Abstract
Introduction
The overall outcome of septic shock has been recently improved. We sought to determine whether this survival gain extends to the high-risk subgroup of patients with cirrhosis.
Methods
Cirrhotic patients with septic shock admitted to a medical intensive care unit (ICU) during two consecutive periods (1997-2004 and 2005-2010) were retrospectively studied.
Results
Forty-seven and 42 cirrhotic patients presented with septic shock in 1997-2004 and 2005-2010, respectively. The recent period differed from the previous one by implementation of adjuvant treatments of septic shock including albumin infusion as fluid volume therapy, low-dose glucocorticoids, and intensive insulin therapy. ICU and hospital survival markedly improved over time (40% in 2005-2010 vs. 17% in 1997-2004, P = 0.02 and 29% in 2005-2010 vs. 6% in 1997-2004, P = 0.009, respectively). Furthermore, this survival gain in the latter period was sustained for 6 months (survival rate 24% in 2005-2010 vs. 6% in 1997-2004, P = 0.06). After adjustment with age, the liver disease stage (Child-Pugh score), and the critical illness severity score (SOFA score), ICU admission between 2005 and 2010 remained an independent favorable prognostic factor (odds ratio (OR) 0.09, 95% confidence interval (CI) 0.02-0.4, P = 0.004). The stage of the underlying liver disease was also independently associated with hospital mortality (Child-Pugh score: OR 1.42 per point, 95% CI 1.06-1.9, P = 0.018).
Conclusions
In the light of advances in management of both cirrhosis and septic shock, survival of such patients substantially increased over recent years. The stage of the underlying liver disease and the related therapeutic options should be included in the decision-making process for ICU admission.
Introduction
The overall incidence of severe sepsis and septic shock is steadily increasing due to the aging of the population and to the growing prevalence of underlying co-morbidities including chronic organ dysfunctions and immunosuppression [1,2]. Several studies have highlighted the major influence of cirrhosis on the susceptibility to severe bacterial infections [3,4]. Indeed, the overall mortality rate of septic shock remains particularly high in cirrhotic patients, ranging from 60% to 100% [5-7], raising the question of indications of aggressive and extensive organ failure support in such patients.
The overall outcome of septic shock has clearly improved over the recent years, related to improved supportive care and rapid and protocolized treatment interventions supported by international guidelines [8]. Of note, the most significant improvements in survival from septic shock have been achieved in vulnerable subgroups including elderly patients, those with malignancies [9,10] or neutropenia [11]. Cirrhotic patients are usually excluded from interventional trials in sepsis, but it is likely that such a high-mortality subgroup would particularly benefit from therapeutic advances in septic shock. In addition, great strides have also been recently achieved in the management of specific complications of cirrhosis. Whether the survival gain achieved by therapeutic advances in septic shock also extends to cirrhotic patients has not been assessed. In order to determine the trend in mortality of cirrhotic patients with septic shock and the impact of related therapeutic interventions, we performed a retrospective single-center study over a 14-year time period.
Materials and methods
Patients and setting
The study took place in a 24-bed medical ICU with an average of 1,500 admissions per year. All patients with histological or clinical diagnosis of cirrhosis and presenting with septic shock at the time of ICU admission or within the first 48 h in the ICU were included. Septic shock was defined as a microbiologically proven or clinically suspected infection, associated with acute circulatory failure requiring vasoactive support despite adequate fluid filling [12]. Senior staffing remained quite stable over the 14-year study period. ICU admission decisions were taken on by both the intensivist and the referring hepatologist throughout the study period. Therefore, only patients with end-stage liver disease declined for liver transplantation were not admitted to the ICU. End-of-life decisions to withhold or withdraw life support were taken on collectively when all participants were convinced that maintenance or increase of life-sustaining therapies was futile and that death would irremediably occur in a short-term manner.
Informed consent was waived since the study was retrospective and observational, in accordance with French regulation of clinical research. This epidemiologic study did not require ethical approval, in accordance with the standards of our local institutional review board.
Intended care for cirrhotic patients with septic shock
Systematic screening for infection included clinical features (temperature, signs of shock), biological parameters (leukocytes), chest X-rays, and cultures of blood, sputum, urine, and ascites. As soon as infection was recognized, patients were promptly treated with empirical broad-spectrum antibiotic combination, depending on the site of infection, known colonization and previous antibiotic treatment. Antifungal therapy was added if fungal infection was suspected or documented. Antimicrobial treatment was narrowed after identification of the responsible pathogen. In addition, source control measures, such as surgery or removal of infected catheters were applied when necessary. Hemodynamic management included fluid resuscitation combined with continuous infusion of vasoactive drugs (mostly norepinephrin, while associated cardiac dysfunction prompted the use of either a combination of norepinephrin and dobutamine or epinephrin). Terlipressin was not used in combination with other vasoactive drugs. Endotracheal intubation and mechanical ventilation were performed in case of respiratory failure or coma. Renal replacement therapy (RRT), through either intermittent hemodialysis or continuous venovenous hemofiltration was initiated in case of acute renal failure, severe metabolic acidosis, or other life-threatening metabolic disorders.
Several adjuvant therapies were progressively implemented in septic patients over the study period. Thus, patients with acute respiratory distress syndrome (ARDS) were mechanically ventilated using a protective strategy with low tidal volume of 6 mL/kg of predicted body weight [13]. More generally, the plateau pressure was limited to 30 cm H2O in all ventilated patients. Intensive insulin therapy was used to maintain blood glucose between 4.4 and 8.1 mmol/L [14]. Low-dose corticosteroids (200 mg hydrocortisone per day) were administrated in vasopressor-dependent septic shock for 5 to 7 days [15]. Patients with at least two organ dysfunctions were considered for treatment with activated protein C in the absence of contraindication [16]. In order to assess whether these therapeutic advances resulted in improved survival in cirrhotic septic patients, we divided the whole cohort in two near-sized period groups, 1997-2004 (first period) and 2005-2010 (second period), in between the first guidelines of the Surviving Sepsis Campaign were published.
Data collection
The following data were collected: demographic characteristics; Charlson co-morbidity index (excluding points for liver disease) [17]; functional status prior to ICU admission as assessed by the Knaus scale (A, prior good health, no functional limitation; B, mild to moderate limitation of activity because of a chronic disease; C, serious but not incapacitating restriction of activity; D, severe restriction of activity, including bedridden or institutionalized persons) [18]; stage of cirrhosis graded using the Child-Pugh classification [19]; and the Model for End-stage Liver Disease (MELD) score [20]. Child-Pugh score was computed prior to the current acute complication whereas MELD score was computed on the first day in the ICU. We also collected the infection characteristics including microbiological and clinical documentation and adequacy of initial antibiotic regimen within the early 48 h following the onset of infection, the organ failures supports including type and volume of fluid loading, mechanical ventilation and RRT, as well as adjuvant treatments of sepsis (intensive insulin therapy, low-dose glucocorticoids and activated protein C). The Simplified Acute Physiology Score 2 (SAPS II) and the Sequential Organ Failure Assessment (SOFA) score were calculated on the first day in the ICU [21,22]. Outcomes were in-ICU, in-hospital, and 6-month survival rates. Vital status was assessed using the medical records or the administrative hospital database. The outcome of patients followed up in another hospital or discharged home was requested to their referring hepatologist or their general practitioner.
Statistical analysis
Results are reported as median (25th-75th percentile) or number (%) as appropriate. Categorical variables were compared with χ2 or Fisher exact tests, and continuous variables were compared with the Mann-Whitney U test. Survival curves were obtained using the Kaplan-Meier method and compared using the log-rank test. To identify characteristics associated with hospital mortality, we used a logistic regression model. Variables that reached a P value < 0.1 were entered into a multivariate analysis. Inclusion of severity scores in the analysis precluded the inclusion of related variables in order to avoid colinearity. The goodness-of-fit of the model was evaluated by the Hosmer-Lemeshow statistic. Odds ratios (OR) and their 95% confidence intervals (CI) were computed. All test were two-sided and P values < 0.05 were considered statistically significant. All calculations were performed with SPSS software version 16 (SPSS, Chicago, IL, USA).
Results
Patients characteristics
During the 14-year study period, 1,632 patients with septic shock were admitted to the ICU, including 89 patients (5.5%) with cirrhosis. Among them, 47 patients were admitted to the ICU during the first period (1997-2004) whereas 42 were admitted during the latter (2005-2010) (Figure 1). Underlying characteristics of patients are presented in Table 1 and were grossly similar between both study periods. Most cirrhosis was caused by chronic alcohol abuse associated or not with chronic viral hepatitis B or C infection. None of the patients presented with acute alcoholic hepatitis. Six patients had hepatocellular carcinoma, at an early stage (stage A, n = 2) or at an intermediate stage (stage B, n = 4), according to the BCLC classification [23].
Figure 1.
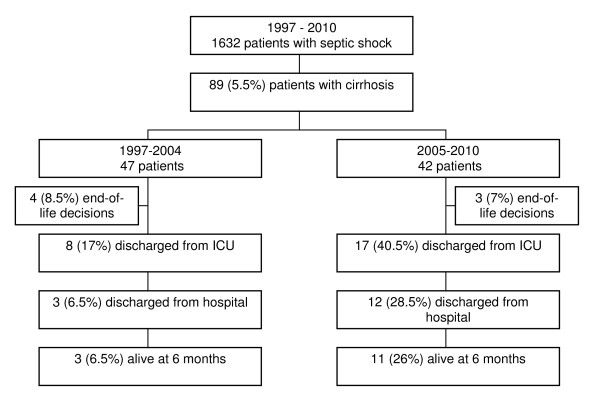
Flow chart of the study.
Table 1.
Baseline characteristics of patients.
| Characteristic | 1997-2004 47 patients |
2005-2010 42 patients |
P |
|---|---|---|---|
| Age (years, IQR) | 55 (46.5-62) | 58 (53-65) | 0.08 |
| Male gender | 36 (76.5) | 26 (62) | 0.49 |
| Knaus scale | 0.15 | ||
| Mild limitation (B) | 6 (12.8) | 10 (23.8) | |
| Important limitation (C) | 25 (53.2) | 17 (40.5) | |
| Severe limitation (D) | 16 (34) | 15 (35.7) | |
| Charlson score (IQR)a | 1 (0-1) | 1 (0-2) | 0.51 |
| Co-morbidities | |||
| Chronic heart failure | 4 (8.5) | 3 (7) | 0.81 |
| COPD | 2 (4.3) | 3 (7) | 0.79 |
| Diabetes | 6 (12.8) | 10 (24) | 0.27 |
| Cancer | 8 (17) | 8 (19) | 0.80 |
| Hepatocellular carcinoma | 3 | 3 | |
| Otherb | 5 | 5 | |
| Immunosuppressionc | 6 (12.8) | 2 (4.8) | 0.27 |
| Cause of cirrhosisd | |||
| Alcohol +/- virus | 38 (80.1) | 34 (80.1) | 1 |
| Chronic Hepatitis B virus infection | 2 (4.3 ) | 0 | 0.50 |
| Chronic Hepatitis C virus infection | 6 (12.8) | 4 (9.5) | 0.74 |
| Primary biliary cirrhosis | 2 (4.3) | 2 (4.8) | 1 |
| Undetermined | 3 (6.4) | 2 (4.8) | 1 |
| Persistent alcohol abuse | 26 (55.3) | 22 (52.3) | 0.83 |
| Nosocomial septic shock | 13 (27.7) | 14 (33.3) | 0.67 |
| Primary source of infection | |||
| Respiratory | 18 (38.3) | 19 (45.2) | 0.53 |
| Abdominal | 10 (21.3) | 16 (38,1) | 0.10 |
| Spontaneous peritonitis | 8 (17) | 14 (33.3) | |
| Secondary peritonitis | 2 (4.3) | 2 (4.8) | |
| Urinary tract | 6 (12.8) | 4 (9.5) | 0.49 |
| Others | |||
| CNS | 3 (6.4) | 0 | 0.10 |
| Arthritis | 1 (2.1) | 1(2.4) | 1 |
| Isolated bacteriemia | 2 (4.2) | 0 | 0.50 |
| Unknown | 7 (14.9) | 2 (4.8) | 0.16 |
| Bacteremia | 20 (42.6) | 14 (33.3) | 0.39 |
| Type of organisms | |||
| Gram-positive cocci | 15 (31.9) | 13 (30.9) | 1 |
| Gram-negative bacilli | 17 (36.2) | 11 (23.8) | 0.25 |
| Fungi | 2 (4.3) | 2 (4.8) | 1 |
| Culture negative | 12 (25.5) | 8 (19) | 0.61 |
| Polymicrobial sepsis | 1 (2.1) | 8 (19) | 0.01 |
| Multi-drug resistant bacteria | 2 (4.3) | 8 (17) | 0.04 |
Variables are expressed as number (percentage) or median (interquartile range (IQR)). aExcluding liver disease points.
bBreast cancer (n = 3), colon cancer (n = 2), laryngeal cancer (n = 2), cholangiocarcinoma (n = 1), bladder cancer (n = 1), myeloma (n = 1).
cInfection by human immunodeficiency virus (n = 2), multiple myeloma (n = 1), hypogammaglobulinemia (n = 1), treatment with mesalazine (n = 1) and recent chemotherapy for cancer (n = 3).
dSum of causes may exceed the number of patients because of concomitant alcoholic and viral cirrhosis.
CNS, Central Nervous System; COPD, Chronic Pulmonary Obstructive Disease.
The main sites of infections were pneumonia (42%), spontaneous or secondary peritonitis (29%), and urinary tract infection (11%). Sixty-nine patients (78%) had microbiologically documented infections balanced between gram-positive cocci (31%) and gram-negative bacilli (31%) (Table 1). Multi-drug resistant bacteria were more frequently involved in the recent period (Table 1).
Management of organ failures
Major differences were noted in the management of septic shock between both study periods, including type and volumes of fluid loading within the first 3 days, ventilatory management, and adjuvant therapies of sepsis (Table 2). Indeed, intravenous albumin was frequently used in the most recent period (57.1% of patients vs. 8.5%, P < 0.001) whereas infusion of crystalloids was markedly reduced in the same time (3 (1.7-4.5) L vs. 6 (3-8.9) L, P < 0.001). Moreover, albumin-resuscitated patients tended to receive a higher albumin dose during the recent period (50 (30-72.5) vs. 20 (17.5-30) g, P = 0.06). RRT was less frequently required in the recent period (52.4 vs. 72.3%, P = 0.08). The ventilatory management also significantly differed between the two periods with smaller tidal volumes used in the period 2005-2010 (8.6 vs. 7 mL/kg, P = 0.001). Intensive insulin therapy and low-dose glucocorticoids were also more frequently used in the second period (83.3% vs. 31.9%, P < 0.001 and 81% vs. 44.7, P < 0.001, respectively). Only two patients were treated with drotrecogin alpha (activated) in the recent period.
Table 2.
Organ failures, ICU management, and outcome.
| Characteristic | 1997-2004 47 patients |
2005-2010 42 patients |
P |
|---|---|---|---|
| Scoring systems (IQR) | |||
| Child-Pugh score | 9 (7-11) | 10 (8.25-11) | 0.22 |
| MELD day 1 | 25 (17-33.8) | 26 (20.2-32.8) | 0.43 |
| SAPS II day 1 | 56 (38-70.5) | 59 (42-76) | 0.10 |
| SOFA day 1 | 14 (8.5-17.5) | 13 (9-15) | 0.18 |
| Biological findings at ICU admission (IQR) | |||
| Serum creatinine level, μmol/L | 140 (96-199) | 147 (105-235) | 0.93 |
| Serum bilirubin level, μmol/L | 48 (35-110) | 68 (44.2-140.5) | 0.23 |
| Arterial blood lactate level, mmol/L | 4.2 (2-6.5) | 4.3 (2.2-7.9) | 0.68 |
| Serum sodium, mmol/L | 134 (130.5-139) | 135.5 (129.2-139) | 0.74 |
| Serum protein level, g/L | 54 (44.5-61) | 59 (50-66.8) | 0.05 |
| Factor V, % | 42 (33-64) | 46 (33-60) | 0.70 |
| INR | 2 (1.5-2.8) | 2 (1.6-3.1) | 0.69 |
| White blood cells, 103/mm3 | 13.6 (6.2-19) | 10.7 (6.4-15.2) | 0.45 |
| C-reactive protein, mg/L | 101 (60-167) | 75 (34-127) | 0.24 |
| Mechanical ventilation | 45 (95.7) | 39 (93) | 0.34 |
| Tidal volume, mL/kg (IQR)a | 8.6 (7.3-9.9) | 7 (6.1-7.9) | <0.001 |
| Lowest Pao2/Fio2 ratio (IQR) | 101 (80-150) | 117 (86-206) | 0.17 |
| ARDS | 16 (34) | 14 (33.3) | 1 |
| Renal replacement therapy | 34 (72.3) | 22 (52.4) | 0.08 |
| Antimicrobial treatment | |||
| β-lactam | 45 (95.7) | 41 (97.6) | 1 |
| Quinolone | 19 (40.4) | 12 (25.5) | 0.27 |
| Aminoglycoside | 23 (49) | 22 (52.4) | 0.83 |
| Glycopeptide | 9 (19.1) | 6 (14.3) | 0.58 |
| Combination therapy | 31 (64) | 34 (81) | 0.15 |
| Inadequacy of initial antimicrobial treatment | 5 (10.6) | 3 (7.1) | 1 |
| Fluid loading within the first 3 days | |||
| Crystalloids, L (IQR) | 6 (3-8.9) | 3 (1.7-4.5) | <0.001 |
| Albumin resuscitation | 4 (8.5) | 24 (57.1) | <0.001 |
| Adjuvant therapies of sepsis | |||
| Intensive insulin therapy | 15 (31.9) | 35 (83.3) | <0.001 |
| Low-dose glucocorticoids | 21 (44.7) | 34 (81) | <0.001 |
| Activated protein C | 0 | 2 (4.8) | 1 |
| ICU survival | 8 (17) | 17 (40) | 0.02 |
| Hospital survival | 3 (6) | 12 (29) | 0.009 |
| 6-month survival | 3 (6) | 9 (21) | 0.06 |
Variables are expressed as number (percentage) or median (interquartile range (IQR)).
aThe predicted body weight was calculated using the following formulas: 50+0.91 (centimeters of height-152.4) (male patients) or 45.5+0.91(centimeters of height-152.4) (female patients).
ARDS, Acute Respiratory Distress Syndrome; INR, International Normalized Ratio; MELD, Model for End Stage Liver Disease; SAPS, Simplified Acute Physiology Score; SOFA, Sequential Organ Failure Assessment.
Short-term and long-term outcomes
The rate of end-of-life decisions was similar between the two periods (Figure 1). The 6-month vital status was obtained for all patients. We observed a marked improvement in ICU and hospital survival rates in the recent period as compared to the 1997-2004 period (40% vs. 17%, P = 0.02 and 29% vs. 6%, P = 0.009, respectively). Most importantly, differences in survival occurred early in the course of the disease (Figure 2A). The benefit in short-term survival was sustained throughout the first 6 months following ICU admission (6-month survival rate 21% vs. 6%, P = 0.06) (Figure 2B). Two survivors of the recent period underwent liver transplantation within 6 months after ICU admission.
Figure 2.
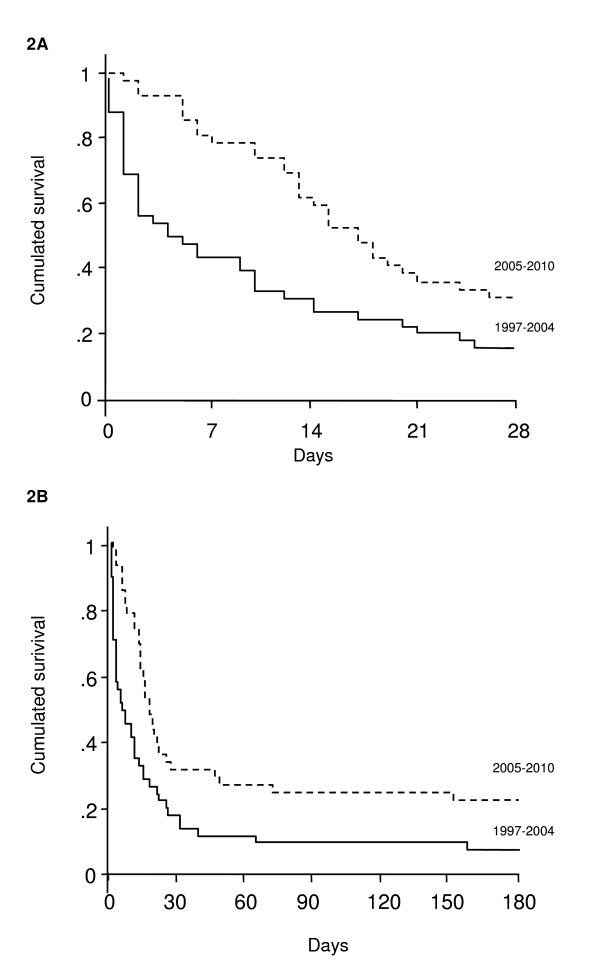
Kaplan Meier estimates of 28-day (A) and 6-month (B) survival according to the period of admission (1997-2004, continuous line; 2005-2010, dotted line). Log-rank test: P = 0.02.
Prognostic factors of hospital mortality
In order to identify the determinants of outcome, we compared the characteristics and treatments of hospital survivors and non-survivors (Table 3). Determinants of hospital mortality were the stage of the liver disease (Child-Pugh score, serum protein, and factor V levels), the extent of organ failures (day-1 SAPS II and SOFA scores, admission serum lactate level, renal replacement therapy), and admission during the first period. Finally, we carried out a multivariate logistic regression analysis taking into account the underlying liver disease stage (Child-Pugh score), the critical illness severity score (for example, SOFA score), and the recent changes in sepsis management (ICU admission period). When adjusted for admission SOFA score and age, the 2005-2010 period remained protective (OR 0.09, 95% CI 0.02-0.4) whereas the stage of cirrhosis prior to the acute complication was independently associated with hospital mortality (Child-Pugh score: OR 1.42 per point, 95% CI 1.06-1.9) (Table 4). Similar results were obtained when adjusted for SAPS II.
Table 3.
Determinants of hospital outcome (univariate analysis).
| Variable | Deceased 74 patients |
Survivors 15 patients |
P |
|---|---|---|---|
| Age (IQR) | 56 (48-65) | 58 (54-62.5) | 0.6 |
| Scoring systems (IQR) | |||
| Child-Pugh score | 10 (8-11) | 7 (7-10) | 0.05 |
| MELD day 1 | 25 (18-33.5) | 26 (18.5-33.5) | 0.19 |
| SAPS II day 1 | 59 (41.5-83) | 50 (42-62.5) | 0.02 |
| SOFA day 1 | 14 (10-17) | 9 (7.5-9) | 0.03 |
| Biological findings at ICU admission (IQR) | |||
| Arterial blood lactate level, mmol/l | 4.2 (2.1-7.3) | 2.8 (2-4.5) | 0.08 |
| Serum protein level, g/L | 54 (45-62) | 62.5 (56-66) | 0.06 |
| Factor V, % | 41 (27.5-62.5) | 53 (48.5-70.5) | 0.02 |
| Renal replacement therapy | 40 (65%) | 5 (36%) | 0.02 |
| ARDS | 22 (35.5%) | 2 (13.5%) | 0.08 |
| Albumin resuscitation | 24 (32.4) | 4 (26.7) | 0.77 |
| Low-dose glucocorticoids | 44 (59.5 ) | 11 (73.3) | 0.39 |
| Intensive insulin therapy | 39 (52.7) | 11 (73.3) | 0.16 |
| Admission period | |||
| 1997-2004 | 44 (59.5) | 3 (20) | 0.009 |
| 2005-2010 | 30 (40.5) | 12 (80) | |
Variables are expressed as number (percentage) or median (interquartile range (IQR)).
ARDS, acute respiratory distress syndrome; MELD, Model For End Stage Liver Disease; SAPS, Simplified Acute Physiology Score; SOFA, Sequential Organ Failure Assessment.
Table 4.
Multivariate analysis of factors associated with in-hospital mortality.
| Variable | Odds ratio | 95% CI | P |
|---|---|---|---|
| SOFA (per point) | 1.15 | 0.99-1.32 | 0.06 |
| Age | 1.02 | 0.96-1.08 | 0.53 |
| Child-Pugh score (per point) | 1.42 | 1.06-1.9 | 0.018 |
| Period 2005-2010 (compared to 1997-2004) | 0.09 | 0.02-0.4 | 0.004 |
CI, confidence interval, Goodness of fit (Hosmer-Lemeshow) chi-square P value = 0.54.
Discussion
Septic shock represents a severe complication of cirrhosis with very low survival rates that question the relevance of life-sustaining therapies in this subgroup of patients. We report here that the current survival rate remains low but substantially improved over time, suggesting that advances in care of septic shock extended to this high-risk subgroup of patients. Most importantly, the short-term improvement in survival was sustained for at least 6 months, suggesting that ICU admission and extensive life support is justified in some patients. In addition to organ failures, the stage of liver disease as assessed by the Child-Pugh score appears to be an independent prognostic factor that should be taken into account in the decision-making process.
Cirrhosis is clearly associated with an increased predisposition to sepsis [4] and has been identified as an independent poor prognostic factor in patients with severe sepsis [24]. In addition, chronic alcohol abuse by itself may contribute to worsening organ failures and has been shown to be an independent risk factor of septic shock [25]. Multiple mechanisms concur to confer an increased susceptibility to bacterial infections and subsequently to multiple organ failure in cirrhotic patients. Bacterial translocation increases with the severity of liver disease and represents the main mechanism of spontaneous bacterial peritonitis. Furthermore, it may sustain the septic process in every type of infection as suggested by the increased levels of endotoxin observed in cirrhotic patients [26]. In addition, innate immune cells display functional abnormalities such as increased production of pro-inflammatory cytokines in response to LPS [27] or alcohol-induced defective phagocytosis [28]. As a consequence, plasma TNF-α and IL-6 levels are higher in cirrhotic patients with bacterial infection than in non-cirrhotic patients [29].
The overall outcome of severe sepsis and septic shock has been improved over the last decade concomitant to the emergence of adjuvant therapeutic interventions such as early-goal directed therapy [30], protective mechanical ventilation with low tidal volumes [13], low-dose corticosteroids [15], intensive insulin therapy [14], or activated protein C [16] supported by positive randomized controlled trials. Although the true benefit of some of these interventions have been addressed by additional studies, they have been included in the Surviving Sepsis Campaign guidelines [8]. This international guideline-based performance improvement program promoted early recognition and management of sepsis and showed that increased compliance with the guidelines was associated with an improved survival rate [31]. In the same way, a continuous decrease in the mortality of severe sepsis and septic shock patients has been reported in large unselected cohorts that mostly comprised non-cirrhotic patients [1,32,33]. Of note, some significant progress in septic shock has also been achieved in highly vulnerable subgroups of patients such as immunocompromised patients with malignancies [9].
Differences in survival between the two periods occurred early in the course of the disorder and were thereafter maintained. The rate of inadequate antibiotic treatment was low and similar between both periods. Most patients received an initial combination antimicrobial treatment of β-lactam associated with either aminoglycosides or fluoroquinolones. An initial single dose of aminoglycosides was commonly subsequently replaced by fluoroquinolones to limit harmful side-effects. Indeed, some retrospective studies suggest a benefit of combination antibiotherapy, most especially of betalactams and aminoglycosides, for septic shock in general cohorts as well as in cirrhotic patients [7,34]. Survival improvement was more likely related to changes in the early management of shock and organ failures. With respect to fluid resuscitation strategies, the majority of patients from the recent period received albumin and consequently received less crystalloids. Albumin resuscitation was associated with higher Child-Pugh and MELD scores (P = 0.04 and P = 0.03 respectively, data not shown), suggesting that albumin was preferentially indicated to patients with advanced stages of cirrhosis. The role of albumin resuscitation in sepsis remains challenging. A meta-analysis suggested that fluid resuscitation with albumin compared to crystalloids was associated with improved survival in patients with severe sepsis [35]. Specifically, albumin resuscitation has been shown to reduce mortality and renal impairment in cirrhotic patients treated for spontaneous bacterial peritonitis [36]. Accordingly, frequent albumin resuscitation in the recent period of our study was also associated with less frequent recourse to renal replacement therapy. Altogether, these results and ours suggest a possible benefit of albumin resuscitation in cirrhotic patients with severe sepsis, that remains to be prospectively investigated. The frequent use of low-dose corticosteroids was also a hallmark of sepsis management during the second period. Indeed, adrenal dysfunction frequently occurs in patients with septic shock and is associated with hemodynamic instability, renal dysfunction, and increased mortality [37]. However, the use of low-dose corticosteroids in septic shock remains controversial because of discrepant efficacy data and a possible higher risk of nosocomial infections [15]. Of note, this treatment has been specifically addressed in cirrhotic patients with septic shock. In a case-control study, resolution of shock and survival were higher in hydrocortisone-treated cirrhotic patients [38]. However a randomized controlled trial in septic shock cirrhotic patients was stopped for futility at interim analysis because hydrocortisone did not reduce mortality and was associated with an increase in adverse effects such as shock relapse and gastrointestinal bleeding [39]. In addition to sepsis-specific therapies, general measures for ICU patients such as ventilation with low tidal volumes and glucose control with intravenous insulin therapy were also routinely implemented during the second period.
Cirrhotic patients are commonly perceived as poor candidates for ICU admission because of the very high mortality rates associated with organ failures [40,41]. A general improvement in outcome for cirrhotic patients in the ICU has been recently reported regardless of the type of acute complication [42,43]. Several factors may explain this progress, including a better selection of patients on the basis of previous functional status and advances in the management of acute complications such as variceal bleeding [44], hepatorenal syndrome [45], or septic shock as highlighted in the present study. The extent of organ failures clearly represents a major determinant of outcome, and the performance of SOFA score that nearly reached significance in our multivariate model is in accordance with previous studies [6,46]. Most importantly, we also identified the stage of the underlying liver disease assessed by the Child-Pugh score as an independent prognostic factor of septic shock. Until now, the discrimination of the Child-Pugh score calculated at the time of ICU admission had remained inferior to organ failure scores [6,46,47]. As a matter of fact, we computed Child-Pugh score prior to the acute complication in order to reliably assess the stage of liver disease without any interference from new-onset organ failures. This finding carries major practical implications for the decision-making process. Indeed, this could allow a better selection of patients likely to benefit from intensive care, on the basis of underlying disease's status and realistic therapeutic options including liver transplantation. Nevertheless, an accurate individual prognosis prediction of cirrhotic patients is often difficult at the time of ICU admission, but can be markedly refined after a few days [6,46]. In the light of improved outcomes and limited performance of initial prognostic prediction, critically-ill cirrhotic patients with a reasonable long-term prognosis should be offered a broad intensive care access policy with subsequent reappraisal based on the nature of the acute complication and the evolution of organ failures.
Our study has several limitations that we acknowledge. First, the design was retrospective despite most data were prospectively collected through computerized patient data management system. Therefore, we can only report an association between changes in care and patient outcome over the study period. Second, it was carried out in a single center, with a hepatology unit that is closely involved in the decision-making process and in the management of critically ill cirrhotic patients while in the ICU and after discharge. Third, the limited number of patients may limit the external validity of our findings. However, our survival rates in the latter period are similar to those reported by Arabi et al. (hospital survival 24%) [7] and Levesque et al. (ICU survival 36%) [6]. Fourth, indications for ICU admission or end-of-life decisions might have evolved over the study period, and we cannot exclude that patients from the recent period were more carefully selected or referred earlier to the ICU. Nevertheless, the functional status, the stage of the underlying liver disease and the severity scores were similar between both periods. In addition, arterial blood lactate level as an indicator of prolonged systemic hypoperfusion also suggested similar duration and severity of shock before ICU admission. Altogether, these results suggest that improvements in survival were more likely related to changes in care. Fourth, we failed to link the recent improvement in survival with a single therapeutic change, suggesting that it is more likely related to a combination of interventions. Alternatively, some unrecognized or non-collected data might also influence the outcome. For instance, the functional status assessed by the performance status prior to the acute complication is a major prognostic factor in critically-ill cancer patients [48], and might be more accurate than the Knaus scale in this setting. In the same way, the nutritional status might also be of importance in these patients [49].
Conclusions
This study reports an encouraging improvement in survival in cirrhotic patients with septic shock that needs to be confirmed in a larger multicenter cohort. Implementation of therapeutic advances in sepsis probably accounted for this result. In addition, the stage of the underlying liver disease appears as an important prognostic factor. Delineation of the long-term prognosis of cirrhosis and the related therapeutic options thus appears essential in order to determine the indications for life-sustaining therapies.
Key messages
• The current survival rate of septic shock in cirrhotic patients remains low but has improved over the recent years.
• Cirrhotic patients could have benefited from recent advances in the management of septic shock.
• The stage of liver disease prior to the acute complication as assessed by the Child-Pugh score appears to be an independent prognostic factor of hospital mortality.
List of abbreviations
ARDS: Acute Respiratory Distress Syndrome; CI: Confidence Interval; COPD: Chronic Obstructive Pulmonary Disease; ICU: Intensive Care Unit; IL: Interleukin; INR: International Normalized Ratio; MELD: Model for End-stage Liver Disease; OR: Odds Ratio; RRT: Renal Replacement Therapy; SAPS: Simplified Acute Physiology Score; SOFA: Sequential Organ Failure Assessment; TNF: Tumor Necrosis Factor.
Competing interests
JC is consultant for LFB and received lecture fees. JPM is consultant and member of the scientific board of LFB, and received lecture fees from LFB, Fresenius and Baxter. JC and JPM were the main investigators of an interventional trial on albumin resuscitation in septic shock. FP received lecture fees from LFB. The authors declare that they have no other competing interests relevant to the field of the manuscript.
Authors' contributions
BS, BC, and FP designed the study. BS, BC, AS, and NM extracted the data. BS and FP performed the statistical analysis. BS, BC, AS, NM, JC, AC, JDC, VM, JPM, and FP contributed to data analysis. BS and FP drafted the manuscript. All authors read and approved the final version of the manuscript.
Contributor Information
Bertrand Sauneuf, Email: bertrand.sauneuf@cch.aphp.fr.
Benoit Champigneulle, Email: bchampig@hotmail.com.
Alexis Soummer, Email: alexis.soummer@cch.aphp.fr.
Nicolas Mongardon, Email: nicolas.mongardon@cch.aphp.fr.
Julien Charpentier, Email: julien.charpentier@cch.aphp.fr.
Alain Cariou, Email: alain.cariou@cch.aphp.fr.
Jean-Daniel Chiche, Email: jean-daniel.chiche@cch.aphp.fr.
Vincent Mallet, Email: vincent.mallet@cch.aphp.fr.
Jean-Paul Mira, Email: jean-paul.mira@cch.aphp.fr.
Frédéric Pène, Email: frederic.pene@cch.aphp.fr.
Acknowledgements
Presented at the 40th Congress of the Société de Réanimation de Langue Française, 18-20 January 2012, Paris, France.
References
- Annane D, Aegerter P, Jars-Guincestre MC, Guidet B. Current epidemiology of septic shock: the CUB-Rea Network. Am J Respir Crit Care Med. 2003;17:165–172. doi: 10.1164/rccm.2201087. [DOI] [PubMed] [Google Scholar]
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;17:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- Fernandez J, Navasa M, Gomez J, Colmenero J, Vila J, Arroyo V, Rodes J. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;17:140–148. doi: 10.1053/jhep.2002.30082. [DOI] [PubMed] [Google Scholar]
- Foreman MG, Mannino DM, Moss M. Cirrhosis as a risk factor for sepsis and death: analysis of the National Hospital Discharge Survey. Chest. 2003;17:1016–1020. doi: 10.1378/chest.124.3.1016. [DOI] [PubMed] [Google Scholar]
- Moreau R, Hadengue A, Soupison T, Kirstetter P, Mamzer MF, Vanjak D, Vauquelin P, Assous M, Sicot C. Septic shock in patients with cirrhosis: hemodynamic and metabolic characteristics and intensive care unit outcome. Crit Care Med. 1992;17:746–750. doi: 10.1097/00003246-199206000-00008. [DOI] [PubMed] [Google Scholar]
- Levesque E, Hoti E, Azoulay D, Ichai P, Habouchi H, Castaing D, Samuel D, Saliba F. Prospective evaluation of the prognostic scores for cirrhotic patients admitted to an intensive care unit. J Hepatol. 2011;17:95–102. doi: 10.1016/j.jhep.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Arabi YM, Dara SI, Memish Z, Al-Abdulkareem A, Tamim HM, Al-Shirawi N, Parrillo JE, Dodek P, Lapinsky S, Feinstein D, Wood G, Dial S, Zanotti S, Kumar A. Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group. Antimicrobial therapeutic determinants of outcomes in cirrhotic patients with septic shock. Hepatology. 2012;17:2305–2315. doi: 10.1002/hep.25931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008;17:17–60. doi: 10.1007/s00134-007-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber B, Tran TC, Aegerter P, Grimaldi D, Charpentier J, Guidet B, Mira JP, Pène F. Impact of case volume on survival of septic shock in patients with malignancies. Crit Care Med. 2012;17:55–62. doi: 10.1097/CCM.0b013e31822d74ba. [DOI] [PubMed] [Google Scholar]
- Pène F, Percheron S, Lemiale V, Viallon V, Claessens YE, Marque S, Charpentier J, Angus DC, Cariou A, Chiche JD, Mira JP. Temporal changes in management and outcome of septic shock in patients with malignancies in the intensive care unit. Crit Care Med. 2008;17:690–696. doi: 10.1097/CCM.0B013E318165314B. [DOI] [PubMed] [Google Scholar]
- Legrand M, Max A, Peigne V, Mariotte E, Canet E, Debrumetz A, Lemiale V, Seguin A, Darmon M, Schlemmer B, Azoulay E. Survival in neutropenic patients with severe sepsis or septic shock*. Crit Care Med. 2012;17:43–49. doi: 10.1097/CCM.0b013e31822b50c2. [DOI] [PubMed] [Google Scholar]
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;17:530–538. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- The Acute Respiratory Distress Syndrom Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;17:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;17:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach JM, Capellier G, Cohen Y, Azoulay E, Troche G, Chaumet-Riffaud P, Bellissant E. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;17:862–871. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ Jr. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;17:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;17:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Knaus WA, Zimmerman JE, Wagner DP, Draper EA, Lawrence DE. APACHE-acute physiology and chronic health evaluation: a physiologically based classification system. Crit Care Med. 1981;17:591–597. doi: 10.1097/00003246-198108000-00008. [DOI] [PubMed] [Google Scholar]
- Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;17:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;17:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;17:2957–2963. doi: 10.1001/jama.1993.03510240069035. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;17:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;17:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- Brun-Buisson C, Meshaka P, Pinton P, Vallet B. EPISEPSIS: a reappraisal of the epidemiology and outcome of severe sepsis in French intensive care units. Intensive Care Med. 2004;17:580–588. doi: 10.1007/s00134-003-2121-4. [DOI] [PubMed] [Google Scholar]
- O'Brien JM Jr, Lu B, Ali NA, Martin GS, Aberegg SK, Marsh CB, Lemeshow S, Douglas IS. Alcohol dependence is independently associated with sepsis, septic shock, and hospital mortality among adult intensive care unit patients. Crit Care Med. 2007;17:345–350. doi: 10.1097/01.CCM.0000254340.91644.B2. [DOI] [PubMed] [Google Scholar]
- Lin RS, Lee FY, Lee SD, Tsai YT, Lin HC, Lu RH, Hsu WC, Huang CC, Wang SS, Lo KJ. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol. 1995;17:165–172. doi: 10.1016/0168-8278(95)80424-2. [DOI] [PubMed] [Google Scholar]
- Deviere J, Content J, Denys C, Vandenbussche P, Schandene L, Wybran J, Dupont E. Excessive in vitro bacterial lipopolysaccharide-induced production of monokines in cirrhosis. Hepatology. 1990;17:628–634. doi: 10.1002/hep.1840110416. [DOI] [PubMed] [Google Scholar]
- Fiuza C, Salcedo M, Clemente G, Tellado JM. In vivo neutrophil dysfunction in cirrhotic patients with advanced liver disease. J Infect Dis. 2000;17:526–533. doi: 10.1086/315742. [DOI] [PubMed] [Google Scholar]
- Byl B, Roucloux I, Crusiaux A, Dupont E, Deviere J. Tumor necrosis factor alpha and interleukin 6 plasma levels in infected cirrhotic patients. Gastroenterology. 1993;17:1492–1497. doi: 10.1016/0016-5085(93)90361-f. [DOI] [PubMed] [Google Scholar]
- Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;17:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, Schorr C, Artigas A, Ramsay G, Beale R, Parker MM, Gerlach H, Reinhart K, Silva E, Harvey M, Regan S, Angus DC. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med. 2010;17:367–374. doi: 10.1097/CCM.0b013e3181cb0cdc. [DOI] [PubMed] [Google Scholar]
- Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;17:1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- Harrison DA, Welch CA, Eddleston JM. The epidemiology of severe sepsis in England, Wales and Northern Ireland, 1996 to 2004: secondary analysis of a high quality clinical database, the ICNARC Case Mix Programme Database. Crit Care. 2006;17:R42. doi: 10.1186/cc4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Zarychanski R, Light B, Parrillo J, Maki D, Simon D, Laporta D, Lapinsky S, Ellis P, Mirzanejad Y, Martinka G, Keenan S, Wood G, Arabi Y, Feinstein D, Kumar A, Dodek P, Kravetsky L, Doucette S. Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group. Early combination antibiotic therapy yields improved survival compared with monotherapy in septic shock: a propensity-matched analysis. Crit Care Med. 2010;17:1773–1785. doi: 10.1097/CCM.0b013e3181eb3ccd. [DOI] [PubMed] [Google Scholar]
- Delaney AP, Dan A, McCaffrey J, Finfer S. The role of albumin as a resuscitation fluid for patients with sepsis: a systematic review and meta-analysis. Crit Care Med. pp. 386–391. [DOI] [PubMed]
- Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M, Ginès P, Rodés J. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;17:403–409. doi: 10.1056/NEJM199908053410603. [DOI] [PubMed] [Google Scholar]
- Tsai MH, Peng YS, Chen YC, Liu NJ, Ho YP, Fang JT, Lien JM, Yang C, Chen PC, Wu CS. Adrenal insufficiency in patients with cirrhosis, severe sepsis and septic shock. Hepatology. 2006;17:673–681. doi: 10.1002/hep.21101. [DOI] [PubMed] [Google Scholar]
- Fernandez J, Escorsell A, Zabalza M, Felipe V, Navasa M, Mas A, Lacy AM, Gines P, Arroyo V. Adrenal insufficiency in patients with cirrhosis and septic shock: Effect of treatment with hydrocortisone on survival. Hepatology. 2006;17:1288–1295. doi: 10.1002/hep.21352. [DOI] [PubMed] [Google Scholar]
- Arabi YM, Aljumah A, Dabbagh O, Tamim HM, Rishu AH, Al-Abdulkareem A, Knawy BA, Hajeer AH, Tamimi W, Cherfan A. Low-dose hydrocortisone in patients with cirrhosis and septic shock: a randomized controlled trial. CMAJ. 2010;17:1971–1977. doi: 10.1503/cmaj.090707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Cheyron D, Bouchet B, Parienti JJ, Ramakers M, Charbonneau P. The attributable mortality of acute renal failure in critically ill patients with liver cirrhosis. Intensive Care Med. 2005;17:1693–1699. doi: 10.1007/s00134-005-2842-7. [DOI] [PubMed] [Google Scholar]
- Goldfarb G, Nouel O, Poynard T, Rueff B. Efficiency of respiratory assistance in cirrhotic patients with liver failure. Intensive Care Med. 1983;17:271–273. doi: 10.1007/BF01691253. [DOI] [PubMed] [Google Scholar]
- O'Brien AJ, Welch CA, Singer M, Harrison DA. Prevalence and outcome of cirrhosis patients admitted to UK intensive care: a comparison against dialysis-dependent chronic renal failure patients. Intensive Care Med. 2012;17:991–1000. doi: 10.1007/s00134-012-2523-2. [DOI] [PubMed] [Google Scholar]
- Galbois A, Trompette ML, Das V, Boelle PY, Carbonell N, Thabut D, Housset C, Ait-Oufella H, Offenstadt G, Maury E, Guidet B. Improvement in the prognosis of cirrhotic patients admitted to an intensive care unit, a retrospective study. Eur J Gastroenterol Hepatol. 2012;17:897–904. doi: 10.1097/MEG.0b013e3283544816. [DOI] [PubMed] [Google Scholar]
- Carbonell N, Pauwels A, Serfaty L, Fourdan O, Levy VG, Poupon R. Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology. 2004;17:652–659. doi: 10.1002/hep.20339. [DOI] [PubMed] [Google Scholar]
- Moreau R, Durand F, Poynard T, Duhamel C, Cervoni JP, Ichai P, Abergel A, Halimi C, Pauwels M, Bronowicki JP, Giostra E, Fleurot C, Gurnot D, Nouel O, Renard P, Rivoal M, Blanc P, Coumaros D, Ducloux S, Levy S, Pariente A, Perarnau JM, Roche J, Scribe-Outtas M, Valla D, Bernard B, Samuel D, Butel J, Hadengue A, Platek A. et al. Terlipressin in patients with cirrhosis and type 1 hepatorenal syndrome: a retrospective multicenter study. Gastroenterology. 2002;17:923–930. doi: 10.1053/gast.2002.32364. [DOI] [PubMed] [Google Scholar]
- Das V, Boelle PY, Galbois A, Guidet B, Maury E, Carbonell N, Moreau R, Offenstadt G. Cirrhotic patients in the medical intensive care unit: early prognosis and long-term survival. Crit Care Med. 2010;17:2108–2116. doi: 10.1097/CCM.0b013e3181f3dea9. [DOI] [PubMed] [Google Scholar]
- Cholongitas E, Senzolo M, Patch D, Shaw S, Hui C, Burroughs AK. Review article: scoring systems for assessing prognosis in critically ill adult cirrhotics. Aliment Pharmacol Ther. 2006;17:453–464. doi: 10.1111/j.1365-2036.2006.02998.x. [DOI] [PubMed] [Google Scholar]
- Soares M, Caruso P, Silva E, Teles JM, Lobo SM, Friedman G, Dal Pizzol F, Mello PV, Bozza FA, Silva UV, Torelly AP, Knibel MF, Rezende E, Netto JJ, Piras C, Castro A, Ferreira BS, Rea-Neto A, Olmedo PB, Salluh JI. Characteristics and outcomes of patients with cancer requiring admission to intensive care units: a prospective multicenter study. Crit Care Med. 2010;17:9–15. doi: 10.1097/CCM.0b013e3181c0349e. [DOI] [PubMed] [Google Scholar]
- Huisman EJ, Trip EJ, Siersema PD, van Hoek B, van Erpecum KJ. Protein energy malnutrition predicts complications in liver cirrhosis. Eur J Gastroenterol Hepatol. 2011;17:982–989. doi: 10.1097/MEG.0b013e32834aa4bb. [DOI] [PubMed] [Google Scholar]


