Abstract
Opioid use in HIV infection has been associated with an increased frequency of neurological disease and cognitive impairment and vitamin A deficiency has been linked to progressive HIV disease in drug users. In this report the potential effects of these factors, alone and in combination, on gamma amino butyric acid (GABA)-expression interneurons in hippocampus in the HIV-1 transgenic rat (TG) model were studied. TG and wild-type (WT) F344 Fisher rats that were vitamin A deficient from birth were implanted either with a 37.5 mg morphine tablet or with a matching placebo and total numbers of neurons and of parvalbumin+ neurons were quantitated and parvalbumin expression was quantitated in the CA1 hippocampal region of the rats. These studies showed that total neuronal numbers were decreased in the TG versus WT Fisher rats and that this decrease was enhanced by the vitamin A deficient diet and by treatment with morphine. In contrast, there was no significant change noted in numbers of parvalbumin+ neurons. However, levels of parvalbumin expression were decreased for vitamin A deficient and morphine-treated WT rats as compared to WT rats on the normal diet and placebo-treated WT rats. For TG rats, parvalbumin expression was higher for vitamin A deficient TG rats treated with either placebo or morphine than for WT vitamin A deficient rats treated with placebo, and placebo treated vitamin A deficient TG rats showed higher expression than morphine treated vitamin A deficient rats. Expression was also higher for vitamin A deficient morphine-treated rats than for the corresponding WT rat groups and for vitamin A deficient TG rats treated with placebo. For the remaining groups, parvalbumin was similar for the TG and WT rats. These findings suggest that in hippocampus vitamin A deficiency and morphine can increase parvalbumin expression, perhaps as a manifestation of a stress response. Parvalbumin-expressing GABA-ergic interneurons regulate the primary neuronal output from hippocampus that is important for memory and behavior. Therefore, these studies suggest that vitamin A deficiency and morphine might have effects that may impact such outputs and thereby have lasting effects on cognitive status.
INTRODUCTION
HIV-associated dementia is a severe complication of primarily late HIV infection that is associated with debilitating impairment of cognition and increased mortality (1). Recent statistics in Western countries show stabilization in the number of new cases of HIV infection (2). With the availability of effective antiretroviral therapy there has been an increased survival of HIV+ individuals accompanied by a decreased incidence of HIV-associated dementia (3–5). Although the rate of new cases of HIV infection and incidence of dementia has decreased, the longer survival of infected individuals has resulted in higher numbers of persons with less severe cognitive impairment. Opioid use in individuals with advanced HIV disease has been associated with more frequently development of HIV encephalitis and neurocognitive impairment (6), and a longer lifetime use of heroin by HIV-infected individuals may be associated with increased deficits in memory as compared to non-drug users (7). In addition vitamin A deficiency, which is prevalent in drug users and occurs at very high levels in developing areas of the world, can be associated with more rapid progression of HIV infection, particularly in drug users (8), and as well as with impaired cognition in children and adults (9, 10).
In previous studies, the HIV transgenic rat has been shown to effectively model the clinical, immunological and pathological abnormalities that can occur with HIV infection in humans (11–16). These include the occurrence of cognitive, motor and behavioral abnormalities in association with increased expression of HIV proteins. In addition, the observed clinical abnormalities can develop in the context of dysfunction of specific neuronal populations, the significance of which is supported by the demonstration of such populations being also affected in HIV infected humans with neurocognitive impairment. These include interneurons that express the marker parvalbumin (13). These cells have been shown to be decreased in brains of humans with HIV infection and impaired cognition. In addition the numbers of these cells are further decreased in brains of individuals with a history or methamphetamine use (17). Little information has been published related to effects of opioids on parvalbumin+ neurons. However, in studies modeled in the transgenic rat, these cells were decreased in frontal cortex of these animals as compared to controls (13). On formal neuropsychological testing of individuals with HIV-related neurocognitive impairment the regions that are frequently involved include brain frontal and prefrontal cortex as well as hippocampus (18). In this report we describe analyses of parvalbumin+ hippocampal neurons in HIV TG rat brains to determine whether numbers of these neurons and expression of parvalbumin might be altered by vitamin A deficiency or by morphine.
METHODS
Animals
All experiments were performed using 3–6 month old specific pathogen free TG and age-matched WT Fisher 344/NHsd control rats. The details on the construction of the HIV-1 TG rat have been previously described (19). The TG and WT rats were administered a diet previously used to induce VA deficiency in mice (20), except that the rats were fed the Bio-Serv AIN-93M rodent maintenance diet (Bio-Serv; Frenchtown, NJ), which contains 400,000 IU/kg of retinyl palmitate, the major dietary form of VA, or the same diet mix formulated minus retinyl palmitate. Female rats maintained on the normal maintenance diet were mated then randomly divided into two groups at 2 wks gestation. One group of pregnant females was subsequently fed a VA deficient diet and the other was fed the VA-sufficient diet. Weanlings were maintained on the same diets as their dams. For collection of brains the animals were euthanized then perfused with 10% formalin then the brains were removed and kept in 10% formalin for 24 hours, then equilibrated in 30% glycerol. Blocks produced by sectioning the brains from −2 to −6 Bregma were frozen in OCT until use. All studies were approved by the Institutional Animal Care and Use Committee of the University of Maryland, Baltimore.
Treatments
75 mg morphine tablets and placebo control tablets were obtained through the NIDA Drug Supply Program. TG and WT control rats were anesthetized with Ketamine then implanted subcutaneously with initially either a whole morphine or placebo tablet. Subsequently, with the occurrence of unanticipated deaths in WT rats implanted with the morphine 75 mg tablet, the morphine dose was decreased to 37.5 mg (one half tablets). The rats were then observed for 5 days and then euthanized using Ketamine inhalation, perfused with 1% paraformaldehyde, and the brains then removed, fixed in 4% paraformaldehyde and embedded with paraffin for subsequent immunocytochemical analysis.
Immunocytochemistry
Immunoperoxidase staining
The tissue sections were deparaffinized and hydrated through xylene and graded alcohol then rinse for 5 min in tap water. The slides were then incubated with 0.3% H2O2 in methanol for 30min then wash in phosphate buffered saline (PBS) for 5min. Antigen retrieval was performed by incubating in 10mM citrate buffer pH 6.0. The slides were then washed in cold water then the sections incubated with blocking solution (10% horse serum in PBS + 0.1% Triton-X) for 30 min. The slides were then washed and incubated with blocking solution for 1 hour then incubated overnight at 4 degrees Celsius with mouse anti-rat parvalbumin, (SWant, cat#235) diluted 1:50,000 in PBS. The following day the slides were washed three times with PBS then incubated for 1 hour with horse anti-mouse antibody diluted 1:500 with blocking solution (Vectastain ABC kit, cat# PK-4002), washed, incubated with AB reagent and then with diaminobenzadine according to the manufacturer’s protocol. The slides were then processed through graded alcohols and xylene coverslipped.
NeuN
The sections were deparaffinized and incubated through graded alcohols, xylene, methanol, antigen retrieval buffer as described above, and washed as described above. NeuN staining was performed by incubating overnight at 4 degrees Celsius with mouse anti-NeuN (US Biological cat# N2173) antibody diluted 1:500 dilution in PBS. The slides were then washed with PBS for 5 min and then incubated for 1 hr with anti-mouse IgG (Vectastain ABC kit peroxidase Mouse IgG, cat# 4002) diluted 1:200 in blocking serum. The slides were then washed in PBS for 5 min and incubated for 30min. with AB reagent, washed, then incubated with diaminobenzadine as recommended by the manufacturer (Vector Laboratories). After rinsing the slides were counterstained with hematoxylin then rinsed, dehydrated and coverslipped.
Quantitative Analysis
NeuN+ and parvalbumin+ cell numbers in sections from hippocampal CA1 regions and the intensity of parvalbumin stains were determined. For determining cell numbers, nine 300 × 300 pixel regions in 10X fields were imaged using ImageJ software (NIH) and the positive cells in sections from at least 5 rats per group were manually counted and the numbers averaged. The distribution of the intensity of staining for parvalbumin was determined for three to five 300 × 300 pixel regions in 10x images from a representative TG and WT rat using ImageJ to adjust the color threshold to the level of the background, convert the image to grayscale and perform histogram analysis to determine the distribution of the gray values for the thresholded regions. Mean staining intensity was determined by multiplying the number of pixels at each grayscale value > 0 on the histogram plot x the grayscale value, calculating the sum of the products, and then dividing that number by the total number of values.
Statistical Analyses
Mean numbers of NeuN+ and parvalbumin+ cells and mean parvalbumin cell grayscale values for the thresholded images were compared using repeated measures analysis of variance. The Bonferroni post-test was applied to correct for multiple comparisons with statistical significance set at p < 0.05. Statistical analyses were performed using GraphPad Prism statistical software (GraphPad Software, Inc.).
RESULTS
Quantitation of NeuN+ Cells in Rat Hippocampus
Hippocampal neurons were quantitated as described the materials and methods section. These studies showed that for WT rats mean numbers of NeuN+ cells progressively decreased with morphine exposure and with placing the rats on a vitamin A deficient diet (figure 1). For WT rats there was an overall decrease in NeuN+ neurons with exposure to morphine and VA deficiency. There was also a numerical decrease in the numbers of NeuN+ neurons for the WT rats on the normal diet on morphine as compared to untreated controls; however, this difference was not statistically significant. In contrast, rats on the VA deficient diet had significantly lower numbers of these neurons than untreated rats on the normal diet, and this difference was not affected by treatment with morphine.
Figure 1.
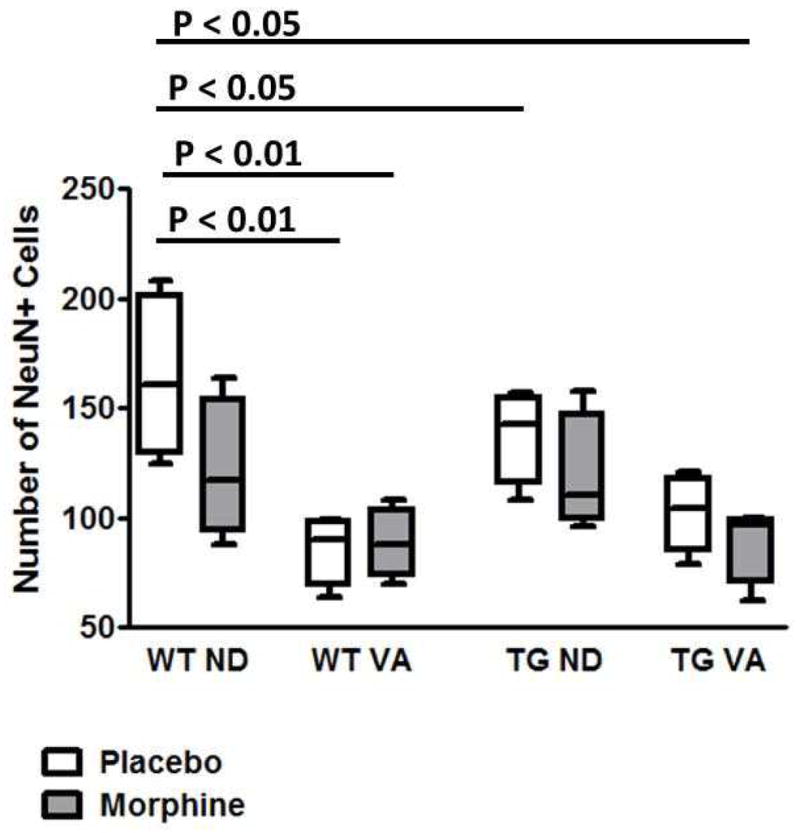
Comparison of total numbers (NeuN+) neuron in hippocampus CA1 region for TG and WT rats on a normal or vitamin A deficient diet and treated with either placebo or morphine (WTND = wild type rat, normal diet; WTVA = wild type rat, vitamin A deficient diet; TGND = transgenic rat, normal diet; TGTVA = transgenic, vitamin A deficient diet). Cell numbers were higher for WTND rats than for both WTVA rat groups and for TGND placebo treated rats and for TGVA rats treated with morphine.
Analysis of Numbers of Parvalbumin+ Cells in Rat Hippocampus
Changes in numbers of parvalbumin+ cells were analyzed in CA1 region of hippocampus with exposing the WT and TG rats to the vitamin A deficient diet and to morphine (figure 2). For WT rats on the normal diet, there was an overall decrease in mean cell numbers with exposing the rats to the deficient diet and to morphine. Also, for TG rats mean numbers of the cells decrease slightly with vitamin A deficiency and increased with morphine exposure. None of these changes were, however, statistically significant.
Figure 2.

Comparison of total number of parvalbumin+ in hippocampus CA1 region for TG and WT rats on a normal or vitamin A deficient diet and treated with either placebo or morphine (WTND = wild type rat, normal diet; WTVA = wild type rat, vitamin A deficient diet; TGND = transgenic rat, normal diet; TGTVA = transgenic, vitamin A deficient diet). Mean cell numbers were similar for the genotype, dietary and treatment groups.
Parvalbumin Expression by CA1 Hippocampal Neurons
To examine relative expression of parvalbumin by CA1 hippocampal neurons, the distribution of the mean grayscale values were determined for parvalbumin stained cells in imaged 10x fields of hippocampus from WT and TG rats on either a normal or a vitamin A deficient diet and treated with either morphine or placebo (figure 3), and these values were compared as described in the Materials and Methods section (table 1). These studies showed that the mean density values for the WT rats on the vitamin A deficient diet were lower than for the WT rats on the normal diet. For WT rats in both dietary groups this relationship was also noted whether the animals were treated with either placebo or morphine.
Figure 3.
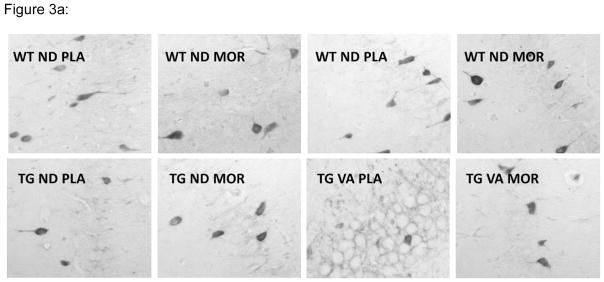
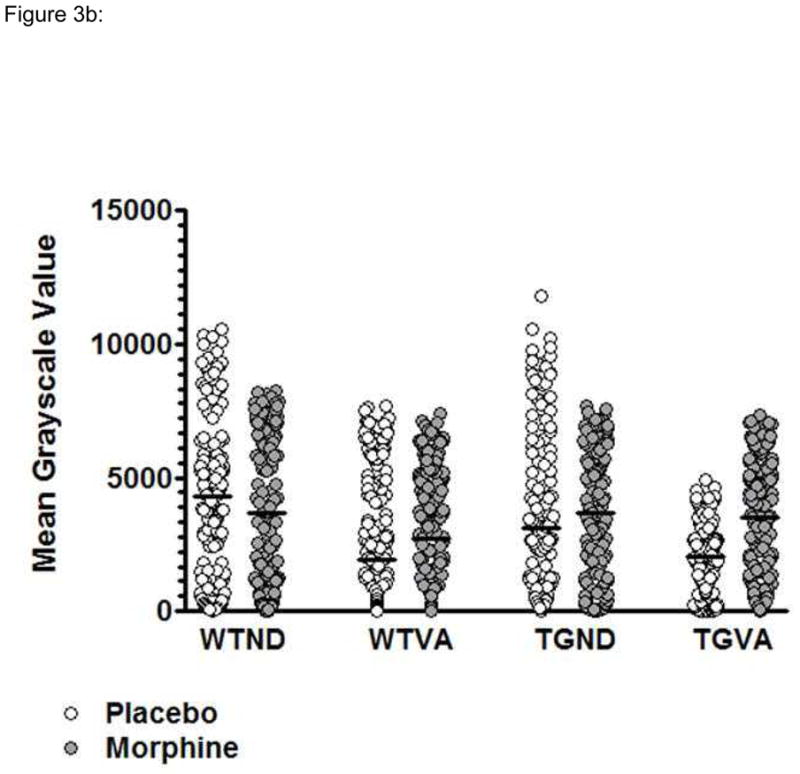
Comparison of mean levels of parvalbumin expression in hippocampus CA1 region neurons for TG and WT rats on a normal or vitamin A deficient diet and treated with either placebo or morphine (WTND = wild type rat, normal diet; WTVA = wild type rat, vitamin A deficient diet; TGND = transgenic rat, normal diet; TGTVA = transgenic, vitamin A deficient diet). (A) Representative 10x fields from Immunoperoxidase stained sections. (B) Plots of the distribution of mean density values for a representative animal from each group.
Table 1.
ANOVA P-values for WT versus TG Hippocampal Parvalbumin+ Neuron Cell Density Measurements for the Dietary and Morphine Treatment Groups
| WT ND PLA | WT ND MOR | WT VA PLA | WT VA MOR | TG ND PLA | TG ND MOR | TG VA PLA | TG VA MOR | |
|---|---|---|---|---|---|---|---|---|
| WT ND PLA | 0.001 (2392) | 0.001 (−1626) | 0.001 (2303) | |||||
| WT ND MOR | 0.001 (1749) | 0.001 (969.2) | 0.001 (1660) | |||||
| WT VA PLA | 0.001 (−2392) | 0.001 (−1749) | 0.01 (−765.7) | 0.001 (−1936) | 0.001 (−1735) | 0.001 (−1598) | ||
| WT VA MOR | 0.001 (1626) | 0.001 (−969.2) | 0.01 (765.7) | 0.001 (−1170) | 0.01 (676.6) | 0.01 (−832.3) | ||
| TG ND PLA | 0.001 (1936) | 0.001 (1170) | 0.001 (1846) | |||||
| TG ND MOR | 0.001 (1735) | 0.001 (1646) | ||||||
| TG VA PLA | 0.001 (−2303) | 0.001 (−1660) | 0.01 (−676.6) | 0.001 (−1846) | 0.001 (−1646) | 0.001 (−1509) | ||
| TG VA MOR | 0.001 (1598) | 0.01 (832.3) | 0.001 (1509) |
Value in parentheses = mean difference between the comparison groups.
Gray boxes represent comparisons that were not statistically significant
For the TG rats on the normal diet the mean density values for the placebo and morphine treatment groups were similar to those for the corresponding WT rats. Vitamin A deficient TG rats treated with either placebo or morphine showed higher parvalbumin expression than WT vitamin A deficient rats treated with placebo, and placebo treated vitamin A deficient TG rats showed higher expression than morphine treated vitamin A deficient rats.
For vitamin A deficient TG rats treated with placebo, parvalbumin density measurements were similar to those for the corresponding WT rat group (i.e., vitamin A deficient, placebo WT rats) but less than for all other WT and TG rat groups. Parvalbumin expression was also similar for morphine-treated TG rats on either the normal or on the vitamin A deficient diet. However, expression was higher for vitamin A deficient morphine-treated rats than for the corresponding WT rat groups and for vitamin A deficient TG rats treated with placebo.
DISCUSSION
In these studies we show effects from morphine exposure and vitamin A deficiency on the overall numbers of neurons in the CA1 region of hippocampus. In contrast, for the parvalbumin+ subpopulation of neurons, numbers within dietary and treatment groups did not significantly differ; however a difference in parvalbumin expression was observed. These findings are opposite to those previously described on frontal cortex in this model, where numbers of NeuN+ cells did not correlate but there were changes noted in number of parvalbumin+ cells (13). This apparent discrepancy may be due to the fact that there are simply different susceptibilities to the potential causes of neurodegeneration that may be present in tissue and that expression of these factors may be differentially regulated by the presence of vitamin A deficiency and morphine. On the other hand, there are known to exist specific reciprocal connections between cortical GABAergic interneuron and pyramidal cells in the CA1 region of the hippocampus (21), and it is possible that involvement of such networks may be the reason for the observed pattern of involvement.
Parvalbumin is a calcium-binding protein which is expressed by interneurons that also produce and secrete gamma-aminobutyric acid (GABA), an inhibitory neurotransmitter that regulates neuronal excitability in the central nervous system (22). Neurons that express parvalbumin are “fast-spiking” cells which, through the formation of chemical synapses and electrical gap junctions, promote synchronization of primary neuronal electrical activity (23, 24). Factors that increase parvalbumin expression include activation of the NMDA receptor which, with subsequent increase in intracellular calcium levels, increases expression of GAD67 (glutamic acid decarboxylase 67, the isoform of the enzyme that synthesizes GABA), and activation of Erk1/2 protein kinases and CREB (25, 26) (27). The rats in these studies were exposed to morphine for five days, and the chronic effects from this exposure would be expected to increase adenyl cyclase above baseline levels (28–30). Activation of GABA receptors can be potentially enhanced by synergistic activation of protein kinase A by retinoids in the presence of cAMP (31). Such activation would be likely impaired in the context of vitamin A deficiency, which could enhance the development of clinical abnormalities in the presence of pathologic states as those which occur in HIV infection.
In studies of brain cortex in this model, vitamin A deficiency and morphine treatment resulted in changes in numbers of parvalbumin+ neurons which could in part be explained by the occurrence of altered parvalbumin expression (13). These changes included decreased expression with vitamin A deficiency and morphine exposure in WT rats and an overall opposite trend in TG animals. To investigate whether this effect might also occur in hippocampus the range of density values for cells positively stained for parvalbumin in tissue sections were measured and compared for the dietary and treatment groups. These studies showed that in the WT rat group there was an overall decrease in expression with both vitamin A deficiency and morphine exposure. For these animals the median staining intensity for the deficient rats was lower than that for rats only exposed to morphine and not to a vitamin A deficient diet; however, this difference was not statistically significant. This suggests that these two factors had similar effects on parvalbumin expression and potentially the absence of a further decrease with the combination of the two implies the absence of an additive effect. In the TG rat on the normal diet there was a less prominent decrease in parvalbumin expression with exposure to morphine and vitamin A deficiency, which could occur due to separate the induction of activation of parvalbumin expression by HIV gene products. With morphine treatment, parvalbumin expression increased in the vitamin A deficient TG rats, which could occur due to morphine either relieving the suppression induced by the deficient diet or activating expression through a separate pathway. It is also possible that inflammatory responses that occur secondary to these conditions might play a role in the regulation of parvalbumin expression, as suggested by studies where parvalbumin expression was decreased by activation of nuclear factor-κB (NFκB) (32).
These above possibilities are, of course, speculative, and further studies are required to identify the specific mechanisms that are involved in these effects. Also to be considered is the possibility that the observed changes do not reflect direct effects of vitamin A deficiency and morphine exposure on parvalbumin+ neurons. Although these cells clearly play an important role in memory and behavior, they are only one of four GABAergic interneuron subtypes that are present in brain. The others can be classified by the expression the neurotransmitter cholecyctokinin and by the presence of either dense axonal aborization or long-range projections. Just as it is possible that the low vitamin A diet and morphine treatments are able to directly stimulate parvalbumin expression by the GABAergic cells, it is also possible that expression occurs indirectly as a compensatory response to the loss of the other inhibitory neuronal populations.
In addressing issues of how parvalbumin+ GABAergic neurons might play a role in the pathophysiological mechanisms that underlie neurocognitive impairment that occurs related to HIV infection, larger questions inevitably arise related to the role of vitamin A deficiency in HIV disease progression and mechanisms related to neurotoxicity induced by opioids. Although it is clear that vitamin A deficiency is detrimental to HIV+ individuals, approaches for vitamin A supplementation that are clearly both safe have yet to be developed (33, 34). Therefore, as future studies are pursued to understand the role of vitamin A deficiency in the risk of nervous system complications, it is important to also develop approaches for correcting the deficiency in affected individuals.
Acknowledgments
Supported by: R01DA15311 (WR; JB), R01MH086356 (WR) and R21NS070708 (WR)
References
- 1.Boisse L, Gill MJ, Power C. HIV infection of the central nervous system: clinical features and neuropathogenesis. Neurol Clin. 2008 Aug;26:799–819. x. doi: 10.1016/j.ncl.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Word Health Organization. GLOBAL HIV/AIDS RESPONSE - Epidemic update and health sector progress towards Universal Access. 2011. [Google Scholar]
- 3.Kraft-Terry SD, Stothert AR, Buch S, Gendelman HE. HIV-1 neuroimmunity in the era of antiretroviral therapy. Neurobiol Dis. 2010 Mar;37:542–548. doi: 10.1016/j.nbd.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sacktor N, Lyles RH, Skolasky R, et al. HIV-associated neurologic disease incidence changes:: Multicenter AIDS Cohort Study, 1990–1998. Neurology. 2001 Jan 23;56:257–260. doi: 10.1212/wnl.56.2.257. [DOI] [PubMed] [Google Scholar]
- 5.Sacktor N, McDermott MP, Marder K, et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002 Apr;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- 6.Bell JE, Brettle RP, Chiswick A, Simmonds P. HIV encephalitis, proviral load and dementia in drug users and homosexuals with AIDS. Effect of neocortical involvement. Brain. 1998 Nov;121( Pt 11):2043–2052. doi: 10.1093/brain/121.11.2043. [DOI] [PubMed] [Google Scholar]
- 7.Byrd DA, Fellows RP, Morgello S, et al. Neurocognitive impact of substance use in HIV infection. J Acquir Immune Defic Syndr. 2011 Oct 1;58:154–162. doi: 10.1097/QAI.0b013e318229ba41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Semba RD, Graham NM, Caiaffa WT, Margolick JB, Clement L, Vlahov D. Increased mortality associated with vitamin A deficiency during human immunodeficiency virus type 1 infection. Arch Intern Med. 1993 Sep 27;153:2149–2154. [PubMed] [Google Scholar]
- 9.Chen K, Zhang X, Wei XP, Qu P, Liu YX, Li TY. Antioxidant vitamin status during pregnancy in relation to cognitive development in the first two years of life. Early Hum Dev. 2009 Jul;85:421–427. doi: 10.1016/j.earlhumdev.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Lane MA, Bailey SJ. Role of retinoid signalling in the adult brain. Prog Neurobiol. 2005 Mar;75:275–293. doi: 10.1016/j.pneurobio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Royal W, III, Wang H, Jones O, Tran H, Bryant JL. A vitamin A deficient diet enhances proinflammatory cytokine, Mu opioid receptor, and HIV-1 expression in the HIV-1 transgenic rat. J Neuroimmunol. 2007 Apr;185:29–36. doi: 10.1016/j.jneuroim.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.June HL, Tzeng Yang AR, Bryant JL, Jones O, Royal W., III Vitamin A deficiency and behavioral and motor deficits in the human immunodeficiency virus type 1 transgenic rat. J Neurovirol. 2009 Sep;15:380–389. doi: 10.3109/13550280903350200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sultana S, Li H, Puche A, Jones O, Bryant JL, Royal W. Quantitation of parvalbumin+ neurons and human immunodeficiency virus type 1 (HIV-1) regulatory gene expression in the HIV-1 transgenic rat: effects of vitamin A deficiency and morphine. J Neurovirol. 2010 Feb;16:33–40. doi: 10.3109/13550280903555712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vigorito M, Lashomb AL, Chang SL. Spatial learning and memory in HIV-1 transgenic rats. J Neuroimmune Pharmacol. 2007 Dec;2:319–328. doi: 10.1007/s11481-007-9078-y. [DOI] [PubMed] [Google Scholar]
- 15.Peng J, Vigorito M, Liu X, Zhou D, Wu X, Chang SL. The HIV-1 transgenic rat as a model for HIV-1 infected individuals on HAART. J Neuroimmunol. 2010 Jan 25;218:94–101. doi: 10.1016/j.jneuroim.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Rao JS, Kim HW, Kellom M, et al. Increased neuroinflammatory and arachidonic acid cascade markers, and reduced synaptic proteins, in brain of HIV-1 transgenic rats. J Neuroinflammation. 2011;8:101. doi: 10.1186/1742-2094-8-101. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Langford D, Adame A, Grigorian A, et al. Patterns of selective neuronal damage in methamphetamine-user AIDS patients. J Acquir Immune Defic Syndr. 2003 Dec 15;34:467–474. doi: 10.1097/00126334-200312150-00004. [DOI] [PubMed] [Google Scholar]
- 18.Maki PM, Cohen MH, Weber K, et al. Impairments in memory and hippocampal function in HIV-positive vs HIV-negative women: a preliminary study. Neurology. 2009 May 12;72:1661–1668. doi: 10.1212/WNL.0b013e3181a55f65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reid W, Sadowska M, Denaro F, et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A. 2001 Jul 31;98:9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carman JA, Hayes CE. Abnormal regulation of IFN-gamma secretion in vitamin A deficiency. J Immunol. 1991;147:1247–1252. [PubMed] [Google Scholar]
- 21.Klausberger T. GABAergic interneurons targeting dendrites of pyramidal cells in the CA1 area of the hippocampus. Eur J Neurosci. 2009 Sep;30:947–957. doi: 10.1111/j.1460-9568.2009.06913.x. [DOI] [PubMed] [Google Scholar]
- 22.Gerfen CR, Baimbridge KG, Miller JJ. The neostriatal mosaic: compartmental distribution of calcium-binding protein and parvalbumin in the basal ganglia of the rat and monkey. Proc Natl Acad Sci U S A. 1985 Dec;82:8780–8784. doi: 10.1073/pnas.82.24.8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999 Nov 4;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 24.Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001 Jul;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 25.Cochran SM, Fujimura M, Morris BJ, Pratt JA. Acute and delayed effects of phencyclidine upon mRNA levels of markers of glutamatergic and GABAergic neurotransmitter function in the rat brain. Synapse. 2002 Dec 1;46:206–214. doi: 10.1002/syn.10126. [DOI] [PubMed] [Google Scholar]
- 26.Keilhoff G, Becker A, Grecksch G, Wolf G, Bernstein HG. Repeated application of ketamine to rats induces changes in the hippocampal expression of parvalbumin, neuronal nitric oxide synthase and cFOS similar to those found in human schizophrenia. Neuroscience. 2004;126:591–598. doi: 10.1016/j.neuroscience.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 27.Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM. A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J Neurosci. 2006 Feb 1;26:1604–1615. doi: 10.1523/JNEUROSCI.4722-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma SK, Nirenberg M, Klee WA. Morphine receptors as regulators of adenylate cyclase activity. Proc Natl Acad Sci U S A. 1975 Feb;72:590–594. doi: 10.1073/pnas.72.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Traber J, Gullis R, Hamprecht B. Influence of opiates on the levels of adenosine 3′:5′-cyclic monophosphate in neuroblastoma X glioma hybrid cells. Life Sci. 1975 Jun 15;16:1863–1868. doi: 10.1016/0024-3205(75)90292-1. [DOI] [PubMed] [Google Scholar]
- 30.Benalal D, Bachrach U. Opiates and cultured neuroblastoma x glioma cells. Effect on cyclic AMP and polyamine levels and on ornithine decarboxylase and protein kinase activities. Biochem J. 1985 Apr 15;227:389–395. doi: 10.1042/bj2270389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hohmann P, Greene RS. Retinoid induced changes in cAMP-dependent protein kinase activity detected by a new minigel assay. FEBS Lett. 1990 Feb 12;261:81–84. doi: 10.1016/0014-5793(90)80641-u. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Zhou Z, Yang C, Xu J, Yang J. Nuclear factor-kappaB is involved in the phenotype loss of parvalbumin-interneurons in vitro. Neuroreport. 2011 Apr 20;22:264–268. doi: 10.1097/WNR.0b013e3283451787. [DOI] [PubMed] [Google Scholar]
- 33.Forrester JE, Sztam KA. Micronutrients in HIV/AIDS: is there evidence to change the WHO 2003 recommendations? Am J Clin Nutr. 2011 Dec;94:1683S–1689S. doi: 10.3945/ajcn.111.011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irlam JH, Visser MM, Rollins NN, Siegfried N. Micronutrient supplementation in children and adults with HIV infection. Cochrane Database Syst Rev. 2010:CD003650. doi: 10.1002/14651858.CD003650.pub3. [DOI] [PubMed] [Google Scholar]


