1186/1744-8069-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G, Neugebauer V. Differential effects of CRF1 and CRF2 receptor antagonists on pain-related sensitization of neurons in the central nucleus of the amygdala. J Neurophysiol. 2007;97:3893–3904. doi: 10.1152/jn.00135.2007. [DOI] [PubMed] [Google Scholar]
- Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an internet-based survey. J Pain. 2010;11:1230–1239. doi: 10.1016/j.jpain.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci. 2006;26:5894–5900. doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SK, Min BI, Kim JH, Hwang BG, Yoo GY, Park DS, Na HS. Effects of alpha1- and alpha2-adrenoreceptor antagonists on cold allodynia in a rat tail model of neuropathic pain. Brain Res. 2005;1039:207–210. doi: 10.1016/j.brainres.2005.01.051. [DOI] [PubMed] [Google Scholar]
- King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields HL, Porreca F. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12:1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O’Dell LE, Parsons LH, Sanna PP. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Laulin JP, Larcher A, Celerier E, Le Moal M, Simonnet G. Long-lasting increased pain sensitivity in rat following exposure to heroin for the first time. Eur J Neurosci. 1998;10:782–785. doi: 10.1046/j.1460-9568.1998.00083.x. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Ahmed SH. Supply of a nondrug substitute reduces escalated heroin consumption. Neuropsychopharmacology. 2008;33:2272–2282. doi: 10.1038/sj.npp.1301602. [DOI] [PubMed] [Google Scholar]
- Liang DY, Liao G, Wang J, Usuka J, Guo Y, Peltz G, Clark JD. A genetic analysis of opioid-induced hyperalgesia in mice. Anesthesiology. 2006;104:1054–1062. doi: 10.1097/00000542-200605000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang DY, Shi X, Li X, Li J, Clark JD. The beta2 adrenergic receptor regulates morphine tolerance and physical dependence. Behav Brain Res. 2007;181:118–126. doi: 10.1016/j.bbr.2007.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Schulteis G. Brain reward deficits accompany naloxone-precipitated withdrawal from acute opioid dependence. Pharmacol Biochem Behav. 2004;79:101–108. doi: 10.1016/j.pbb.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Maldonado R. Participation of noradrenergic pathways in the expression of opiate withdrawal: biochemical and pharmacological evidence. Neurosci Biobehav Rev. 1997;21:91–104. doi: 10.1016/0149-7634(95)00061-5. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Eisenach JC. Pharmacology of opioid and nonopioid analgesics in chronic pain states. J Pharmacol Exp Ther. 2001;299:811–817. [PubMed] [Google Scholar]
- Martin TJ, Kim SA, Buechler NL, Porreca F, Eisenach JC. Opioid self-administration in the nerve-injured rat: relevance of antiallodynic effects to drug consumption and effects of intrathecal analgesics. Anesthesiology. 2007;106:312–322. doi: 10.1097/00000542-200702000-00020. [DOI] [PubMed] [Google Scholar]
- McNally GP, Akil H. Role of corticotropin-releasing hormone in the amygdala and bed nucleus of the stria terminalis in the behavioral, pain modulatory, and endocrine consequences of opiate withdrawal. Neuroscience. 2002;112:605–617. doi: 10.1016/s0306-4522(02)00105-7. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Tan KS, Fecho K, Flood P, Diatchenko L, Maixner W. Catechol-O-methyltransferase inhibition increases pain sensitivity through activation of both beta2- and beta3-adrenergic receptors. Pain. 2007;128:199–208. doi: 10.1016/j.pain.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park PE, Vendruscolo LF, Schlosburg JE, Edwards S, Schulteis G, Koob GF. Corticotropin-releasing factor (CRF) and alpha 2 adrenergic receptors mediate heroin withdrawal-potentiated startle in rats. Int J Neuropsychopharmacol. 2013:1–9. doi: 10.1017/S1461145713000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Zhao Y, Fekete EM, Funk CK, Wirsching P, Janda KD, Zorrilla EP, Koob GF. MPZP: a novel small molecule corticotropin-releasing factor type 1 receptor (CRF1) antagonist. Pharmacol Biochem Behav. 2008;88:497–510. doi: 10.1016/j.pbb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ririe DG, Eisenach JC. Age-dependent responses to nerve injury-induced mechanical allodynia. Anesthesiology. 2006;104:344–350. doi: 10.1097/00000542-200602000-00021. [DOI] [PubMed] [Google Scholar]
- Rothwell PE, Thomas MJ, Gewirtz JC. Distinct profiles of anxiety and dysphoria during spontaneous withdrawal from acute morphine exposure. Neuropsychopharmacology. 2009;34:2285–2295. doi: 10.1038/npp.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safieh-Garabedian B, Poole S, Haddad JJ, Massaad CA, Jabbur SJ, Saade NE. The role of the sympathetic efferents in endotoxin-induced localized inflammatory hyperalgesia and cytokine upregulation. Neuropharmacology. 2002;42:864–872. doi: 10.1016/s0028-3908(02)00028-x. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Heyser CJ, Koob GF. Differential expression of response-disruptive and somatic indices of opiate withdrawal during the initiation and development of opiate dependence. Behav Pharmacol. 1999;10:235–242. doi: 10.1097/00008877-199905000-00001. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Koob GF. Reinforcement processes in opiate addiction: a homeostatic model. Neurochemical research. 1996;21:1437–1454. doi: 10.1007/BF02532385. [DOI] [PubMed] [Google Scholar]
- Self DW, Nestler EJ. Relapse to drug-seeking: neural and molecular mechanisms. Drug Alcohol Depend. 1998;51:49–60. doi: 10.1016/s0376-8716(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Highfield D, Delfs J, Leung S, Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. Eur J Neurosci. 2000;12:292–302. doi: 10.1046/j.1460-9568.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- Shurman J, Koob GF, Gutstein HB. Opioids, pain, the brain, and hyperkatifeia: a framework for the rational use of opioids for pain. Pain Med. 2010;11:1092–1098. doi: 10.1111/j.1526-4637.2010.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonnet G, Rivat C. Opioid-induced hyperalgesia: abnormal or normal pain? Neuroreport. 2003;14:1–7. doi: 10.1097/00001756-200301200-00001. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain structure & function. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe GN, Vendruscolo LF, Edwards S, Schlosburg JE, Misra KK, Schulteis G, Mayorov AV, Zakhari JS, Koob GF, Janda KD. A vaccine strategy that induces protective immunity against heroin. Journal of medicinal chemistry. 2011;54:5195–5204. doi: 10.1021/jm200461m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney C, Nadaud D, Koenig-Berard E, Stinus L. Effects of two alpha 2 agonists, rilmenidine and clonidine, on the morphine withdrawal syndrome and their potential addictive properties in rats. The American journal of cardiology. 1988;61:35D–38D. doi: 10.1016/0002-9149(88)90462-6. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Schlosburg JE, Misra KK, Chen SA, Greenwell TN, Koob GF. Escalation patterns of varying periods of heroin access. Pharmacol Biochem Behav. 2011;98:570–574. doi: 10.1016/j.pbb.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Schulteis G. Withdrawal from acute morphine dependence is accompanied by increased anxiety-like behavior in the elevated plus maze. Pharmacol Biochem Behav. 2008;89:392–403. doi: 10.1016/j.pbb.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]