Abstract
Lithocholic acid (LCA) supplementation in the diet results in intrahepatic cholestasis and bile infarcts. Previously we showed that an innate immune response is critical for cholestatic liver injury in the bile duct ligated mice. Thus, the purpose of this study was to investigate the role of neutrophils in the mechanism of liver injury caused by feeding mice a diet containing LCA. C57BL/6 mice were given control or 1% LCA containing diet for 24–96h and then examined for parameters of hepatotoxicity. Plasma ALT levels were significantly increased by 48h after LCA feeding, which correlated with both neutrophil recruitment to the liver and upregulation of numerous pro-inflammatory genes. The injury was confirmed by histology. Deficiency in intercellular adhesion molecule-1 (ICAM-1) expression or inhibition of neutrophil function failed to protect against the injury. Bile acid levels were quantified in plasma and bile of LCA-fed mice after 48 and 96h. Only the observed biliary levels of taurochenodeoxycholic acid and potentially tauro-LCA caused direct cytotoxicity in mouse hepatocytes. These data support the conclusion that neutrophil recruitment occurs after the onset of bile acid-induced necrosis in LCA-fed animals, and is not a primary mechanism of cell death when cholestasis occurs through accumulation of hydrophobic bile acids.
Keywords: bile acid hepatotoxicity, lithocholic acid, cholestasis, innate immunity, neutrophils
1. INTRODUCTION
Cholestasis occurs as a result of a number of different pathologies including obstruction of the common bile duct by gall stones, biliary atresia, compression of the common bile duct from tumor growths such as cholangiocarcinoma, or during intrahepatic cholestasis of pregnancy (Zollner and Trauner, 2008; Jüngst and Lammert, 2013). During cholestasis, bile acids accumulate in hepatocytes, which have the potential to cause cytotoxicity (Guicciardi and Gores, 2002; Perez and Briz, 2009). Studies with rat hepatocytes have shown that high concentrations of toxic bile acids such as glycochenodeoxycholic acid (GCDCA) or taurolithocholic acid (TLCA) result in hepatocellular apoptosis mediated by mitochondrial and lysosomal dysfunction (Spivey et al., 1993; Botla et al., 1995; Graf et al., 2002). However, more recent data suggest these bile acids might not reach concentrations during obstructive cholestasis that result in hepatocellular toxicity in vivo (Trottier et al., 2011, 2012; Zhang et al., 2012), leading to alternate hypotheses for mechanisms of injury (Woolbright and Jaeschke, 2012).
Liver injury induced by obstructive cholestasis (bile duct ligation, BDL) in rodents is characterized by areas of focal necrosis (bile infarcts) and extensive neutrophil accumulation (Kountouras et al., 1984; Saito and Maher, 2000; Gujral et al., 2003). Animals with impaired neutrophil function developed significantly less liver injury after BDL suggesting neutrophils caused the majority of the cell damage (Gujral et al., 2003; 2004b). Prerequisite for neutrophil-induced liver injury is the activation and recruitment of neutrophils into sinusoids and a chemotactic signal for extravasation into the parenchyma (Jaeschke and Smith, 1997; Jaeschke and Hasegawa, 2006). Recent studies demonstrated that cleaved osteopontin in bile is a critical chemotactic factor for the early neutrophil-induced injury mechanisms after BDL (Yang et al., 2014). The increased biliary pressure during obstructive cholestasis results in rupture of cholangioles causing the leakage of bile back into the parenchyma, which is responsible for the focal nature of the liver damage (Fickert et al., 2002). In addition, the exposure of hepatocytes to high levels of the typical bile acids present in mice, i.e. taurocholic acid (TCA), β-muricholic acid (βMCA) and TβMCA, does not cause directly cell death (Allen et al., 2011; Zhang et al., 2012). However, these bile acids trigger intercellular adhesion molecule-1 (ICAM-1) gene expression in hepatocytes and CXC chemokine formation that provides an additional chemotactic gradient for neutrophil recruitment (Allen et al., 2011). Thus, the acute liver injury during obstructive cholestasis in mice is a neutrophilic inflammatory injury triggered by the biliary leakage of osteopontin and the generation of CXC chemokines by hepatocytes exposed to bile acids. Given the strong evidence for a neutrophil-mediated liver injury after BDL (Gujral et al., 2003, 2004b; Kim et al., 2006; O’Brien et al., 2013; Licata et al., 2013), it remains unclear if bile acids directly cause cell death under pathophysiologically relevant conditions in vivo.
Recently, lithocholic acid (LCA) feeding was used as a model of liver injury (Fickert et al., 2006). LCA is a hydrophobic bile acid generated by bacterial reduction of chenodeoxycholic acid (CDCA) in the gut (Hofmann, 2004). The accumulation of high biliary concentrations of LCA and its metabolites after feeding this bile acid results in the precipitation of LCA in cholangioles and the clogging of the biliary system resembling the obstructive cholestasis caused by BDL (Fickert et al., 2006). In addition to the focal areas of necrosis, an extensive accumulation of neutrophils was also observed (Fickert et al., 2006). This raises the question whether in this model, similar to BDL, a neutrophilic inflammatory response is also the main mechanism of liver injury or if the increased levels of the hydrophobic bile acid LCA can trigger direct cell death in hepatocytes. This issue may be clinically important as hydrophobic bile acids such as LCA and CDCA and their conjugates are more prevalent in humans (Trottier et al., 2011) than in mice (Zhang et al., 2012). Moreover, there are concerns that LCA may derive from UDCA (Sinakos et al., 2010; Hofmann, 2004), in particular when used at high doses in cholestatic conditions such as primary sclerosing cholangitis where it has been associated with adverse clinical outcomes (Lindor et al., 2009; Sinakos et al., 2010). Notably, LCA may also result in sclerosing cholangitis in some mouse strains (Fickert et al., 2006). Thus, the objective of this investigation was to evaluate the pathophysiological role of neutrophils in the LCA feeding model and, after analysis of LCA levels in bile and serum, assess the relevance of these concentrations for cytotoxicity in cultured mouse hepatocytes.
2. MATERIALS AND METHODS
2.1 Animals and experimental protocol
C57Bl/6J, gp91 phox−/− (NOX-2)-deficient, and ICAM-1-deficient mice on C57Bl/6 background were purchased from Jackson Laboratories (Bar Harbor, ME). All animals received humane care according to the criteria outlined in Guide for the Care and Use of Laboratory Animals. All experimental protocols were approved by the Institutional Animal Care and Usage Committees of the University of Kansas Medical Center. Mice were fed either 1% LCA mixed into control diet (Purina, St. Louis) or normal control diet and allowed food and water ad libitum for 0–96 hours. There was no statistical difference in total intake of food for animals on each diet across experiments. Mice treated with the NADPH oxidase (NOX 2) inhibitor diphenylene iodonium chloride (DPI) (Sigma, St. Louis, MO) were given subcutaneously 10 mg DPI/kg daily (in 0.2 ml of 5% glucose). Some mice were treated with 700 mg/kg galactosamine and 100 μg/kg Salmonella abortus equi endotoxin (Sigma) for 6 hours as positive control for apoptotic cell death (Jaeschke et al., 1998). Animals were sacrificed by cervical dislocation after isoflurane anesthesia. Blood and liver samples were collected at this time. Plasma was used for determination of alanine transaminase (ALT) activities and bile acid concentrations. Pieces of the liver were snap-frozen in liquid nitrogen for mRNA and protein analyses or fixed in phosphate-buffered formalin for immunohistochemistry and histology. ALT activities were determined in plasma by using the Pointe Scientific Serum ALT kit (Canton, MI) according to the manufacturer’s instructions. Plasma alkaline phosphatase (ALP) activities were determined with an ALP kit from Thermo Scientific (Waltham, MA).
2.2 Immunohistochemistry
Formalin-fixed tissue samples were embedded in paraffin and 5 μm sections were cut. Sections were stained with hemotoxylin and eosin (H&E) and evaluated for necrosis by the pathologist (A. Farhood) or stained by the TUNEL assay according to manufacturer’s protocol (Roche, Basel, Switzerland).
2.3 Western Blotting
Frozen liver samples were homogenized and centrifuged at 14,000 x g for 10 minutes. Protein concentrations were normalized using the BCA assay, and then loaded into an Invitrogen Mini-Blot system for gel electrophoresis (Invitrogen, Carlsbad, CA). An anti-ICAM-1 antibody (Santa Cruz Biotechnology, Dallas, TX) was used at 1:1000 dilution and the proteins were visualized using a horseradish peroxidase conjugated secondary antibody.
2.4 Caspase Activity
Quantification of hepatic caspase-3 activity was performed as previously described in detail (Jaeschke et al., 1998). Liver tissue was homogenized and protein concentration was normalized by BCA protein assay (Thermo Scientific, Waltham, MA). The homogenate was assayed with a fluorogenic substrate cleavable by caspase-3 (Enzo Life Sciences, Plymouth Meeting, PA) for change in fluorescence intensity over time using a Spectramax Gemini fluorescence plate reader (Molecular Devices, Sunnyvale, CA). The enzyme activity that was inhibitable by a pancaspase inhibitor was reported as caspase-3 activity.
2.5 Hepatic neutrophil sequestration
Neutrophil accumulation in the livers was assessed by staining tissue sections with an anti-neutrophil antibody (Ly-6b) (AbD Serotec, Raleigh, NC) followed by visualization with the Vectastain Elite ABC kit (Vector Labs, Burlingame, CA). The numbers of neutrophils present in sinusoids and extravasated into parenchymal tissue were counted in either 10 or 20 high-power fields (HPF). The sum of the sinusoidal and extravasated neutrophils was expressed as total neutrophil count in liver.
2.6 RT-PCR analysis
Expression of the selected genes was quantified by using real-time RT-PCR analysis as described. Briefly, total RNA was reverse transcribed with Moloney murine leukemia virus reverse transcriptase and oligo(dT) primers. The forward and reverse primers for selected genes were designed by using Primer Express software (Applied Biosystems, Foster City, CA). The SYBRgreen DNA PCR kit (Applied Biosystems, Foster City, CA) was used for real-time PCR analysis. Genes are expressed as percent of GAPDH control.
2.7 Bile acid analysis
Plasma and biliary bile acid extraction and quantification were described previously (Zhang et al., 2012). Liver BA concentrations were quantified using ultra performance liquid chromatography- tandem mass spectrometry (UPLC/MS/MS) as previously described (Zhang et al., 2012).
2.8 Isolation and culture of mouse hepatocytes
A multi-step collagenase perfusion method was used to isolate hepatocytes as described (Bajt et al., 2004). In brief, the liver was perfused in situ for 10 min at 37°C with calcium and magnesium free Hank’s Balanced Salt Solution (HBSS: Lonza/04-315Q) with EGTA (1 mM) followed by perfusion with HBSS and Liberase™. The liv- er was digested with 0.025 mg/ml of Liberase™ (Roche # 05401127001) over 8 minutes. The cell viability was evaluated by the trypan blue exclusion method. Cells were plated on 6-well plates in Williams’s Medium E (Gibco) containing 10% fetal bovine serum (Gibco), 100 U/ml penicillin/streptomycin, and insulin and cultured at 37°C with 5% CO2. After an initial 3 hour attachment period, cultures were washed with phosphate-buffered saline (PBS) and then culture medium + vehicle (DMSO) or media containing the indicated concentration of LCA, TLCA, TCA, TCDCA or a mixture these bile acids was added for 6 or 24 h. Cell death was assessed by lactate dehydrogenase (LDH) release as described (Bajt et al., 2004).
2.9 Statistics
Data are expressed as means ± S.E. Comparison between two groups were performed with Student’s t-test or one-way ANOVA followed by Dunn’s multiple comparisons for comparisons between a control and the Student-Neumann-Keul’s post-hoc test for comparisons between groups. P<0.05 was considered significant.
3. RESULTS
3.1 Lithocholic acid feeding causes extensive hepatic damage and neutrophil recruitment
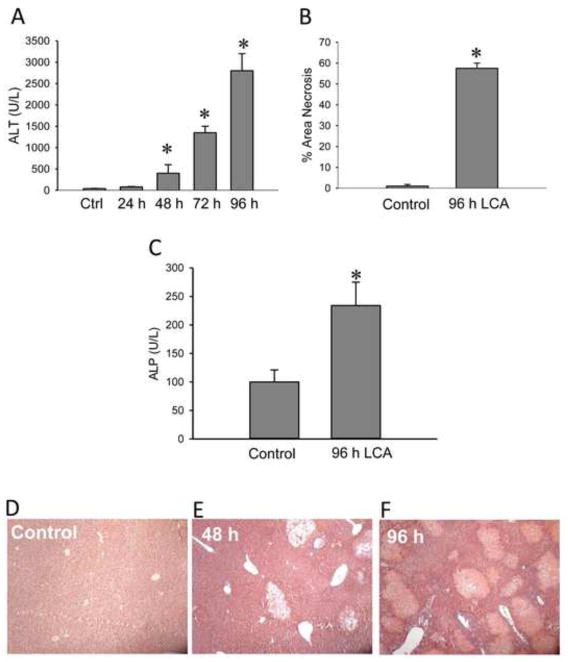
C57Bl/6 mice were fed a diet containing 1% LCA or a control diet ad libitum for up to 96 hours. In line with our previous report (Fickert et al., 2006), severe liver injury developed within 48–72 h after exposure to the LCA diet. A significant increase in plasma ALT activities as indicator of liver injury was observed as early as 48 hours after the initial change in diet. The injury progressively increased up to 96 h (Figure 1A). Mean liver necrosis was found to be 55% by 96 hours (Figure 1B). Plasma ALP activities were significantly increased by 96 hours after the onset of LCA diet feeding, consistent with a cholestatic phenotype (Figure 1C). H&E staining of liver sections confirmed focal hepatocellular necrosis (bile infarcts) and the progressive increase in injury (Figure 1D–F).
Figure 1.
LCA-induced liver injury. Plasma alanine aminotransferase (ALT) activities (A) and area of necrosis (B) were determined after feeding animals a diet supplemented with 1% LCA for 0–96 hours. The area of necrosis was estimated from H&E stained liver sections. Alkaline phosphatase (ALP) activity (C) in control or mice fed 1% LCA for 96 hours. Representative images of control (D) and LCA feeding for 48 hours (E) and 96 hours (F) are shown here at 100x magnification. Data represent means ± SE of n = 4 animals per group. *P<0.05 (compared to controls, Ctrl).
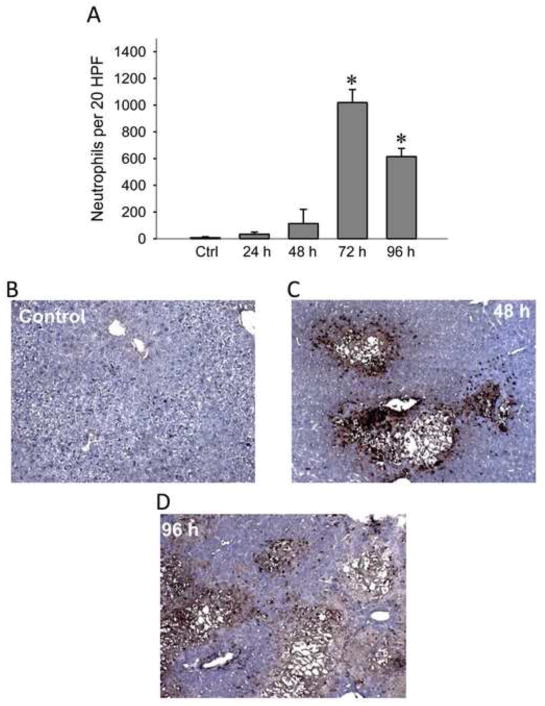
To visualize the presence of neutrophils, liver sections of controls and LCA-fed mice were stained with an anti-neutrophil antibody (Ly-6b) to evaluate the inflammatory infiltrate in the liver (Figure 2A). Concurrent with the increase in ALT values, there was a dramatic increase in neutrophil recruitment to the liver by 72 hours on the LCA diet (Figure 2B), although this value had declined slightly by 96 hours. However, the presence of neutrophils alone does not establish a primary role for inflammation in the injury process (Jaeschke and Hasegawa, 2006; Williams et al., 2010).
Figure 2.
Hepatic neutrophil recruitment during LCA feeding. Neutrophil accumulation in the liver was quantified during up to 96 hours of feeding animals a diet supplemented with 1% LCA. Neutrophils were counted in 20 randomly selected high powered fields and totaled for each time point (A). Representative sections of controls (B) and animals fed 48 hours (C) and 96 hours (D) with the LCA diet were stained with the neutrophil marker Ly-6G. Data represent means ± SE of n = 4 animals per group. *P<0.05 (compared to controls, Ctrl).
3.2 LCA feeding results in hepatocellular necrosis
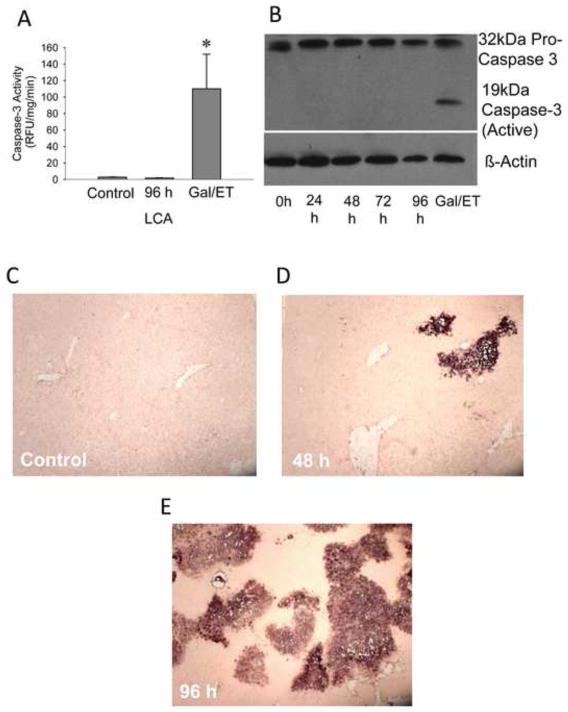
A large part of the current literature focuses on apoptosis as the mechanism for bile acid-induced cell death (Spivey et al., 1993; Faubion et al., 1999; Rust et al., 2009). Active caspase-3 levels, a hallmark of apoptosis, were measured via a caspase activity assay (Figure 3A) or Western blotting (Figure 3B). There was no apparent increase in caspase-3 activity in the LCA fed mice. While there was an abundant amount of procaspase-3 in the liver at every time point, there was no cleavage into the active caspase-3 fragment in any of the samples from LCA-treated animals. In contrast, high levels of caspase-3 activities (Figure 3A) correlated with the presence of active caspase-3 fragments in galactosamine/endotoxin-treated mice used as a positive control (Jaeschke et al., 2000). We also examined TUNEL-positive cells during LCA feeding. The TUNEL assay (Figure 3C–E) showed widespread nuclear and cytosolic staining indicative of DNA strand breaks in necrotic cells (Gujral et al., 2002).
Figure 3.
Necrosis versus apoptosis in LCA treated mice. Caspase activity (A) in LCA fed or galactosamine/endotoxin treated mice. Western Blot (B) for caspase activation after LCA feeding. Representative TUNEL images (x100 magnification) for control (C), 48 h (D), or 96 h (E) show widespread staining indicative of necrotic DNA damage.
3.3 LCA upregulates multiple genes involved in inflammation
Real time PCR analysis was used to analyze expression of inflammatory genes previously established to be involved in cholestatic liver injury (Copple et al., 2010). Multiple inflammatory genes were strongly upregulated including the interleukins IL-1β, IL-6, and IL-10, and the CXC chemokines MIP-2 and mKC (Table 1) and the adhesion molecule ICAM-1 (Figure 4A). In addition to the inflammatory genes, the acute phase proteins HO-1 and especially MT-1 were induced (Table 1). These data strongly implicated inflammation in the pathology of LCA-induced liver injury. The key question remained whether these inflammatory events are a consequence of the necrotic damage caused by LCA feeding or are a critical part of the mechanism of toxicity.
Table 1.
Gene Changes after LCA Feeding
| Inflammatory Markers (Gene) | Control | 24 h LCA | 48 h LCA | 72 h LCA | 96 h LCA |
|---|---|---|---|---|---|
| MIP2 | 1 ± 0.1 | 7.4 ± 1.2* | 6.0 ± 0.6* | 27.4 ± 9.3* | 46.4 ± 7.1* |
| mKC | 1 ± 0.4 | 2.8 ± 1.1* | 1.2 ± 0.2 | 4.9 ± 3.0* | 5.1 ± 1.2* |
| TSP-1 | 1 ± 0.4 | 24.5 ± 2.2* | 19.3± 6.8* | 23.7± 3.5* | 1.6 ± 0.5 |
| IL-1β | 1 ± 0.1 | 2.0 ± 0.5* | 1.9 ± 0.2 | 2.5 ± 0.7* | 4.4 ± 0.3* |
| IL-6 | 1 ± 0.1 | 3.8 ± 0.9* | 3.8 ± 0.5* | 7.5 ± 1.5* | 4.6 ± 1.9* |
| IL-10 | 1 ± 0.2 | 11.8 ± 3.1* | 9.1 ±1.0* | 15.8 ± 2.3* | 0.9 ± 0.4 |
| iNOS | 1 ± 0.3 | 56.0 ± 12.4* | 35.9 ± 9.8* | 65.2 ± 9.3* | 3.7 ± 1.0* |
| TNF-α | 1 ± 0.1 | 0.9 ± 0.2 | 0.7 ± 0.1 | 1.9 ± 0.3* | 3.1 ± 0.3* |
| TNFR1 | 1 ± 0.2 | 2.2 ± 0.4* | 1.8 ± 0.6 | 2.8 ± 0.4* | 1.2 ± 0.1 |
| Cox2 | 1 ± 0.4 | 2.0 ± 0.3* | 2.0 ± 0.2* | 3.8 ± 0.5* | 1.2 ± 0.1 |
| VCAM-1 | 1 ± 0.1 | 0.8 ± 0.1 | 0.5 ± 0.1 | 1.2 ± 0.6 | 1.7 ± 0.1* |
| Acute Phase Proteins | |||||
| HO-1 | 1 ± 0.2 | 1.5 ± 0.1 | 1.5 ± 0.1 | 2.3 ± 0.3* | 2.6 ± 0.5* |
| hsp86 | 1 ± 0.1 | 1.2 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.1 | 0.4 ± 0.8 |
| Gpx1 | 1 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.2 | 0.5 ± 0.7 | 0.4 ± 0.5 |
| MT-1 | 1 ± 0.1 | 2.0 ± 0.8 | 6.2 ± 3.0* | 55.8 ± 9.4* | 73.1 ± 24.7* |
RT-PCR was used to assess gene changes after LCA feeding. Data represent means ± SE of n=3 animals per group and time points.
P<0.05 (compared to controls).
ICAM-1, intercellular adhesion molecule-1, iNOS, inducible nitric oxide synthase; mKC, mouse keratinocyte chemoattractant; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-10, interleukin-10; TNF-α, tumor necrosis factor α; TNFR1, tumor necrosis factor alpha receptor 1; Cox-2, cyclooxygenase-2; VCAM-1, vascular adhesion molecule-1; HO-1, heme oxygenase-1; hsp86, heat shock protein-86; Gpx-1, glutathione peroxidase-1; MT-1, metallothionein-1; MIP-2, macrophage inflammatory protein 2; TSP-1, thrombospondin 1;
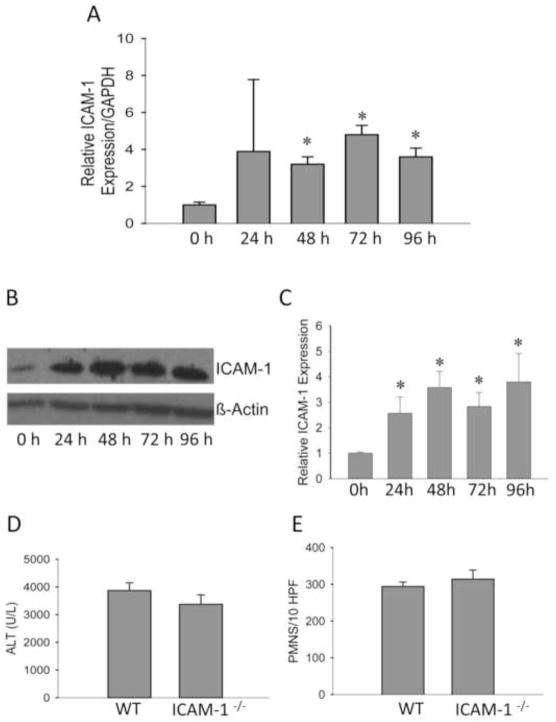
Figure 4.
No protection against LCA induced liver injury in ICAM-1-deficient mice. ICAM-1 mRNA (A) and protein (B and C) levels were measured after 0–96 hours of LCA exposure. Plasma ALT activities (D) and neutrophil infiltration (E) were determined in ICAM-1-deficient and WT mice after 96 h of LCA feeding. Data represent means ± SE of n = 4 animals per group. *P<0.05 (compared to controls, Ctrl). Protein expression quantification represents means ± SE of n=3 animals per group. *P<0.05 (compared to controls, Ctrl).
3.4 No protection against LCA treatment in ICAM-1-deficient mice
ICAM-1 expression is critical for neutrophil cytotoxicity in the liver (Essani et al., 1995a). After LCA feeding, hepatic ICAM-1 mRNA (Figure 4A) and protein levels (Figure 4B,C) were strongly induced. To test the pathophysiological role of neutrophils in LCA-induced liver injury, ICAM-1-deficient mice or WT animals were fed 1% LCA for 96 hours as in previous experiments. In contrast to the extensive protection of ICAM-1-deficient mice after BDL (Gujral et al., 2004b), plasma ALT values showed no significant difference between ICAM-1-deficient and WT mice after LCA feeding (Figure 4D); similarly, the number of neutrophils in the liver of both KO and WT animals was not different (Figure 4E).
3.5 No protection against LCA treatment by inhibition of neutrophil-induced ROS formation
Neutrophils kill invading pathogens through ROS formation (Weiss, 1989; Nathan, 2006). This is largely mediated through the formation of superoxide via NAPDH oxidase and the subsequent generation of secondary ROS such as hypochlorous acid by neutrophil myeloperoxidase (Jaeschke, 2011). Thus, inhibition of NADPH oxidase results in a dramatically reduced potential for cytotoxicity of neutrophils. The gp91phox−/− knockout mice, deficient for the catalytic subunit of NADPH oxidase, were fed the 1% LCA diet for 72 hours, but no difference was found in plasma ALT activities or neutrophil recruitment (Table 2). To confirm these findings, a pharmacological inhibitor of NADPH oxidase was used. LCA-fed mice were given diphenylene iodonium (DPI) chloride daily, starting at the onset of the LCA feeding. Despite the effectiveness of this treatment regimen against neutrophil-induced injury during endotoxemia (Gujral et al., 2004a), DPI had no effect on the LCA-induced liver injury (Table 2). Together, these data strongly suggest that the liver injury caused by LCA feeding is not mediated by an inflammatory mechanism involving neutrophils.
Table 2.
Ablation of functional NADPH oxidase failed to protect against LCA induced liver injury
| ALT | PMNs/10 HPF | |
|---|---|---|
| WT | 1748 ± 409 | 361 ± 49 |
| gp91phox−/− | 1650 ± 453 | 446 ± 53 |
| Vehicle | 2191 ± 326 | 292 ± 43 |
| DPI | 2208 ± 324 | 366 ± 33 |
Plasma ALT activities and neutrophil recruitment were assessed in WT and gp91phox−/− mice and vehicle- or DPI (10mg/kg daily)-treated WT mice after 72 hours of LCA diet feeding. Data represent means ± SE of n = 4 animals per group.
3.6 Bile acid toxicity in cultured mouse hepatocytes
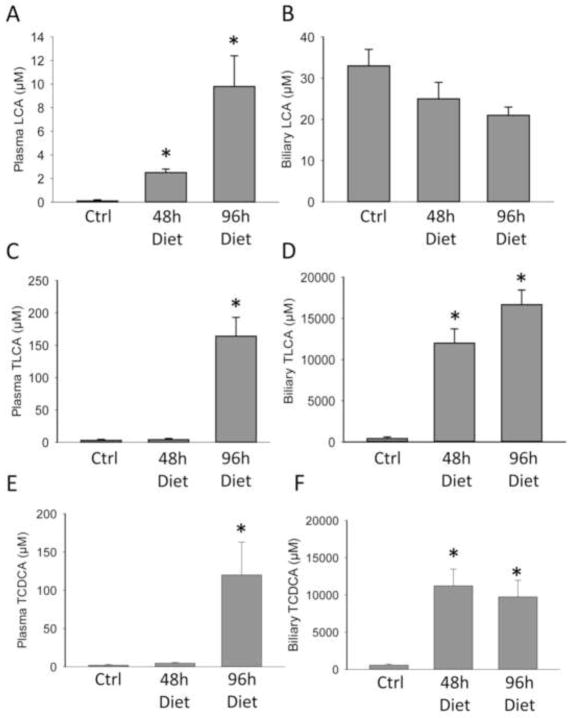
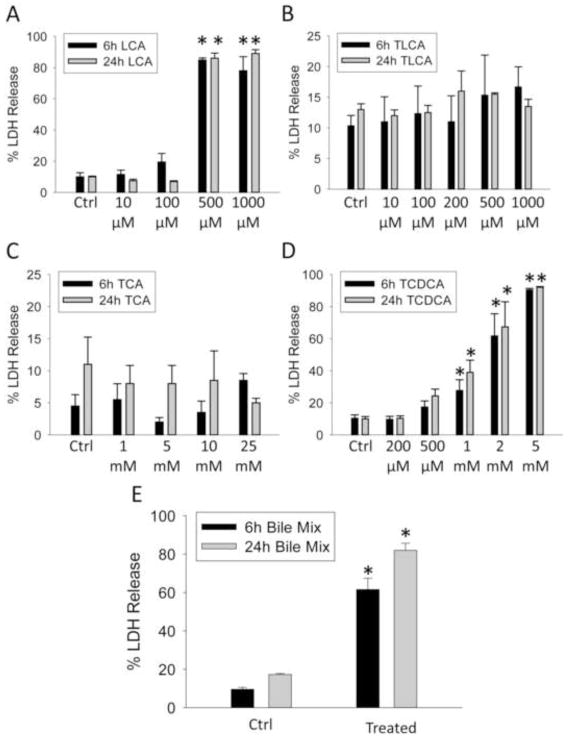
To test the hypothesis that LCA or a metabolite may directly cause cell death in hepatocytes in vivo, we first measured bile acid levels in plasma and bile of animals on the control and LCA diet at 48 and 96 h after LCA feeding. After LCA feeding, increases were seen in TCA, TLCA, and TCDCA in plasma, with smaller increases seen in CDCA, DCA and CA levels (Figure 5, Supplementary Table 1). Biliary TLCA and TCDCA levels rose to concentrations of ~10mM and ~16mM respectively (Figure 5D, F). Additionally, the biliary levels of TCA, GCA, and CA levels dropped, whereas the other bile acids largely stayed the same over 96 h (Supplementary Table 2). To test if these concentrations were consistent with toxicity, in vitro dose response curves were established for LCA (Figure 6A), TLCA (Figure 6B), TCA (Figure 6C) and TCDCA (Figure 6D) using primary murine hepatocytes. Measured plasma concentrations of these bile acids did not correspond with toxicity in the case of any bile acid tested (Figure 6). While LCA was toxic at concentrations ≥ 500μM, these levels were never achieved in bile in the study. Thus, LCA is unlikely the toxic bile acid in these experiments. In contrast, biliary TLCA levels rose to the greatest degree in the study; however, issues with solubility prevented testing concentrations above 1mM. However, at levels ≤ 1 mM, TLCA did not cause cytotoxicity in hepatocytes at 6 or 24h (Figure 6B). Thus, the hypothesis that biliary TLCA levels were toxic was not testable in vitro. However, these findings may suggest that TLCA was the bile acid precipitating in bile and clogging the cholangioles (Fickert et al., 2006). While TCA levels were non-toxic at doses up to 25mM (Figure 6C), TCDCA was highly toxic at doses of 1mM and above (Figure 6D). As biliary TCDCA concentrations were close to 10mM during the study, it is likely that TCDCA mediates a portion of the toxicity. To further test this hypothesis, we created a biliary mixture of bile acids including 10mM TCA, 10mM TCDCA and 1mM TLCA and treated murine hepatocytes with this dose. Significant toxicity was present at this dose, confirming the hypothesis that biliary values of bile acids mediate the injury after LCA feeding (Figure 6E). These conclusions are consistent with the pattern of injury (focal necrosis).
Figure 5.
Major bile acid changes induced by LCA feeding. Plasma and biliary concentrations of LCA (A and B), TLCA (C and D), and TCDCA (E and F) were measured in control mice or 1% LCA feeding after 48 or 96 h. Data represent means ± SE of n = 3 individual mice.*P<0.05 (compared to controls, Ctrl).
Figure 6.
Direct toxicity of bile acids in mouse hepatocytes. LCA (A), TLCA (B), TCA (C) and TCDCA (D) were applied to primary murine hepatocytes 3h after plating at the indicated concentration for 6 or 24h. In addition, a mixture of bile acids including TLCA, TCDCA, and TCA (Bile Mix) was applied to cells at the concentrations measured at 96 h post LCA feeding to recapitulate how cells would respond to the total biliary mixture (Figure 5). These bile acids were chosen as they account for 99% of the total biliary mix. This mixture was applied for 6 h or 24 h. Cell death was assessed by LDH release. Data represent means ± SE of n = 3 individual cell isolation experiments..*P<0.05 (compared to vehicle control).
4. DISCUSSION
The main objective of the current investigation was to evaluate the detailed mechanisms of LCA-induced hepatotoxicity in vivo. In particular, it was tested if the toxicity involved neutrophilic inflammatory mechanisms or was mainly caused by the direct cytotoxicity of LCA and its metabolites. LCA is a toxic hydrophobic bile acid, especially in humans. LCA hepatotoxicity was studied extensively over the past 50 years (Hofmann, 2004). Feeding various concentrations of LCA in the diet has been shown to cause focal necrosis in mice but the actual mechanism of cell death in vivo remained unclear (Fickert et al., 2006; Song et al., 2011).
4.1 The role of neutrophils in LCA-induced cholestatic liver injury
Our current study confirmed previous findings that feeding of high levels of LCA caused focal necrosis similar to the liver injury observed after BDL (Fickert et al., 2006). This injury pattern is caused by the precipitation of hydrophobic LCA or its metabolites in cholangioles resulting in the blockage of these biliary structures (Fickert et al., 2006). Based on the measured biliary levels of LCA, TLCA and TCDCA, and the fact that only TLCA was insoluble at these concentrations in an aqueous medium, it is most likely that TLCA was the bile acid which precipitated in bile and clogged the cholangioles. Similar to BDL, the blocked bile ducts lead to an increased pressure build-up in the biliary system, which ultimately causes ruptures and leakage of bile back into the parenchyma (Fickert et al., 2006). Similar to BDL, a substantial number of neutrophils can be observed within and around the area of necrosis. However, in striking contrast to BDL, various interventions or gene deficiencies, which impair the cytotoxic capabilities of neutrophils, did not affect the injury. This included ICAM-1, an inducible adhesion molecule expressed on sinusoidal endothelial cells and on hepatocytes (Essani et al., 1995b). In the liver, ICAM-1 is mainly involved in the extravasation of neutrophils from sinusoids and the adhesion to hepatocytes (Essani et al., 1995a). Blocking ICAM-1 is highly effective in preventing neutrophil-induced liver injury by inhibiting neutrophil extravasation during endotoxemia (Essani et al., 1995a) or BDL (Gujral et al., 2004b). Despite the extensive protection against BDL-induced focal necrosis in ICAM-1-deficient mice (Gujral et al., 2004b), these animals experienced the same injury during LCA feeding as the wild type animals, which showed significant induction of ICAM-1 gene expression. These data support the conclusion that neutrophils are not significant contributors to the injury in LCA-fed animals.
It is well established that neutrophils kill target cells by reactive oxygen species, which starts with the formation of superoxide and hydrogen peroxide by NADPH oxidase and subsequently hypochlorous acid by myeloperoxidase (Weiss, 1989; Nathan, 2006). Consistent with these observations, neutrophil-induced liver injury depends on oxidant stress (Jaeschke, 2011; Jaeschke and Woolbright, 2012). In fact, there is direct evidence that neutrophil-derived reactive oxygen species diffuse into hepatocytes and cause cell death in ischemia-reperfusion injury (Hasegawa et al., 2005), endotoxemia (Gujral et al., 2004a) and BDL (Gujral et al., 2003). Therefore, gp91phox−/− animals with a non-functional NADPH oxidase or wild type animals treated with DPI, an inhibitor of NADPH oxidase, are highly protected if the injury depends on neutrophils as was shown for ischemia (Abdelrahman et al., 2005; Lehnert et al., 2003; Harada et al., 2004) and for endotoxemia (Gujral et al., 2004a). However, neither gp91phox−/− mice nor animals treated with DPI showed reduced liver injury or neutrophil recruitment after LCA feeding. These data together with the lack of protection in ICAM-1 deficient mice strongly suggest that neutrophils are not relevant for the development of liver injury in this model. The lack of protection was especially interesting in light of the fact that LCA has been shown to act as a priming agent for neutrophils when exposed to low μM concentrations in vitro (Dahm and Roth, 1990). It seems that even though neutrophils are likely primed for oxidative burst and phagocytosis, the injury occurs through other mechanisms, indicating many of the damaged cells were necrotic or committed to cell death before the arrival of the neutrophils.
4.2 Direct cytotoxicity of bile acids
The second mechanism of cellular injury would be direct cytotoxicity of bile acids (Delzenne et al., 1992; Sokol et al., 1993). Our data indicate that plasma levels of LCA were significantly elevated in LCA-fed animals compared to controls (Figure 6) or BDL animals (Zhang et al., 2012), whereas biliary levels were slightly decreased. The lack of increase in LCA in bile is likely caused by the preferential export of conjugated bile acids and increased conjugation present during cholestasis. Dose-response experiments of LCA toxicity in isolated mouse hepatocytes showed an increase in cell death at concentrations between 100 and 500 μM LCA in the culture medium (Figure 6A). However, LCA concentrations never reached these levels in plasma or bile during the study. Instead, biliary TLCA levels were dramatically elevated, consistent with the liver conjugating LCA in order to enhance excretion of this bile acid (Figure 5). Plasma concentrations of TLCA never exceeded 200 μM and TLCA was generally non-toxic up to 1mM (Figure 5, 6B). Unfortunately, due to solubility problems, we were unable to test the hypothesis that biliary concentrations of TLCA were consistent with toxicity as levels above 1mM were insoluble in the aqueous culture medium. It is important to note though, that the tested concentrations were 10-fold higher than previous levels tested in rat hepatocytes (Sokol et al., 1993). However, TLCA concentrations of ≤1 mM did not cause cell death in mouse hepatocytes, which is in strong contrast to previous data in rat hepatocytes where TLCA was shown to be highly cytotoxic (Sokol et al., 1993). These observations further exemplify differences in the susceptibility to bile acid between rat and mouse hepatocytes (Woolbright and Jaeschke, 2012). Thus, our data do not preclude the idea that biliary concentrations of TLCA are directly cytotoxic, it only suggests rats are more susceptible compared to mice in regards to TLCA toxicity. Instead, TCDCA levels rose dramatically in bile and were toxic to isolated hepatocytes at concentrations reached in the bile (Figure 5, 6D). While it was somewhat surprising that TCDCA reached concentrations of 10mM in bile, previous reports have suggested that LCA can be re-oxidized to CDCA in the liver and then conjugated with taurine to form TCDCA (Zhang and Klaassen, 2010). Given that TCDCA levels were approximately on par with TLCA levels, and that this aspect is not recapitulated in other models of cholestasis (Zhang et al., 2012), it seems likely that direct conversion is the source of the high levels of TCDCA. A mixture of bile acids present in the bile after LCA feeding was also applied to hepatocytes (Figure 6E). Interestingly, while this mixture was acutely toxic, it was slightly less toxic than TCDCA alone, lending credence to the idea that some of the less toxic, hydrophilic bile acids such as TCA might help ameliorate toxicity (Figure 6E). The selective leakage of the biliary system after LCA feeding (Fickert et al., 2006) and the focal areas of necrosis (bile infarcts) strongly argue for the biliary bile acid concentrations as the cause of cell death. Although neutrophils were recruited into the liver and were mainly present in the area of necrosis, the ineffectiveness of multiple interventions against neutrophils suggests that direct bile acid cytotoxicity dominates under these conditions and the neutrophil response has no relevant impact.
Moderate (50–100 μM) concentrations of hydrophobic bile acids can cause apoptotic cell death in cultured rat hepatocytes (Guicciardi and Gores, 2002; Perez and Briz, 2009). However, morphological evidence and ALT release together with the lack of caspase-3 activity and caspase-3 processing clearly suggests that necrotic cell death dominates after LCA feeding in vivo. Although the dying cells stain positive with the TUNEL assay, which recognizes DNA strand breaks, the pattern of staining (nucleus and cytosol) is typical for necrotic cell death (Gujral et al., 2002; Jaeschke and Lemasters, 2003). In addition, the in vitro experiments with extensive LDH release support the conclusion that TCDCA and potentially TLCA cause necrotic cell death in mouse hepatocytes. The characteristics of cell death after LCA feeding are very similar to the necrotic cell death observed after BDL, which includes morphological evidence of cell swelling, vacuolation, and karyolysis. In addition, lack of caspase activation (based on enzyme activity, western blotting, immunohistochemistry, caspase-cleaved cytokeratin-18 fragments), the massive release of cell content (ALT, miR-122, full length cytokeratin-18 and high mobility group box 1 protein), and the TUNEL staining of nucleus and cytosol further supports the conclusion of necrotic cell death (Schoemaker et al., 2003; Gujral et al, 2004c; Fickert et al., 2005; Nalapareddy et al., 2009; Mitchell et al., 2011; Woolbright et al., 2013). Together these observations strongly suggest that the direct cytotoxicity of the hydrophobic bile acids LCA, TCDCA and potentially TLCA in mice in vivo and in cultured hepatocytes in vitro is caused by necrosis and not apoptosis.
4.3 Summary and Conclusions
Previous studies demonstrated the critical role of neutrophils in causing bile infarcts after obstructive cholestasis (BDL) in mice. The main reason for the limited direct toxicity of bile acids is that the dominant bile acids in mice are not cytotoxic at pathophysiological relevant concentrations. However, if the composition of the mouse bile is changed by feeding the hydrophobic bile acid LCA, the biliary concentrations of LCA metabolites TCDCA and TLCA, are sufficient to directly cause necrotic cell death in mouse hepatocytes. In addition, the extensive focal necrosis in vivo, which was correlated with neutrophil infiltration, was not affected by various interventions against neutrophils. Together, these data indicate that the toxicity of bile leaking from ruptured cholangioles during obstructive cholestasis is dependent on the bile acid composition. Thus, if bile contains sufficient levels of toxic hydrophobic bile acids, direct cytotoxicity is the main cause of hepatic necrosis with little impact of neutrophils. These findings do not reflect the mechanism of BDL injury in mice due to their high percentage of hydrophilic bile acids in bile. However, the observations may have implications for obstructive cholestasis in humans because of their higher levels of hydrophobic bile acids. This may in particular apply to patients with obstructive cholangiopathies who have higher LCA levels due to treatment with high doses of UDCA (Sinakos et al., 2010; Lindor et al., 2009). Studies with human hepatocytes exposed to relevant concentrations of human bile acids are needed to address this question.
Supplementary Material
Highlights.
Lithocholic acid (LCA) feeding results in significant hepatotoxicity and neutrophil recruitment
Ablation of neutrophil function and activity does not protect against LCA induced injury
Tauro-LCA and Tauro-chenodeoxycholic acid accumulate to toxic levels after LCA feeding
Direct bile acid toxicity can occur when toxic bile acid species are administered
Species differences in bile acid composition may determine mechanistic differences in injury
Acknowledgments
This work was supported in part by the National Institutes of Health [R01 DK070195 and R01 AA12916 to H.J.], grants from the National Center for Research Resources (5P20RR021940-07) and the National Institute of General Medical Sciences (8 P20 GM103549-07) from the National Institutes of Health, and grant F3517-B20 from the Austrian Science Foundation to MT). B.L. Woolbright was supported by the “Training Program in Environmental Toxicology” (T32 ES007079-26) from the National Institute of Environmental Health Sciences.
Abbreviations
- BA
bile acid
- CA
cholic acid
- GCDCA
glycochenodeoxycholic acid
- LCA
lithocholic acid
- αMCA
α-muricholic acid
- βMCA
β-muricholic acid
- DCA
deoxycholic acid
- TCDCA
taurochenodeoxycholic acid
- GCA
glycocholic acid
- TCA
taurocholic acid
- CDCA
chenodeoxycholic acid
- TLCA
taurolithocholic acid
- FXR
farnesoid X receptor
- Egr-1
early growth response factor 1
- ICAM-1
intercellular adhesion molecule-1
- iNOS
inducible nitric oxide synthase
- mKC
mouse keratinocyte chemoattractant
- IL-1β
interleukin-1 β
- IL-6
interleukin-6
- IL-10
interleukin-10
- TNF-α
tumor necrosis factor α
- Cox-2
cyclooxygenase-2
- VCAM-1
vascular adhesion molecule-1
- HO-1
heme oxygenase-1
- hsp86
heat shock protein-86
- Gpx-1
glutathione peroxidase-1
- MT-1
metallothionein-1
- MIP-2
macrophage inflammatory protein 2
- TSP-1
thrombospondin 1
- TNFR1
tumor necrosis factor alpha receptor 1
Footnotes
Conflict of Interest
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelrahman M, Mazzon E, Bauer M, Bauer I, Delbosc S, Cristol JP, Patel NS, Cuzzocrea S, Thiemermann C. Inhibitors of NADPH oxidase reduce the organ injury in hemorrhagic shock. Shock. 2005;23:107–114. doi: 10.1097/01.shk.0000151028.15377.f7. [DOI] [PubMed] [Google Scholar]
- Allen K, Jaeschke H, Copple BL. Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am J Pathol. 2011;178:175–186. doi: 10.1016/j.ajpath.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajt ML, Knight TR, Lemasters JJ, Jaeschke H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol Sci. 2004;80:343–349. doi: 10.1093/toxsci/kfh151. [DOI] [PubMed] [Google Scholar]
- Botla R, Spivey JR, Aguilar H, Bronk SF, Gores GJ. Ursodeoxycholate (UDCA) inhibits the mitochondrial membrane permeability transition induced by glycochenodeoxycholate: a mechanism of UDCA cytoprotection. J Pharmacol Exp Ther. 1995;272:930–938. [PubMed] [Google Scholar]
- Copple BL, Jaeschke H, Klaassen CD. Oxidative stress and the pathogenesis of cholestasis. Semin Liver Dis. 2010;30:195–204. doi: 10.1055/s-0030-1253228. [DOI] [PubMed] [Google Scholar]
- Dahm LJ, Roth RA. Differential effects of lithocholate on rat neutrophil activation. J Leukoc Biol. 1990;47:551–560. doi: 10.1002/jlb.47.6.551. [DOI] [PubMed] [Google Scholar]
- Delzenne NM, Calderon PB, Taper HS, Roberfroid MB. Comparative hepatotoxicity of cholic acid, deoxycholic acid and lithocholic acid in the rat: in vivo and in vitro studies. Toxicol Lett. 1992;61:291–304. doi: 10.1016/0378-4274(92)90156-e. [DOI] [PubMed] [Google Scholar]
- Essani NA, Fisher MA, Farhood A, Manning AM, Smith CW, Jaeschke H. Cytokine-induced upregulation of hepatic intercellular adhesion molecule-1 messenger RNA expression and its role in the pathophysiology of murine endotoxin shock and acute liver failure. Hepatology. 1995a;21:1632–1639. [PubMed] [Google Scholar]
- Essani NA, McGuire GM, Manning AM, Jaeschke H. Differential induction of mRNA for ICAM-1 and selectins in hepatocytes, Kupffer cells and endothelial cells during endotoxemia. Biochem Biophys Res Commun. 1995b;211:74–82. doi: 10.1006/bbrc.1995.1780. [DOI] [PubMed] [Google Scholar]
- Faubion WA, Guicciardi ME, Miyoshi H, Bronk SF, Roberts PJ, Svingen PA, Kaufmann SH, Gores GJ. Toxic bile salts induce rodent hepatocyte apoptosis via direct activation of Fas. J Clin Invest. 1999;103:137–145. doi: 10.1172/JCI4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fickert P, Fuchsbichler A, Marschall HU, Wagner M, Zollner G, Krause R, Zatloukal K, Jaeschke H, Denk H, Trauner M. Lithocholic acid feeding induces segmental bile duct obstruction and destructive cholangitis in mice. Am J Pathol. 2006;168:410–422. doi: 10.2353/ajpath.2006.050404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fickert P, Trauner M, Fuchsbichler A, Zollner G, Wagner M, Marschall HU, Zatloukal K, Denk H. Oncosis represents the main type of cell death in mouse models of cholestasis. J Hepatol. 2005;42:378–385. doi: 10.1016/j.jhep.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Fickert P, Zollner G, Fuchsbichler A, Stumptner C, Weiglein AH, Lammert F, Marschall HU, Tsybrovskyy O, Zatloukal K, Denk H, Trauner M. Ursodeoxycholic acid aggravates bile infarcts in bile duct-ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology. 2002;123:1238–1251. doi: 10.1053/gast.2002.35948. [DOI] [PubMed] [Google Scholar]
- Graf D, Kurz AK, Reinehr R, Fischer R, Kircheis G, Häussinger D. Prevention of bile acid-induced apoptosis by betaine in rat liver. Hepatology. 2002;36:829–839. doi: 10.1053/jhep.2002.35536. [DOI] [PubMed] [Google Scholar]
- Guicciardi ME, Gores GJ. Bile acid-mediated hepatocyte apoptosis and cholestatic liver disease. Dig Liver Dis. 2002;34:387–392. doi: 10.1016/s1590-8658(02)80033-0. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Farhood A, Bajt ML, Jaeschke H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology. 2003;38:355–363. doi: 10.1053/jhep.2003.50341. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Hinson JA, Farhood A, Jaeschke H. NADPH oxidase-derived oxidant stress is critical for neutrophil cytotoxicity during endotoxemia. Am J Physiol Gastrointest Liver Physiol. 2004a;287:G243–252. doi: 10.1152/ajpgi.00287.2003. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci. 2002;67:322–328. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Liu J, Farhood A, Hinson JA, Jaeschke H. Functional importance of ICAM-1 in the mechanism of neutrophil-induced liver injury in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol. 2004b;286:G499–507. doi: 10.1152/ajpgi.00318.2003. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Liu J, Farhood A, Jaeschke H. Reduced oncotic necrosis in Fas receptor-deficient C57BL/6J-lpr mice after bile duct ligation. Hepatology. 2004c;40:998–1007. doi: 10.1002/hep.20380. [DOI] [PubMed] [Google Scholar]
- Harada H, Hines IN, Flores S, Gao B, McCord J, Scheerens H, Grisham MB. Role of NADPH oxidase-derived superoxide in reduced size liver ischemia and reperfusion injury. Arch Biochem Biophys. 2004;423:103–108. doi: 10.1016/j.abb.2003.08.035. [DOI] [PubMed] [Google Scholar]
- Hasegawa T, Malle E, Farhood A, Jaeschke H. Generation of hypochlorite-modified proteins by neutrophils during ischemia-reperfusion injury in rat liver: attenuation by ischemic preconditioning. Am J Physiol Gastrointest Liver Physiol. 2005;289:G760–767. doi: 10.1152/ajpgi.00141.2005. [DOI] [PubMed] [Google Scholar]
- Hofmann AF. Detoxification of lithocholic acid, a toxic bile acid: relevance to drug hepatotoxicity. Drug Metab Rev. 2004;36:703–722. doi: 10.1081/dmr-200033475. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J Gastroenterol Hepatol. 2011;26(Suppl 1):173–179. doi: 10.1111/j.1440-1746.2010.06592.x. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Farhood A, Cai SX, Tseng BY, Bajt ML. Protection against TNF-induced liver parenchymal cell apoptosis during endotoxemia by a novel caspase inhibitor in mice. Toxicol Appl Pharmacol. 2000;169:77–83. doi: 10.1006/taap.2000.9035. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Fisher MA, Lawson JA, Simmons CA, Farhood A, Jones DA. Activation of caspase 3 (CPP32)-like proteases is essential for TNF-alpha-induced hepatic parenchymal cell apoptosis and neutrophil-mediated necrosis in a murine endotoxin shock model. J Immunol. 1998;160:3480–3486. [PubMed] [Google Scholar]
- Jaeschke H, Hasegawa T. Role of neutrophils in acute inflammatory liver injury. Liver Int. 2006;26:912–919. doi: 10.1111/j.1478-3231.2006.01327.x. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology. 2003;125:1246–1257. doi: 10.1016/s0016-5085(03)01209-5. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J Leukoc Biol. 1997;61:647–653. doi: 10.1002/jlb.61.6.647. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Woolbright BL. Current strategies to minimize hepatic ischemia-reperfusion injury by targeting reactive oxygen species. Transplant Rev (Orlando) 2012;26:103–114. doi: 10.1016/j.trre.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jüngst C, Lammert F. Cholestatic liver disease. Dig Dis. 2013;31:152–154. doi: 10.1159/000347210. [DOI] [PubMed] [Google Scholar]
- Kim ND, Moon JO, Slitt AL, Copple BL. Early growth response factor-1 is critical for cholestatic liver injury. Toxicol Sci. 2006;90:586–595. doi: 10.1093/toxsci/kfj111. [DOI] [PubMed] [Google Scholar]
- Kountouras J, Billing BH, Scheuer PJ. Prolonged bile duct obstruction: a new experimental model for cirrhosis in the rat. Br J Exp Pathol. 1984;65:305–311. [PMC free article] [PubMed] [Google Scholar]
- Lehnert M, Arteel GE, Smutney OM, Conzelmann LO, Zhong Z, Thurman RG, Lemasters JJ. Dependence of liver injury after hemorrhage/resuscitation in mice on NADPH oxidase-derived superoxide. Shock. 2003;19:345–351. doi: 10.1097/00024382-200304000-00009. [DOI] [PubMed] [Google Scholar]
- Licata LA, Nguyen CT, Burga RA, Falanga V, Espat NJ, Ayala A, Thorn M, Junghans RP, Katz SC. Biliary obstruction results in PD-1-dependent liver T cell dysfunction and acute inflammation mediated by Th17 cells and neutrophils. J Leukoc Biol. 2013;94:813–823. doi: 10.1189/jlb.0313137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindor KD, Kowdley KV, Luketic VA, Harrison ME, McCashland T, Befeler AS, Harnois D, Jorgensen R, Petz J, Keach J, Mooney J, Sargeant C, Braaten J, Bernard T, King D, Miceli E, Schmoll J, Hoskin T, Thapa P, Enders F. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology. 2009;50:808–814. doi: 10.1002/hep.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C, Mahrouf-Yorgov M, Mayeuf A, Robin MA, Mansouri A, Fromenty B, Gilgenkrantz H. Overexpression of Bcl-2 in hepatocytes protects against injury but does not attenuate fibrosis in a mouse model of chronic cholestatic liver disease. Lab Invest. 2011;91:273–282. doi: 10.1038/labinvest.2010.163. [DOI] [PubMed] [Google Scholar]
- Nalapareddy PD, Schüngel S, Hong JY, Manns MP, Jaeschke H, Vogel A. The BH3-only protein bid does not mediate death-receptor-induced liver injury in obstructive cholestasis. Am J Pathol. 2009;175:1077–1085. doi: 10.2353/ajpath.2009.090304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- O’Brien KM, Allen KM, Rockwell CE, Towery K, Luyendyk JP, Copple BL. IL- 17A synergistically enhances bile acid-induced inflammation during obstructive cholestasis. Am J Pathol. 2013;183:1498–1507. doi: 10.1016/j.ajpath.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MJ, Briz O. Bile-acid-induced cell injury and protection. World J Gastroenterol. 2009;15:1677–1689. doi: 10.3748/wjg.15.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust C, Wild N, Bernt C, Vennegeerts T, Wimmer R, Beuers U. Bile acid-induced apoptosis in hepatocytes is caspase-6-dependent. J Biol Chem. 2009;284:2908–2916. doi: 10.1074/jbc.M804585200. [DOI] [PubMed] [Google Scholar]
- Saito JM, Maher JJ. Bile duct ligation in rats induces biliary expression of cytokine- induced neutrophil chemoattractant. Gastroenterology. 2000;118:1157–1168. doi: 10.1016/s0016-5085(00)70369-6. [DOI] [PubMed] [Google Scholar]
- Schoemaker MH, Gommans WM, Conde de la Rosa L, Homan M, Klok P, Trautwein C, van Goor H, Poelstra K, Haisma HJ, Jansen PL, Moshage H. Resistance of rat hepatocytes against bile acid-induced apoptosis in cholestatic liver injury is due to nuclear factor-kappa B activation. J Hepatol. 2003;39:153–161. doi: 10.1016/s0168-8278(03)00214-9. [DOI] [PubMed] [Google Scholar]
- Sinakos E, Marschall HU, Kowdley KV, Befeler A, Keach J, Lindor K. Bile acid changes after high-dose ursodeoxycholic acid treatment in primary sclerosing cholangitis: Relation to disease progression. Hepatology. 2010;52:197–203. doi: 10.1002/hep.23631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol RJ, Devereaux M, Khandwala R, O’Brien K. Evidence for involvement of oxygen free radicals in bile acid toxicity to isolated rat hepatocytes. Hepatology. 1993;17:869– 881. [PubMed] [Google Scholar]
- Song P, Zhang Y, Klaassen CD. Dose-response of five bile acids on serum and liver bile Acid concentrations and hepatotoxicty in mice. Toxicol Sci. 2011;123:359–367. doi: 10.1093/toxsci/kfr177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivey JR, Bronk SF, Gores GJ. Glycochenodeoxycholate-induced lethal hepatocellular injury in rat hepatocytes. Role of ATP depletion and cytosolic free calcium. J Clin Invest. 1993;92:17–24. doi: 10.1172/JCI116546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier J, Bia ek A, Caron P, Straka RJ, Heathcote J, Milkiewicz P, Barbier O. Metabolomic profiling of 17 bile acids in serum from patients with primary biliary cirrhosis and primary sclerosing cholangitis: a pilot study. Dig Liver Dis. 2012;44:303–310. doi: 10.1016/j.dld.2011.10.025. [DOI] [PubMed] [Google Scholar]
- Trottier J, Bia ek A, Caron P, Straka RJ, Milkiewicz P, Barbier O. Profiling circulating and urinary bile acids in patients with biliary obstruction before and after biliary stenting. PLoS One. 2011;6:e22094. doi: 10.1371/journal.pone.0022094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Williams CD, Bajt ML, Farhood A, Jaeschke H. Acetaminophen-induced hepatic neutrophil accumulation and inflammatory liver injury in CD18-deficient mice. Liver Int. 2010;30:1280–1292. doi: 10.1111/j.1478-3231.2010.02284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolbright BL, Antoine DJ, Jenkins RE, Bajt ML, Park BK, Jaeschke H. Plasma biomarkers of liver injury and inflammation demonstrate a lack of apoptosis during obstructive cholestasis in mice. Toxicol Appl Pharmacol. 2013;273:524–531. doi: 10.1016/j.taap.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolbright BL, Jaeschke H. Novel insight into mechanisms of cholestatic liver injury. World J Gastroenterol. 2012;18:4985–4993. doi: 10.3748/wjg.v18.i36.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Ramachandran A, Yan HM, Woolbright BL, Copple BL, Fickert P, Trauner M, Jaeschke H. Osteopontin is an initial mediator of inflammation and liver injury during obstructive cholestasis after bile duct ligation in mice. Toxicol Lett. 2014;224:186–195. doi: 10.1016/j.toxlet.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hong JY, Rockwell CE, Copple BL, Jaeschke H, Klaassen CD. Effect of bile duct ligation on bile acid composition in mouse serum and liver. Liver Int. 2012;32:58–69. doi: 10.1111/j.1478-3231.2011.02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Klaassen CD. Effects of feeding bile acids and a bile acid sequestrant on hepatic bile acid composition in mice. J Lipid Res. 2010;51:3230–42. doi: 10.1194/jlr.M007641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollner G, Trauner M. Mechanisms of cholestasis. Clin Liver Dis. 2008;12:1–26. doi: 10.1016/j.cld.2007.11.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.