Abstract
Background. High blood pressure (BP) poses a major risk for cognitive decline. Aim of the study was to highlight the relationship between cognitive assessment scores and an effective therapeutic BP control.
Methods. By medical visit and ambulatory BP monitoring (ABPM), we studied 302 treated hypertensives, subdivided according to office/daytime BP values into 120 with good (GC) and 98 poor (PC) BP control, 40 with “white coat hypertension” (WCH) and 44 a “masked-hypertension” phenomenon (MH). Patients underwent neuropsychological assessment to evaluate global cognitive scores at the Mini Mental State Examination (MMSE) and Frontal Assessment Battery (FAB) and attention/executive functions (Delayed Recall, Digit Span Forwards, Digit Span Backwards, Selective Attention, Verbal Fluency, Stroop Test and Clock Drawing). Carotid intima-media thickness (IMT) served as the index of vascular damage.
Results. There were no differences among the groups in terms of gender, age, education, metabolic assessment, clinical history and hypertension treatment. GC presented lower office and ambulatory BP values and IMT. PC performed worse than GC on global executive and attention functions, especially executive functions. In PC, office systolic BP (SBP) was significantly associated to the MMSE and FAB scores and, in particular, to Verbal Fluency, Stroop Errors and Clock Drawing tests. Office diastolic BP (DBP) was associated to Selective attention, nocturnal SBP to Digit Span backwards and Verbal Fluency. Worse cognitive assessment scores were obtained in WCH than GC.
Conclusions. The findings showed that in adult treated hypertensives, a poor BP control, as both doctor's office and daytime scores, is associated to impaired global cognitive and especially executive/attention functions.
Keywords: cognitive dysfunction, executive impairment, blood pressure control, ambulatory blood pressure monitoring.
Introduction
In recent years many studies have been focused on delineating the relationship between blood pressure (BP) and cognitive function, in view of the evidence that hypertension (HTN) is a risk factor for cognitive decline 1. The findings are consistent for both vascular dementia and Alzheimer's disease 2 but, in particular, for the wider category of mild cognitive impairment 3. The association between BP values and cognitive performances, in both adult hypertensives 4 and normotensives 5, is demonstrated by the progressive pathophysiological role of normal-to-high BP in worsening cognitive abilities. Nevertheless, the description of cognitive patterns associated with high BP is still controversial and largely debated. In fact, the cognitive tests employed and differences in patients' selection in the diverse studies may account for the heterogeneity of results 4-6.
According to a Cochrane review 6, the HYVET-COG study 7 and the SCOPE study 8 do not provide convincing evidence that pharmacological BP reduction can prevent the development of dementia in hypertensives without a prior diagnosis of cerebrovascular disease. Interestingly, only few studies have explored the different domains of cognitive functions that may be improved by effective BP control 9 but no study has compared cognitive scores with the hypertensive state findings during the medical visit and ambulatory BP monitoring (ABPM).
The aim of the study was to investigate the effect of BP control, both in the doctor's office and during daily activities, on cognitive functions in adults treated for hypertension. To assess cognitive function, we used specific tests that explore different cognitive domains.
Methods
Subjects. After obtaining approval for the study from the local Ethics Committee, between May 2009 and January 2012 we recruited 302 consecutive patients referred to the Cerebrovascular Prevention and Hypertension Clinic of the University of Bari Medical School.
The inclusion criteria were: a) age ≤ 69 years, b) a history of primary hypertension, c) use of antihypertensive medications for at least six months, d) willingness to undergo a battery of neuropsychological tests and to give informed consent, e) Italian as native language, f) no previous history of psychiatric or neurological disorders including neuropsychological deficits, such as dementia, stroke or transient ischemic attack, g) no previous history of other cardiovascular diseases such as angina or myocardial ischemia, h) no history of episodes suggestive of cerebrovascular impairment, i) no atherothrombotic disease; l) no history of diabetes and similar metabolic assessment. The "history of HTN" was defined as referred BP values, when available, and as estimated duration of the hypertensive condition in months. Patients in all four groups were taking antihypertensive treatment.
Study Protocol. Within one week of enrolment, patients presented at the clinic, between 8.00 and 9.00 a.m., after 12 hours fasting, for a routine medical visit, blood tests and electrocardiogram, to confirm the hypertensive state and to reduce the effect of anxiety on the BP measurement. Systolic (SBP) and diastolic (DBP) blood pressure and heart rate (HR) were considered as the mean of three measurements made using a standard sphygmomanometer (AND UM-101, A&D Medical Ltd., Oxford, U.K), at 2 min intervals and at both arms, after the subject had been sitting comfortably for 10 min. The patients underwent carotid ultrasonography, ambulatory blood pressure monitoring and cognitive assessment.
Carotid Ultrasonography. On the following day, carotid ultrasonography was performed, by echodoppler (Toshiba Aplio XV) with a multi-frequency 6.2-8.4 MHz pulse wave probe, to check in both common carotid arteries for signs of atherosclerosis and measure the intima-media thickness (IMT), which served as the index of preclinical vascular damage, according to the Mannheim IMT Consensus 10.
Ambulatory Blood Pressure Monitoring. Within four days, the patients underwent twenty-four-hour ambulatory blood pressure monitoring (ABPM) to evaluate the BP state during awake and sleeping hours (AND TM 2430, A&D Instruments Ltd., Oxford, U.K), with the tailored cuff fastened on the non-dominant arm. Because all of them had already previously undergone the test, it is reasonable to assume that the technique did not arouse any particular distress. Measures were taken every 20' throughout the test. Awake and sleeping time were based on diary card entries and interview. Eight patients underwent a repeat ABPM because of an insufficient number (<80%) of measurements 11.
Following the ESC-ESH 2007 guidelines, based upon both the diagnosis of hypertension in the clinic (SBP/DBP> 140/90 mmHg) and during awake hours by ABPM (SBP/DBP> 135/85 mmHg) 11,12, the patients were subdivided into four groups: a) 120 with good (GC) and b) 98 with poor BP control (PC), both with a confirmed diagnosis of a BP condition in the office and during awake hours, and, c) 40 hypertensives with a positive office-day ABPM BP change, corresponding to the “white-coat hypertension” phenomenon, better defined as “isolated clinic hypertension” (WCH) and d) 44 with a negative office-day ABPM BP change, corresponding to the “masked hypertension” phenomenon (MH), both with an inconsistent diagnosis of a BP condition between office and daytime awake scores.
Cognitive Assessment. The next day, the patients underwent neuropsychological assessment by trained psychologists under the supervision of a clinical neuropsychologist. The test battery was designed to assess a full range of cognitive functions, standardized for the Italian population, and consisted of reliable and sensitive analytical tests for cognitive impairment, validated by the scientific literature and generally associated to a specific cerebral area. In particular, memory functions are under the control of the temporal lobes, whereas executive and attention functions are controlled by the prefrontal cerebral regions. The examination included measures of global cognitive and frontal functions, by the Mini Mental State Examination (MMSE) 13 and Frontal Assessment Battery (FAB) tests 14. The MMSE consists of a brief 30-point questionnaire that is used to screen the general cognitive index, while the FAB consists of a rapid tool including six subtests that can be used to discriminate between a frontal dys-executive phenotype and Alzheimer's Type dementia. Memory was assessed by the Prose Memory Immediate and Delayed Recall 15 and Digit Span Forwards 16 tests, used to investigate verbal long-term memory and verbal short-term memory, respectively. The cognitive domains of attention and executive functions were measured by the Digit Span Backwards test 16 that examines verbal working memory. The Selective Attention Test was used to measure the ability to focus on specific stimuli excluding those that are not relevant to the properties of the target 16, and the Verbal Fluency test to analyze the speed of verbal production 17. The Stroop Test investigates the ability, time (T) and errors made (E), when asked to inhibit and suppress automatic responses 18 and the Clock Drawing Test is used to assess visual-constructive abilities and mental planning 19, requiring the involvement of executive functions. The tests were not limited by a ceiling or floor effect and were, therefore, sensitive to subtle changes in cognitive performance in the range of BP values in all patients. Raw scores were adjusted for age and education. The neuropsychological assessment lasted about 45'.
Statistical Analysis. The findings were analyzed by the SPSS 18.0 statistical package assuming a p<0.05 limit of significance. Data are expressed as mean ± standard deviation. The chi-squared test was used to explore differences between two groups on gender. Between-group analyses of BP, metabolic and neuropsychological variables were operated by independent t-test in the patients with a confirmed (GC vs PC) or unconfirmed office high BP condition (PC vs WCH and GC vs MH) by ABPM. The Mann-Whitney U-test was applied for not normally distributed data. To highlight further peculiarities, ANOVA, followed by Bonferroni post hoc test (p<0.05) were also performed. Partial Pearson test, adjusted for IMT, blood glucose, LDL and HDL cholesterol, triglycerides, waist circumference and history of hypertension, as potential confounders, was used to explore the relationships between neuropsychological variables and BP values.
Results
To examine the results in patients with a confirmed diagnosis of a BP condition, both in the doctor's office and during daily hours, we compared GC to PC. By contrast, to evaluate the results in patients with inconsistent hypertensive scores, we compared WCH to PC, who showed higher office BP values, and MH to GC, who presented lower office BP values. There were no differences among the groups in terms of gender, level of education and estimated history of hypertension and referred first-diagnosed SBP/DBP values. Only the WCH were older and had received less education than the PC. As expected, during the medical visit, the PC group presented significantly higher SBP/DBP values than the GC. (Table 1a). There were no significant differences in the number of pills taken (GC 1.83±0.76, PC 1.80±0.70, WCH 1.85±0.71 and MH 1.81±0.74, p:n.s.), nor in the dosages and anti-hypertensive drugs, such as ACE-inhibitors, AT1-antagonists, calcium-entry blockers, beta-blockers, and diuretics, employed at the time of the study (data not shown). The metabolic assessment was also similar among the patients although the WCH group showed a lower BMI and abdominal circumference than the PC who, as expected, showed higher microalbuminuria and IMT than the GC (Table 1b).
Table 1.
Patients characteristics at enrollment
| GC | PC | WCH | MH | |
|---|---|---|---|---|
| A | ||||
| M/F (n) | 53/67 | 44/54 | 18/22 | 20/24 |
| Age (years) | 56.38±9.61 | 56.70±11.02 | 61.79±9.93°° | 55.65±10.48 |
| Education (years) | 10.99±4.49 | 11.30±4.10 | 9.64±4.66° | 11.65±3.97 |
| HTN history (months) | 14.57±33.91 | 15.54±30.83 | 26.67±39.54 | 6.08±4.09 |
| SBP history (mmHg) | 161±14 | 163±11 | 161±10 | 163±10 |
| DBP history (mmHg) | 97±6 | 96±8 | 96±6 | 96±7 |
| SBP office (mmHg) | 123±9 | 147±14*** | 145±10 | 124±8 |
| DBP office (mmHg) | 80±4 | 90±8*** | 89±7 | 80±6 |
| HR office (bpm) | 71±7 | 71±9 | 70±8 | 71±8 |
| B | ||||
| ABDCIRC (cm) | 102.09±10.89 | 102.67±10.01 | 98.44±9.98° | 101.05±10.51 |
| BMI (Kg/m2) | 28.79±4.73 | 28.82±4.35 | 27.25±3.40° | 28.31±4.89 |
| C-TOT (mg/dl) | 128.18±36.07 | 121.42±30.58 | 132.89±49.54 | 119.33±29.80 |
| C-LDL (mg/dl) | 202.86±43.97 | 196.16±29.24 | 211.35±49,29° | 199.00±35.44 |
| C-HDL (mg/dl) | 51.84±11.25 | 51.22±14.21 | 52.78±11.25 | 52.40±12.66 |
| TRIGL (mg/dl) | 118.66±63.98 | 142.44±129.08 | 109.22±49.34 | 126.96±99.37 |
| GLIC (mg/dl) | 99.38±20.04 | 104.83±30.23 | 106.37±34.64 | 94.40±7.29 |
| INSULIN (µUI/ml) | 11.48±7.09 | 11.40±6.52 | 9.12±6.12 | 9.93±5.52 |
| CREAT (mg/dl) | 0.84±0.17 | 0.87±0.24 | 0.87±0.23 | 0.86±0.18 |
| VFG (ml/min) | 100.64±26.26 | 99.85±26.99 | 87.51±31.22 | 102.07±23.47 |
| MICROALB (mg/l) | 15.37±12.24 | 39.99±77.60* | 14.76±11.50 | 24.42±39.56 |
| IMT (mm) | 0.84±0.21 | 0.95±0.26*** | 0.88±0.20 | 0.80±0.17 |
GC: treated hypertensives with a confirmed satisfactorily controlled blood pressure both in the office and during awake hours; PC: treated hypertensives with a confirmed insufficiently controlled blood pressure both in the office and during awake hours; WCH: treated hypertensives with an inconsistent hypertension (“isolate clinic hypertension/white-coat hypertension effect” with BP>140/90 mmHg in the office but diurnal BP<135/85 mmHg; MH: treated hypertensives with an inconsistent hypertension (“masked hypertension effect”) with BP<140/90 mmHg in the office but diurnal BP>135/85 mmHg (*: p<0.05; **:p<0.01; ***: p<0.001). The results in the patients with a confirmed diagnosis of high BP, both in the doctor's office and during daily hours, were analyzed (independent t-test) considering GC and PC patients (*: p<0.05; **:p<0.01; ***: p<0.001). According to the BP values during the medical visit, the results in patients with an inconsistent BP state in the doctor's office and during daily hours were analyzed (independent t-test) in the WCH and PC groups, that both showed higher office BP values, and in the MH and GC groups, that both presented lower office BP values (°: p<0.05; °°:p<0.01; °°°: p<0.001).
General characteristics. M/F:sex distribution; Age (years); Education (years); HTN history (m): history of hypertension (months); SBP history: first-diagnosed systolic hypertensive values (mmHg); DBP history: first-diagnosed diastolic hypertensive values (mmHg); office: BP and HR values measured at the doctor's office.
Metabolic and vascular characteristics. ABDCIRC: abdominal circumference in cm, BMI: body mass index in kg/m2; C-: blood cholesterol in mg/dl; TOT: total; LDL: low-density lipoprotein; HDL: high-density lipoprotein; TRIGL: triglycerides in mg/dl; GLIC: blood glucose in mg/dl; INSULIN: blood insulin in mµ/l; CREAT: creatinine in mg/dl; VFG: estimated glomerular filtration rate by MDRD formula; MICROALB: microalbuminuria as mg/l; IMT: intima-media thickness in mm.
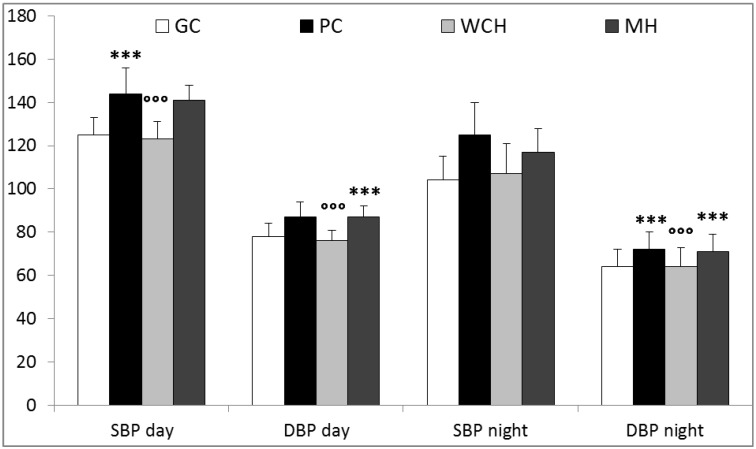
Daytime SBP/DBP was higher in the PC (144±12/87±7 mmHg) than in the GC (125±8/78±6 mmHg; p<0.001) while the WCH (123±8/76±5 mmHg) and MH (141±7/87±5 mmHg) groups presented lower (p<0.001) and higher (p<0.001) values than the PC and GC groups, respectively. The PC group showed higher nocturnal SBP/DBP (125±15/72±8 mmHg) than the GC group (104±11/64±8 mmHg; p<0.001), while the WCH (107±14/64±9 mmHg) and MH (117±11/71±8 mmHg) showed lower (p<0.001) and higher (p<0.001) values than the PC and GC, respectively (Fig.1).
Figure 1.
Ambulatory blood pressure monitoring characteristics. GC: treated hypertensives with a confirmed satisfactorily controlled blood pressure both in the office and during awake hours; PC: treated hypertensives with a confirmed insufficiently controlled blood pressure both in the office and during awake hours; WCH: treated hypertensives with an inconsistent hypertension state (“isolated clinic hypertension/white-coat hypertension” effect with BP>140/90 mmHg in the office but diurnal BP<135/85 mmHg during awake hours; MH: treated hypertensives with an inconsistent hypertension state (“masked hypertension” effect) with BP<140/90 mmHg in the office but diurnal BP>135/85 mmHg awake hours; SBP: systolic blood pressure; DBP: diastolic blood pressure; day: awake hours: night: sleeping hours (***: p<0.001 vs GC; °°°:p<0.001 vs PC).
The nocturnal SBP decrease was lower in the PC (13.65±7.99%) than in the GC (16.40±7.59%; p<0.05). The SBP change during the medical visit in the PC (1.08±8.16%) was higher than in the GC (-2.60±9.33%; p<0.001) and the SBP office-daytime changes in the WCH (14.65±6.35%) and MH (-13.58±7.50%) were, as expected, higher (p<0.001) and lower (p<0.001) than in the PC (-2.60±9.33%) and GC (1.08±7.91%), respectively.
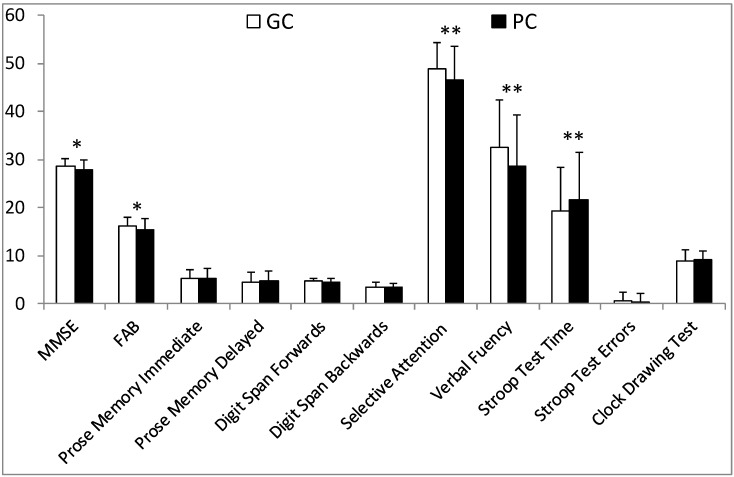
Interestingly, the GC and PC groups, who had a confirmed diagnosis of hypertension in the doctor's office and during awake hours, showed crucial differences in cognitive assessment scores. In fact, the PC performed significantly worse than the GC on global cognitive and frontal tests (MMSE: 27.9±2 vs 28.5±1.6; p<0.05 and FAB: 15.4±2.2 vs 16.2±1.7; p<0.05), on attention function (Selective Attention Test: 46.5±7,1 vs 48.8±5.6; p<0.01), on executive functions (Verbal Fluency Test: 28.7±10.7 vs 32.4±9.9;p<0.01 and Stroop Test -Time: 21.6±9.8 vs 19.2±10.1;p<0.05.). No further significant differences in cognitive tests emerged: Digit Span backwards (3.5±0.7 vs 3.5±0.9; n.s.), Stroop Test-Errors (0.4±1.8 vs 0.5±1.8; n.s.) and Clock Drawing Test (9.2±1.7 vs 8.8±2.3; n.s.) (Fig.2).
Figure 2.
Neuropsychological assessment in treated hypertensives with a confirmed BP state. GC: treated hypertensives with a confirmed satisfactorily controlled blood pressure both in the office and during awake hours; PC: treated hypertensives with a confirmed insufficiently controlled blood pressure both in the office and during awake hours (*: p<0.05 , **: p<0.01 vs WCH).
On the contrary, patients with a fluctuating and inconsistent BP condition exhibited very few differences at the cognitive evaluations. In fact, the WCH performed significantly worse than the PC only on the frontal test examined by the Digit Span Backwards (Table 2). No significant differences emerged between the WCH and MH on neuropsychological variables (Table 3).
Table 2.
Cognitive assessment in PC and in WCH patients. Cognitive assessment, as scores adjusted for age and education, in treated hypertensives.
| PC | WCH | |
|---|---|---|
| Global abilities | ||
| MMSE | 27.9 ± 2 | 27.9 ± 2 |
| FAB | 15.4 ± 2.4 | 14.9 ± 2 |
| Memory | ||
| Prose memory immediate | 5.2 ± 2 | 4.9 ± 1.7 |
| Prose memory delayed | 4.7 ± 2.1 | 4.6 ± 1.9 |
| Digit Span forwards | 4.5 ± 0.8 | 4.3 ± 0.7 |
| Attention and executive functions | ||
| Digit Span backwards | 3.5 ± 0.9 | 3.0 ± 0.8 *** |
| Selective attention | 46.5 ± 7.1 | 48.0 ± 6 |
| Verbal fluency | 28.7 ± 10.7 | 27.7 ± 8.4 |
| Stroop Test -Time | 21.4 ± 9.9 | 23.3± 15.3 |
| Stroop Test - Errors | 0.5 ± 1.8 | 0.3 ± 0.8 |
| Clock Drawing Test | 8.8 ± 2.3 | 8.1 ± 3 |
PC: treated hypertensives with a confirmed insufficiently controlled blood pressure both in the office and during awake hours; WCH: treated hypertensives with an inconsistent hypertension state (“white-coat hypertension effect” with BP>140/90 mmHg in the office but diurnal BP<135/85 mmHg during awake hours (*: p<0.05; **:p<0.01; ***: p<0.001).
Table 3.
Cognitive assessment in GC and in MH patients. Cognitive assessment, as scores adjusted for age and education, in treated hypertensive patients.
| GC | MH | |
|---|---|---|
| Global abilities | ||
| MMSE | 28.5±1.6 | 28.2±1.4 |
| FAB | 16.2±1.7 | 16.1±2 |
| Memory | ||
| Prose memory immediate | 5.1±1.9 | 5.2±1.8 |
| Prose memory delayed | 4.5±2 | 4.5±1.7 |
| Digit Span forwards | 4.6±0.7 | 4.6±0.6 |
| Attention and executive functions | ||
| Digit Span backwards | 3.5±0.7 | 3.3±0.7 |
| Selective attention | 48.8±5.6 | 48.9±4.5 |
| Verbal fluency | 32.4±9.9 | 30.4±6 |
| Stroop Test -Time | 19.4±10.1 | 19.3±8.8 |
| Stroop Test - Errors | 0.4±1.8 | 0.2±0.9 |
| Clock Drawing Test | 9.2±1.7 | 8.8±2.4 |
GC: treated hypertensives with a confirmed satisfactorily controlled blood pressure both in the office and during awake hours; MH: treated hypertensives with an inconsistent hypertension state (“masked hypertension effect”) with BP<140/90 mmHg in the office but diurnal BP>135/85 mmHg during awake hours (*: p<0.05; **:p<0.01; ***: p<0.001).
All the patients completed the neuropsychological assessment and no participants were shown to have dementia or mild cognitive impairment. Although the major focus of the study was the office BP condition, in order to compare patients with a confirmed or no confirmed diagnosis of hypertension, we also performed one-way ANOVA, corrected by the Bonferroni test. This confirmed that the PC performed significantly worse than the GC on attention/executive tests. Interestingly, the WCH performed worse than the GC on the FAB (-1.356; p=0.002), Digit Span backwards (-0.468; p=0.016), Verbal Fluency (-4.688; p=0.037) and Clock Drawing test (-1.123; p=0.028).
Pearson analysis, controlled for age, metabolic variables, history of HTN and first-diagnosed BP values as potential confounders, highlighted significant associations between cognitive test scores and BP values. The GC showed associations between nocturnal DBP and Digit Span forwards (-0.210; p<0.05) and Digit Span backwards (-0.201; p<0.05) and between nocturnal SBP and Stroop test Errors (0.235; p<0.05). The PC demonstrated a significant association between office SBP and the MMSE (-0.304; p<0.01), FAB (-.0226; p<0.05), Verbal Fluency (-0.220; p<0.05), Stroop test Errors (0.356; p<0.001) and Clock Drawing test (-0.372; p<0.001), between office DBP and Selective attention (-0.210; p<0.05) and between nocturnal SBP (-0.211; p<0.05) and Verbal Fluency (0.210; p<0.05). Among the patients with no confirmed diagnosis of hypertension in the office and during awake hours, only the WCH showed a significant association between office DBP and Selective Attention (-0.356; p<0.05) whereas MH did not show any correlation. Interestingly, IMT was associated to frontal capacities, such as selective attention (-0.525; p<0.05) only in the PC group.
Discussion
In the present study, adult treated hypertensives with a similar history of hypertension, metabolic assessment and treatment were evaluated to probe the effect of BP control, measured both in the doctor's office and during daily activities, on global cognitive functions and more definite attention-executive abilities evaluated by trained neuropsychologists.
The findings highlighted that the GC group showed a more effective cognitive performance than the PC. In particular, GC had better performances on global and frontal cognitive indexes, selective attention, and verbal fluency and Stroop time tests. The results suggest that an efficient and consistent BP reduction might be associated to a protective/beneficial effect on the preservation of cognitive functions.
The PC patients exhibited both a greater preclinical vascular damage, in terms of a higher IMT and increased microalbuminuria and the most extensive and strongest association between multiple BP measurements and reduced performances at global and attention/executive cognitive tests.
The findings suggest that the diagnosis of a limited BP control should be extended outside the doctor's office and validated by ABPM, crucial likewise to diagnose isolated clinical or masked hypertension and to highlight the risk for functional and structural cerebro-cardiovascular damage 20. WCH, with similar office BP but lower awake BP values, showed a limited association with the cognitive assessments and worse cognitive scores than GC. The findings confirm that “white -coat” hypertension is not a harmless condition, and is associated to a greater emotional cardiovascular reactivity 21 and enhanced cardiovascular risk 22. Therefore, it is reasonable to assume that treated hypertensives who exhibit a “white-coat phenomenon” may also feature more impaired cognitive performances 23, 24.
Antihypertensive medications seem to reduce the risk for both the development and progression of dementia 25 and to improve cognitive functions in hypertensives without dementia 26. Nevertheless, no relationship between a pharmacological BP reduction and the onset of cognitive impairment was found in elderly and very old hypertensives without prior cerebrovascular disease 6. Indeed, some authors have hypothesized the existence of an age-dependent relation between BP and cognitive function, which would limit the benefits of an efficient BP control only to middle-aged patients but not the elderly 27. Our findings, focusing on similarly treated adult hypertensives, support the hypothesis that a poor cognitive function in old age may be the result of a long-term exposure to vascular risks, such as hypertension, spanning more than 2 to 3 decades 28.
The role of antihypertensive treatment has been widely investigated in the last years with the intent of highlighting the efficacy of specific pharmacological subclasses on hypertension-dependent cognitive impairment 26 and demonstrating an additional “neuro-protective” effect of some molecules 27. Our findings, on the contrary, seem to suggest that regardless of the active agent employed, the treatment may have a true influence on cognitive functions only when the BP control is effectively achieved both inside and outside the doctor's office. The “neuro-protection” should be, therefore, ascribed more to the BP level reached than to any particular antihypertensive drug used.
The previous studies were focused largely on global neuropsychological functions and only few papers combined an evaluation of impairments of the prefrontal functions such as executive function and working memory 29-32. In our study, PC patients showed lower performances not only in terms of global executive capacities but, in particular, of selective attention and verbal fluency, suggesting that high BP values may have an early negative effect on frontal functions.
The relationship between hypertension and cognitive decline, according to the most widely accepted hypothesis, is mediated by cerebral vascular damage such as white matter injuries, detectable as white matter hyperintensities 33, 34. Indeed, the disruption of white matter tracts that connect the frontal lobes to other cortical and subcortical structures 35 may affect attention/executive functions and speed processing 36, 37.
Antihypertensive treatment has been demonstrated to restrain the evolution of white matter lesions 38. It is reasonable, therefore, to hypothesize that a protective effect on deep subcortical white matter pathways, exerted by a proper BP control, might reduce the influence of hypertension on cognition, by improving the cerebral blood flow. The strict relationship between vascular damage and cognitive decline has been proven also by the evidence that the carotid IMT may predict cognitive decline among individuals lacking any vascular or neurological disease 39. Likewise, we found that the IMT was inversely associated to attention functions in the PC but not in the other patients.
Some authors have proposed an additional restorative effect of a BP decrease within normal values on cognitive functions, especially on the attention/executive domain 40 but this was not confirmed by others who, on the contrary, reported poorer cognitive functions in treated mild hypertensives with an effective office BP control 41. The discrepancy may be ascribed to the different consequences of hypertension on the vasculature and on the cerebral blood flow at different ages. This might be attributed to a dysfunction of the cerebral blood flow caused by the thickness and rarefaction of the perforating arterioles. Indeed, adult hypertensives affected by the metabolic syndrome showed a structural microvascular skin rarefaction, strictly associated to an impaired cerebrovascular arterial dilatation 42. As compared to normotensives, hypertensives present a progressive decrease in cerebral blood flow mainly to the hippocampus, anterior cingulate gyrus and prefrontal cortex, areas considered to be involved in memory, executive function and attention 43. Accordingly, the IMT was higher in the PC group despite the similar treatment, age, metabolic assessment and estimated histories of hypertension.
In middle-aged patients, antihypertensive treatment shows a reparative effect on brain perfusion with a major improvement in attention and psychomotor speed 44. Then, in later life, the failure of the cerebral blood flow regulation, due to established endothelial and parietal arterial damage, triggers a compensating counter-regulatory mechanism, i.e. a higher BP state to maintain an optimal cerebral blood flow.
Our findings strongly suggest that a strict control, including ambulatory blood pressure monitoring, of the efficacy of antihypertensive treatments should be exerted to restrain cerebrovascular impairment from the very early stages of hypertension also in adult patients.
We are aware that our study suffers from some limitations. First of all, although we estimated the histories of hypertension and BP values, we cannot exclude that the PC group might have been affected by a more severe hypertension with secondary detrimental consequences or, alternatively, they could have been exposed to a prolonged and/or misdiagnosed resistant hypertension.
We cannot identify a causal effect among the range of variables and, because of the cross-sectional nature of the study, the effect of the estimated history of hypertension and BP levels, as well as the restorative effects exerted by different antihypertensive agents on cognitive function, cannot be estimated. Moreover, we were unable to obtain imaging data for all subjects. Then, the pathophysiology underlying the association of hypertension and attention/executive neuropsychological impairment can only be speculative, based on the most widely accepted hypotheses 33, 34, 45.
To the best of our knowledge, the present study is one of the very few to determine the outcomes of BP values obtained in the office and during the daily activities, on cognitive functions in an adult population. Whereas most of the authors investigated mainly global cognitive functions in older patients 46, 47, the present study was performed by trained psychologists who applied a range of cognitive assessments through a specific and extensive neuropsychological tools. The ABPM and multiple cognitive tools administered by neuropsychologists made it possible to ensure an effective diagnosis of the BP condition and proper testing procedures.
In conclusion, adult treated hypertensives with an insufficient BP control might suffer from an accelerated deterioration of cognitive performances, mainly in the functions regulated by frontal-subcortical circuits. The findings also highlight the critical role of ABPM to obtain a more reliable diagnosis of hypertension, to recognize the individual BP load, the benefit of treatment and any office-daytime BP difference. Further studies are needed to explore the effect of BP control on both peculiar cognitive functions and potential mechanisms whereby hypertension is associated with neuropsychological impairments.
Acknowledgments
We are indebted to Prof. Margherita Fanelli, Dept. of Medicine, University of Bari, for the statistical assistance and to Mary Victoria Pragnell, B.A., for language revision of the manuscript.
Abbreviations
- ABPM
24h ambulatory blood pressure monitoring
- BP
blood pressure
- DBP
diastolic blood pressure
- ESC-ESC
European Society of Cardiology - European Society of Hypertension
- FAB
Frontal Assessment Battery
- GC
treated hypertensives with a good control of hypertension
- HR
heart rate
- HTN
hypertension
- IMT
carotid intima-media thickness
- MH
treated hypertensives with masked-hypertension phenomenon
- MMSE
Mental State Examination
- PC
poorly-controlled treated hypertensives
- SBP
systolic blood pressure
- WCH
treated hypertensives with white-coat (or isolated clinic) hypertension phenomenon
References
- 1.Viswanathan A, Rocca WA, Tzourio C. Vascular risk factors and dementia. How to move forward? Neurology. 2009;72:368–374. doi: 10.1212/01.wnl.0000341271.90478.8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duron E, Hanon O. Vascular risk factors, cognitive decline, and dementia. Vasc Health Risk Manag. 2008;4:363–381. doi: 10.2147/vhrm.s1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cherbuin N, Reglade-Meslin C, Kumar R. et al. Risk factors of transition from normal cognition to mild cognitive disorder: the PATH through Life Study. Dement Geriatr Cogn Disord. 2009;28:47–55. doi: 10.1159/000229025. [DOI] [PubMed] [Google Scholar]
- 4.Kilander L, Nyman H, Boberg M. et al. Hypertension is related to cognitive impairment: a 20-year follow-up of 999 men. Hypertension. 1998;31:780–786. doi: 10.1161/01.hyp.31.3.780. [DOI] [PubMed] [Google Scholar]
- 5.Knecht S, Wersching H, Lohmann H. et al. High-normal blood pressure is associated with poor cognitive performance. Hypertension. 2008;51:663–668. doi: 10.1161/HYPERTENSIONAHA.107.105577. [DOI] [PubMed] [Google Scholar]
- 6.McGuinness B, Todd S, Passmore P. et al. Blood pressure lowering in patients without prior cerebrovascular disease for prevention of cognitive impairment and dementia. Cochrane Database Syst Rev. 2009;7:CD004034. doi: 10.1002/14651858.CD004034.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters R, Beckett N, Forette F, et al.for HYVET investigators. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. The Lancet Neurology. 2008;7:683–689. doi: 10.1016/S1474-4422(08)70143-1. [DOI] [PubMed] [Google Scholar]
- 8.Lithell H, Hansson L, Skoog I. et al. for SCOPE Study Group. The study on cognition and prognosis in the elderly (SCOPE): principal results of a randomized double-blind intervention trial. Journal of Hypertension. 2003;21:875–886. doi: 10.1097/00004872-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Birns J, Morris R, Donaldson N. et al. The effects of blood pressure reduction on cognitive function: a review of effects based on pooled data from clinical trials. J Hypertens. 2006;24:1907–1914. doi: 10.1097/01.hjh.0000244934.81180.16. [DOI] [PubMed] [Google Scholar]
- 10.Touboul PJ, Hennerici MG, Meairs S. et al. Mannheim carotid intima-media thickness consensus (2004-2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. 2007;23:75–80. doi: 10.1159/000097034. [DOI] [PubMed] [Google Scholar]
- 11.Mancia G, De Backer G, Dominiczak A. et al. for ESH-ESC Task Force on the Management of Arterial Hypertension. 2007 ESH-ESC Practice Guidelines for the Management of Arterial Hypertension: ESH-ESC Task Force on the Management of Arterial Hypertension. ESC-ESH 2007 Guidelines. J Hypertens. 2007;25:1751–1762. doi: 10.1097/HJH.0b013e3282f0580f. [DOI] [PubMed] [Google Scholar]
- 12.Pickering TG, Eguchi K, Kario K. Masked hypertension: a review. Hypertens Res. 2007;30:479–488. doi: 10.1291/hypres.30.479. [DOI] [PubMed] [Google Scholar]
- 13.Measso G, Cavarzeran F, Zappalà G. et al. The Mini-Mental State Examination: normative study of a random sample of Italian population. Dev Neuropsychol. 1993;9:77–85. [Google Scholar]
- 14.Appollonio I, Leone M, Isella V. et al. The frontal Assesment Battery (FAB): normative values in an Italian population sample. Neurological Sciences. 2005;26:108–116. doi: 10.1007/s10072-005-0443-4. [DOI] [PubMed] [Google Scholar]
- 15.Bianchi A, Dai Prà M. Twenty years after Spinnler and Tognoni: new instruments in the Italian neuropsychologist's toolbox. Neurological Sciences September. 2008;29:209–217. doi: 10.1007/s10072-008-0970-x. [DOI] [PubMed] [Google Scholar]
- 16.Orsini A, Grossi D, Capitani E. et al. Verbal and spatial immediate memory span: normative data from 1355 adults and 1112 children. Italian Journal of Neurological Sciences. 1987;8:539–548. doi: 10.1007/BF02333660. [DOI] [PubMed] [Google Scholar]
- 17.Carlesimo GA, Caltagirone C, Gainotti G. The Mental Deterioration Battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The Group for the Standardization of the Mental Deterioration Battery. Eur Neurol. 1996;36:378–384. doi: 10.1159/000117297. [DOI] [PubMed] [Google Scholar]
- 18.Caffarra P, Vezzadini G, Dieci F. et al. Una Versione Abbreviata Del Test Di Stroop: Dati Normativi Nella Popolazione Italiana. Nuova Rivista Di Neurologia. 2002;12:111–115. [Google Scholar]
- 19.Mondini S, Mapelli D, Esame Neuropsicologico Breve - una batteria di test per lo screening neuropsicologico. Milano, Italy: Raffaello Cortina Editore; 2003. [Google Scholar]
- 20.Pickering TG, White WB, Giles TD. et al. When and how to use self (home) and ambulatory blood pressure monitoring. J Am Soc Hypertens. 2010;4:56–61. doi: 10.1016/j.jash.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Tanya MS, Thomas GP, Joseph ES. et al. The impact of perceived hypertension status on anxiety and the white coat effect. Annals of Behavioral Medicine. 2007;34:1–9. doi: 10.1007/BF02879915. [DOI] [PubMed] [Google Scholar]
- 22.Muldoon MF, Nazzaro P, Sutton-Tyrrell K. et al. White coat hypertension and carotid artery atherosclerosis: a matching study. Arch Intern Med. 2000;160:1507–1512. doi: 10.1001/archinte.160.10.1507. [DOI] [PubMed] [Google Scholar]
- 23.Suemoto CK, Nitrini R, Grinberg LT. et al. Atherosclerosis and dementia: a cross-sectional study with pathological analysis of the carotid arteries. Stroke. 2011;42:3614–5. doi: 10.1161/STROKEAHA.111.628156. [DOI] [PubMed] [Google Scholar]
- 24.Wetherell JL, Reynolds CA, Gatz M. et al. Anxiety, cognitive performance, and cognitive decline in normal aging. J Gerontol B Psychol Sci Soc Sci. 2002;57:246–55. doi: 10.1093/geronb/57.3.p246. [DOI] [PubMed] [Google Scholar]
- 25.Shah K, Qureshi SU, Johnson M. et al. Does use of antihypertensive drugs affect the incidence or progression of dementia? A systematic review. Am J Geriatr Pharmacother. 2009;7:250–261. doi: 10.1016/j.amjopharm.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Shlyakhto E. Observational Study on Cognitive function And systolic blood pressure Reduction (OSCAR): preliminary analysis of 6-month data from > 10,000 patients and review of the literature. Curr Med Res Opin. 2007;23(Suppl 5):S13–S18. doi: 10.1185/030079907X260719. [DOI] [PubMed] [Google Scholar]
- 27.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 28.Joosten H, van Eersel MEA, Gansevoort RT. et al. Cardiovascular risk profile and cognitive function in young, middle-aged and elderly subjects. Stroke. 2013;44:1543–1549. doi: 10.1161/STROKEAHA.111.000496. [DOI] [PubMed] [Google Scholar]
- 29.Gupta R, Solanki RK, Pathak V. Blood pressure is associated with cognitive impairment in young hypertensives. World J Biol Psychiatry. 2008;9:43–50. doi: 10.1080/15622970601187784. [DOI] [PubMed] [Google Scholar]
- 30.Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behav Neurosci. 2003;117:1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- 31.Harrington F, Saxby BK, McKeith IG. et al. Cognitive performance in hypertensive and normotensive older subjects. Hypertension. 2000;36:1079–1082. doi: 10.1161/01.hyp.36.6.1079. [DOI] [PubMed] [Google Scholar]
- 32.Swan GE, Carmelli D, Larue A. Systolic blood pressure tracking over 25 to 30 years and cognitive performance in older adults. Stroke. 1998;29:2334–2340. doi: 10.1161/01.str.29.11.2334. [DOI] [PubMed] [Google Scholar]
- 33.Sierra C, De La Sierra A, Salamero M. et al. Silent cerebral white matter lesions and cognitive function in middle-aged essential hypertensive patients. Am J Hypertens. 2004;17:529–534. doi: 10.1016/j.amjhyper.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 34.Gons RA, de Laat KF, van Norden AG. et al. Hypertension and cerebral diffusion tensor imaging in small vessel disease. Stroke. 2010;41:2801–2806. doi: 10.1161/STROKEAHA.110.597237. [DOI] [PubMed] [Google Scholar]
- 35.Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- 36.Prins ND, van Dijk EJ, den Heijer T. et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain. 2005;128:2034–2041. doi: 10.1093/brain/awh553. [DOI] [PubMed] [Google Scholar]
- 37.O'Sullivan M, Jarosz JM, Martin RJ. et al. MRI hyperintensities of the temporal lobe and external capsule in patients with CADASIL. Neurology. 2001;56:628–634. doi: 10.1212/wnl.56.5.628. [DOI] [PubMed] [Google Scholar]
- 38.Dufouil C, Chalmers J, Coskun O. et al. PROGRESS MRI Substudy Investigators. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation. 2005;112:1644–1650. doi: 10.1161/CIRCULATIONAHA.104.501163. [DOI] [PubMed] [Google Scholar]
- 39.Saleh C. Carotid artery intima media thickness: a predictor of cognitive impairment? Front Biosci. 2010;2:980–990. doi: 10.2741/e157. [DOI] [PubMed] [Google Scholar]
- 40.Birns J, Kalra L. Cognitive function and hypertension. J Hum Hypertens. 2009;23:86–96. doi: 10.1038/jhh.2008.80. [DOI] [PubMed] [Google Scholar]
- 41.Paran E, Anson O, Reuveni H. Blood pressure and cognitive functioning among independent elderly. Am J Hypertens. 2003;16:818–826. doi: 10.1016/s0895-7061(03)01005-7. [DOI] [PubMed] [Google Scholar]
- 42.Nazzaro P, Schirosi G, Mezzapesa D. et al. Effect of clustering of metabolic syndrome factors on capillary and cerebrovascular impairment. Eur J Intern Med. 2013;24:183–188. doi: 10.1016/j.ejim.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 43.Beason-Held LL, Moghekar A, Zonderman AB. et al. Longitudinal changes in cerebral blood flow in the older hypertensive brain. Stroke. 2007;38:1766–1773. doi: 10.1161/STROKEAHA.106.477109. [DOI] [PubMed] [Google Scholar]
- 44.Efimova IY, Efimova NY, Triss SV. et al. Brain perfusion and cognitive function changes in hypertensive patients. Hypertens Res. 2008;31:673–678. doi: 10.1291/hypres.31.673. [DOI] [PubMed] [Google Scholar]
- 45.Kuller LH, Lopez OL, Newman A. et al. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22:13–22. doi: 10.1159/000067109. [DOI] [PubMed] [Google Scholar]
- 46.Igase M, Kohara K, Miki T. The association between hypertension and dementia in the elderly. Int J Hypertens. 2012. doi 10.1155/2012/320648. [DOI] [PMC free article] [PubMed]
- 47.Hajjar I, Hart M, Chen YL. et al. Effect of antihypertensive therapy on cognitive function in early executive cognitive impairment: a double blind randomized clinical trial. Arch Intern Med. 2012;172:442–444. doi: 10.1001/archinternmed.2011.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]