Abstract
Social defeat leads to selective avoidance of familiar opponents as well as general avoidance of novel, non-threatening intruders. Avoidance of familiar opponents represents a fear-related memory whereas generalized social avoidance indicates anxiety-like behavior. We have previously shown that serotonin signaling alters responses to social defeat in Syrian hamsters, although it is unclear whether serotonin modulates defeat-induced fear, anxiety, or both. In this study we focus on 5-HT1A receptors, in part, because their activation had been linked to the acquisition of conditioned fear. We hypothesized that pharmacological activation of 5-HT1A receptors prior to social defeat would reduce avoidance of familiar opponents, impair Arc expression in the basolateral amygdala (BLA), but not alter anxiety-like behavior. We administered 8-OH-DPAT, a 5-HT1A receptor agonist, prior to 3, 5-minute social defeats and 24-hours later exposed hamsters to a social interaction test to measure the conditioned defeat response immediately followed by either a Y-maze test or an open field test. In a separate experiment, we administered 8-OH-DPAT prior to 3, 5-minute social defeats and later removed brains for Arc immunohistochemistry. Social defeat increased the number of Arc immunopositive cells in the central amygdala (CeA), prelimbic cortex (PL), and BLA, and 8-OH-DPAT treatment reduced Arc immunoreactivity in the PL. These results suggest that 5-HT1A receptor activation impairs the fear memory associated with social defeat, but does not alter defeat-induced anxiety. Overall, 5-HT1A receptor activation may impair Arc expression in select brain regions such as the PL and thereby disrupt the development of a fear memory essential for the conditioned defeat response.
Keywords: social defeat, serotonin, 5-HT1A receptor, anxiety, fear, conditioned defeat
1. Introduction
The type of stress experienced by humans is often psychosocial in nature and stressful events are a contributing factor in the development of affective disorders, such as major depression, generalized anxiety disorder, and post-traumatic stress disorder (Anisman and Zacharko, 1992; Arborelius et al., 1999; Davidson, 2003). Ethologically relevant animal models of social stress are particularly well-suited to investigate the neurobiological mechanisms underlying stress-related psychopathologies (Nestler and Hyman, 2010; Blanchard et al., 1995; Fuchs and Flugge, 2003). Social defeat is a robust stressor that leads to heightened HPA-axis activity (Blanchard et al., 1995) as well as changes in behavior (Ruis et al., 1999; Watt et al., 2009), including increased anxiety-like behavior in the elevated plus maze (Heinrichs et al., 1992; Berton et al., 1998), altered circadian rhythmicity (Tornatzky and Miczek, 1993; Meerlo et al., 1996a), reduced body weight (Bartolomucci et al., 2004; Iio et al., 2012), and reduced locomotor activity (Rygula et al., 2005; Calvo et al., 2011). Following acute social defeat, male Syrian hamsters lose their species-typical territorial aggression and instead show submissive and defensive behavior when a smaller, non-aggressive intruder (NAI) is placed into their home cage (Potegal et al., 1993; Huhman et al., 2003). This change in agonistic behavior is called conditioned defeat and is likely the result of defeat-induced increases in both fear and anxiety.
One advantage of the conditioned defeat model is that because behavioral changes occur following an acute social defeat, neural mechanisms controlling defeat-related memories may be investigated. We know that blockade of NMDA receptors in the basolateral amygdala (BLA) prior to social defeat impairs the acquisition of the conditioned defeat response (Day et al., 2011), whereas overexpression of cAMP response element-binding protein (CREB) in the BLA prior to social defeat increases the acquisition of conditioned defeat (Jasnow et al., 2005). Brain-derived neurotrophic factor (BDNF) mRNA has also been found to increase in the BLA following social defeat, and a TrkB receptor antagonist administered into the BLA prior to social defeat also reduces the acquisition of conditioned defeat (Taylor et al., 2011). Similarly, previous research has shown that NMDA receptors, CREB, and BDNF in the BLA are critical targets controlling the formation of fear-related memories (Rodrigues et al., 2001; Rattiner et al., 2005; Izumi et al., 2011). Altogether, these findings suggest that conditioned defeat is controlled by neural circuitry in the BLA known to regulate fear memories. Activity-regulated cytoskeletal-associated protein (Arc/Arg 3.1) is an immediate early gene induced by neural activity and critical for synaptic plasticity in the hippocampus and the consolidation of long-term memory (Steward et al., 1998; Guzowski et al., 2000; Steward and Worley, 2001 see Ploski et al 2008 for these references). More recently, Arc expression in the BLA was shown to be necessary for both the consolidation (Ploski et al., 2008), and reconsolidation (Maddox and Schafe, 2011), of Pavlovian fear conditioning. In this study we use Arc expression in the amygdala and medial prefrontal cortex as a cellular marker of synaptic plasticity.
Several lines of evidence suggest that serotonin (5-HT), and particularly neural signaling at 5-HT1A receptors, modulates fear-related and anxiety-like behavior. In humans, 5-HT1A receptor binding is negatively correlated with anxiety levels (Tauscher et al., 2001). In animal models, 5-HT1A receptor knockout mice show high levels of anxiety-like behavior compared to controls (Gross et al., 2000; Ramboz et al., 1998), and overexpression of 5-HT1A receptors reduces anxiety-like behavior (Kusserow et al., 2004). Consistent with an anxiolytic role for 5-HT1A receptors, Li et al. (2012) found that viral-mediated knockdown of 5-HT1A receptors in the amygdala resulted in increased anxiety in the elevated plus maze in mice. Activation of 5-HT1A receptors also impairs the formation of fear memories. For example, pharmacological activation of 5-HT1A receptors in the hippocampus has been shown to impair the acquisition of both contextual and cued fear conditioning (Stiedl et al., 2000). We have found that pharmacological activation of 5-HT1A receptors in the dorsal raphe nucleus (DRN) (Cooper et al., 2008) and BLA (Morrison and Cooper, 2012) prior to social defeat impairs the acquisition of conditioned defeat in Syrian hamsters. However, it is unknown whether activation of 5-HT1A receptors alters an anxiety component and/or a fear component of the conditioned defeat response.
In Syrian hamsters acute social defeat results in a specific memory of the aggressive opponent. For example, social defeat results in increased social avoidance of familiar opponents compared to unfamiliar opponents (Petrulis et al., 2004; McCann and Huhman, 2012). Similarly, using a Y-maze test for individual recognition after social defeat, hamsters show increased avoidance of familiar winners compared to unfamiliar winners and familiar neutral animals (Lai and Johnston, 2002; Lai et al., 2005). Also, systemic administration of the protein synthesis inhibitor anisomycin blocks defeat-induced social avoidance in the Y-maze (Huang et al., 2011). Anxiety-like behavior following social defeat has been measured in an open field test in several rodent species. Defeated animals typically show reduced locomotion and reduced time spent in the center of the arena (Raab et al., 1986; Meerlo et al., 1996b; Meerlo et al., 1996c; Kinsey et al., 2007).
The purpose of this study was to investigate the mechanisms by which 5-HT1A receptors modulate the development of conditioned defeat. We hypothesized that activation of 5-HT1A receptors prior to social defeat would impair the acquisition of conditioned defeat by disrupting memory for the defeat experience and decreasing defeat-induced Arc expression in the BLA.
2. Methods
2.1 Animals
We used adult male Syrian hamsters (Mesocricetus auratus) that weighed 130–170 g (3–4 months of age) at the start of the study, and were individually housed for 10–14 days prior to testing. Older hamsters that weighed 180–200 g (>6 months) were individually housed and used as resident aggressors for social defeat training. Younger hamsters that weighed 90–120 g (2 months) were group-housed (4 per cage) and used as nonaggressive intruders for conditioned defeat testing. All animals were housed in polycarbonate cages (12 cm × 27 cm × 16 cm) with corncob bedding, cotton nesting materials, and wire-mesh tops. Animal cages were not changed for at least 1 week prior to testing to allow individuals to scent mark their territory. Animals were housed in a temperature-controlled colony room (20 ± 2 °C) and maintained on a 14:10 h light:dark cycle with food and water available ad libitum. Subjects were handled for 7–10 days prior to social defeat training and all behavioral testing occurred in the first three hours of the dark cycle. All procedures were approved by the University of Tennessee Institutional Animal Care and Use Committee and follow the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2 Behavioral Testing
2.2.1 Social Defeat Training
Social defeat training consisted of three, 5-minute aggressive encounters in the home cage of a larger resident aggressor (RA). To facilitate similar amounts of aggression, the start of social defeat began at the first attack, which usually occurred within the first 60 seconds of the encounter. When drug treatments were administered prior to social defeat training, defeats were digitally recorded to identify any nonspecific effects of drug treatments. The number of attacks and total duration of aggressive behavior received by subjects were later quantified. In experiments where drug treatments altered the duration of conditioned defeat behavior, no defeat controls were used to assess whether the treatments altered agonistic behavior in the absence of social defeat stress. No defeat control animals were exposed to three different empty RA cages for 5 minutes each.
2.2.2 Conditioned Defeat Testing
Conditioned defeat testing occurred 24 hours following social defeat training and consisted of one 5-minute encounter with a novel, non-aggressive intruder (NAI) in the home cage of the subject. Testing was recorded and later scored by a researcher blind to the experimental conditions using Noldus Observer. A second researcher scored a subset of testing sessions, and inter-observer reliability was greater than 90% agreement. We quantified the duration of four categories of behavior: submissive/defensive (flee, avoid, upright and side defensive postures, tail-up, stretch-attend, head flag); aggressive (chase, attack, upright and side offensive postures); non-agonistic social (nose touching, sniff, approach); and nonsocial (locomotion, grooming, nesting, feeding) (Albers et al., 2002). We also quantified the frequency of flees, attacks, and stretch-attend postures displayed by the subject.
2.2.3 Y-maze Testing
In order to evaluate avoidance of the RA, a Y-shaped acrylic maze was used. The Y-maze is divided into eight rectangular regions (10 cm wide × 10 cm high). The base of the Y (89 cm long) is divided into start box (20 cm) and stem (69 cm), and the two arms of the maze are 70 cm long and are divided into 3 sections. The sections closest to the stem are the basal parts (25 cm) of the arm while the compartments farther from the base are the distal parts (25 cm) of the arm. Subjects have access to all compartments of the Y-maze except for the most distal parts of the Y, which are the stimulus boxes (20 cm) where the RA is located during testing. The RA and subject are separated through a perforated Plexiglas wall (0.8 cm thick) to allow for the movement of air throughout the maze. The screen in front of the stimulus box is permanent whereas the screen that separates the start box from the stem is removable to allow the subject to explore the maze. Air is drawn from the stimulus boxes to the start box by a fan mounted on the outside of the start box.
Y-maze testing consisted of two, 3-minute trials as described by Lai et al. (2005). In the first trial, the stimulus box was empty and the subject’s preferred arm was determined. In the second trial, one of the former RAs that defeated the subject was placed in the stimulus box of the subject’s preferred arm to avoid confounding side preferences with avoidance of the RA. The Y-maze was cleaned with 70% ethanol after each subject was tested to remove any residual odors. When scoring the location of the subject within the Y-maze, we divided it into six compartments: the start box, the stem of the Y, the basal part of each arm of the Y, and the distal part of each arm of the Y. The location of the subject within the Y-maze was determined by the location of its nose. We also scored the amount of time spent in olfactory investigation (sniffing), which is defined as time spent with the hamster’s nose within 2 cm of the perforated screen of the stimulus box.
2.2.4 Open Field Testing
The open field arena was an 80 × 80 × 40 cm acrylic box with black sides and a white grid on the floor. The grid was divided into 25 16 × 16 cm2 squares. At the start of the trial subjects were placed underneath a plastic shelter in one corner of the open field to measure latency to withdraw from the shelter into the open field. The open field was cleaned between each trail with 70% ethanol to remove any residual odors left by the subject. Tests were recorded and scored by an observer blind to the experimental conditions. We scored the latency to exit the shelter, the number of line crosses, and the number of center entries within the grid. A line cross was defined as the point at which a hamster’s nose crossed the grid line.
2.3 Drug Treatment
We dissolved (±)-8-Hydroxy-2-(dipropylamino)tetralin hydrobromide (8-OH-DPAT; Sigma-Aldrich) in sterile saline (pH=5.5–6.0), which was used as a vehicle control at the same pH (Ricci et al., 2006). 8-OH-DPAT is a commonly used nonselective 5-HT1A receptor agonist at the doses used here (Cervantes et al., 2010; Fernandez-Guasti and Lopez-Rubalcava, 1995), although it also activates 5-HT7 receptors (Hedlund et al., 2004). Drugs were administered with a 0.3 mL intraperitoneal injection (i.p.).
2.4 Experimental Treatment
2.4.1 Experiment 1
We investigated whether injection of a 5-HT1A receptor agonist would reduce the acquisition of conditioned defeat, reduce avoidance of former opponents, and modulate defeat-induced anxiety in the open field. We injected 8-OH-DPAT (0.25 mg/kg, N=29; or 0.5 mg/kg, N=14) or vehicle (N=31) 20 minutes prior to 3, 5-minute social defeats. For no defeat controls, we injected 8-OH-DPAT (0.25 mg/kg, N=23) or vehicle (N=21) 20 minutes prior to 3, 5-minute exposures to empty RA cages. Twenty-four hours later, animals were tested for conditioned defeat behavior.
Immediately following conditioned defeat testing, about half of the defeated animals (0.25 mg/kg, N=15; or 0.5 mg/kg, N=14; or vehicle, N=16) and half of the non-defeated animals (0.25 mg/kg, N=11; or vehicle N=10) were tested for avoidance of the RA in a Y-maze. The other defeated animals (0.25 mg/kg, N=14; or vehicle N=15) and non-defeated animals (0.25 mg/kg, N=12; or vehicle N=12) were tested for anxiety-like behavior in the open field immediately following conditioned defeat testing (Figure 1). To validate Y-maze testing, a separate set of animals (N = 11) was used to test whether defeated animals avoided familiar winners more than unfamiliar winners.
Figure 1.
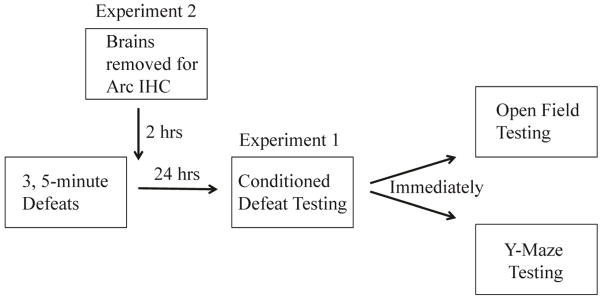
A schematic representation of the experimental design. In experiment 1, animals received conditioned defeat testing immediately followed by either open field testing or Y-maze testing. In experiment 2, animals were euthanized 2 hours following social defeat for Arc immunohistochemistry.
2.4.2 Experiment 2
We investigated whether a 5-HT1A receptor agonist would reduce defeat-induced Arc expression in select brain regions by injecting 8-OH-DPAT (0.25 mg/kg; N=10) or vehicle (N=10) 20 minutes prior to 3, 5-minute social defeats. For no defeat controls, we injected 8-OH-DPAT (0.25 mg/kg; N=8) or vehicle (N=10) 20 minutes prior to 3, 5-minute exposures to empty RA cages. We selected 0.25 mg/kg of 8-OH-DPAT because it was the most effective dose for conditioned defeat.
2.5 Immunohistochemistry
Two hours following the end of social defeat, animals were anesthetized with isoflurane and transcardially perfused with 100 ml of 0.1 M PBS followed by 100 ml of 4% paraformaldehyde. Brains were removed and soaked in 4% paraformaldehyde for 24 hours, followed by 0.1 M PBS/30% sucrose solution for 48 hours, and then were stored in cryoprotectant, all at 4°C. A consecutive series of 30 μm coronal sections were cut on a vibrating microtome and collected into three vials as free floating sections in cryoprotectant. The collected sections were processed for Arc protein immunohistochemistry using the following protocol. Sections were washed five times in PBS + 0.2% Triton before each incubation, which were conducted at room temperature unless otherwise stated. Sections were incubated for 25 minutes in 0.3% hydrogen peroxide and methanol solution. Sections were then incubated with 1% bovine serum (BSA) in PBS + 0.2% Triton for 60 minutes before being incubated at room temperature for 24 hours in mouse anti-Arc polyclonal primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at a final dilution of 1:500 in 1% BSA in PBS + 0.2% Triton. Sections were then washed five times with PBS + 0.2% Triton, incubated for 60 minutes in biotinylated secondary anti-mouse IgG (Vector Laboratories, Burlingame, CA) at a final dilution of 1:200 with 0.1% BSA in PBS + 0.2% Triton. Sections were then incubated in avidin-biotin-complex (Vectastain ABC kit; Vector Laboratories, Burlingame, CA) with PBS for 60 minutes, and the peroxidase reaction was visualized using a 15 minute incubation in 3,3’-diaminobenzidine (DAB tablet, Sigma-Aldrich, St. Louis, MO) and nickel dissolved in PBS. The sections were washed five times with PBS and five times with distilled H2O prior to being mounted onto glass microscope slides. After air-drying, sections were dehydrated using a series of alcohols, cleared with citrisolv and coverslipped using DPX mountant (Sigma-Aldrich, St. Louis, MO). For each brain region, the tissue from all subjects was processed simultaneously.
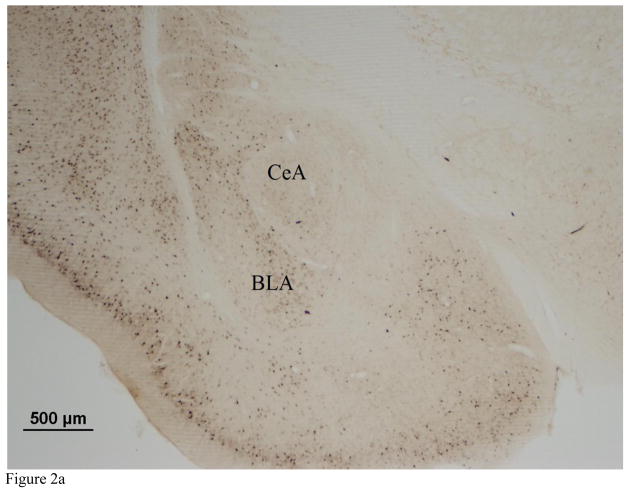
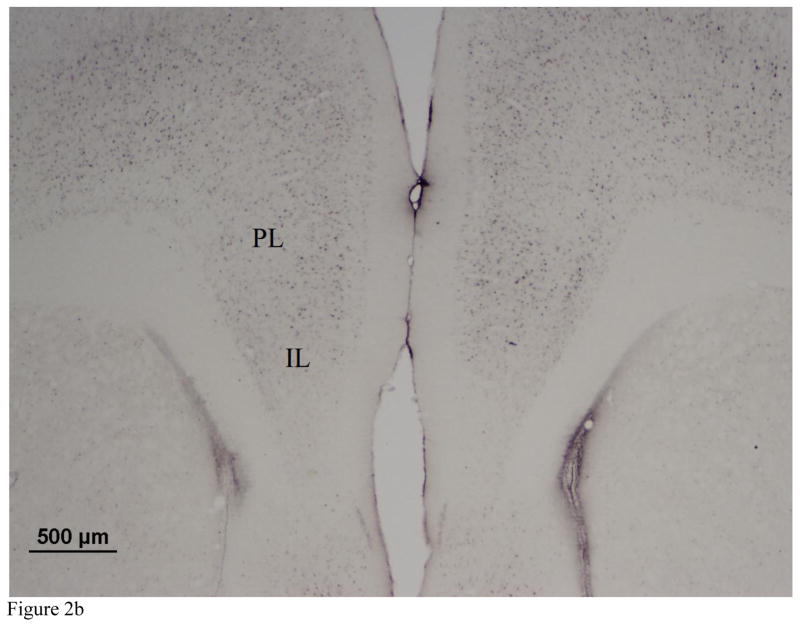
Images were captured at 10X magnification using an Olympus BX41 microscope. The number of Arc immunopositive cells was quantified in the BLA, central amygdala (CeA), prelimic cortex (PL), and infralimbic cortex (IL) using MCID Core image analysis software (InterFocus Imaging, Cambridge, England). Arc immunoreactivity was manually quantified in a subset of sections and software parameters were adjusted to reach 90% agreement with manual cell counts. We recorded background immunoreactivity in unstained regions of each image and defined immunopositive cells as those that showed staining 1.5X darker than the specific background immunoreactivity calculated for each image. Cell counts were restricted to a 350 × 350 μm area within the BLA, a 240 × 240 μm area within the CeA, and a 877 × 660 μm area within the IL and PL (Fig. 2). For each brain region we quantified three to six sections per individual.
Figure 2.
Representative photomicrographs (2X magnification) of the a) amygdala and b) prefrontal cortex showing Arc immunoreactivity. Arc immunopositive cells were quantified in the basolateral amygdala (BLA) central amygdala (CeA) prelimbic cortex (PL) and infralimbic cortex (IL).
2.6 Statistical analysis
Four animals did not receive sufficient aggression from our RAs and were excluded from analysis. One animal was excluded because it was attacked by the RA during Y-maze testing. Two animals were excluded because of damage to brain tissue when sliced on the vibrating microtome. Finally, four animals were excluded from immunohistochemical analysis because of poor and inconsistent staining.
We performed two-way ANOVAs to investigate an interaction between defeat (2 levels) and drug treatment (2 levels) on conditioned defeat, Y-maze, and open field tests. For each behavioral test, we performed planned comparisons to investigate dose-response relationships (one-way ANOVA, LSD post-hoc tests) and non-selective drug effects in non-defeated controls (t-tests). For Arc immunohistochemical data, we performed a two-way ANOVA with defeat experience and drug treatment as independent variables. All statistical tests were two-tailed, the alpha level was p < 0.05, and data are presented as mean ± S.E.
3. Results
3.1 Experiment 1
3.1.1 8-OH-DPAT effects on conditioned defeat
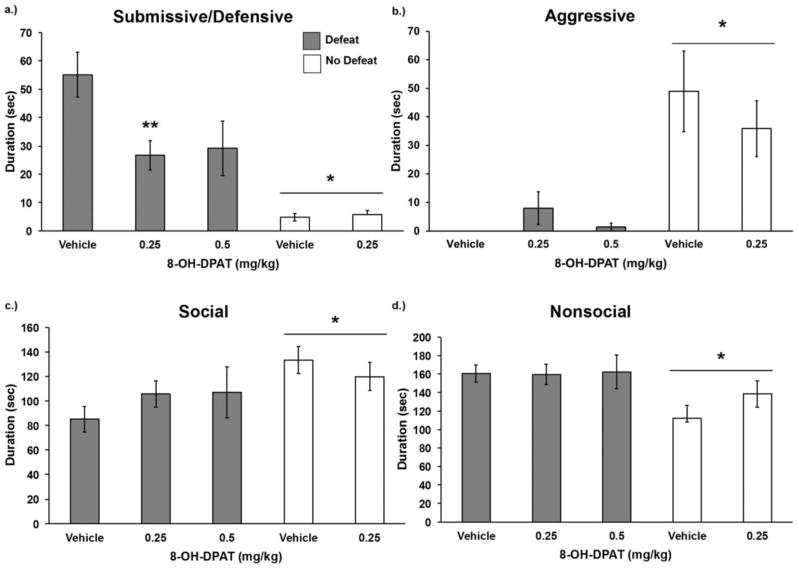
Injection of 8-OH-DPAT prior to social defeat decreased the acquisition of conditioned defeat (Fig 3). We found a significant drug by defeat interaction for the duration of submissive and defensive behavior (F(1,99) = 6.029, p = 0.011) as well as a main effect of defeat (F(1,99) = 37.362 p = 0.000). Specifically, defeated subjects showed a significant decrease in submissive and defensive behavior at the .25 mg/kg dose (F(2,70) = 5.306, p = 0.007, LSD, p = 0.002). Injection of 8-OH-DPAT prior to social defeat did not alter the durations of non-agonistic social (F(1,99) = 2.348, p = 0.129), nonsocial (F(1,99) = 1.336, p = 0.251), or aggressive (F(1,99) = 1.766, p = 0.187) behaviors. We found other effects of social defeat such that defeated animals showed decreased non-agonistic social (F(1,99) = 7.872, p = 0.006), increased nonsocial (F(1,99) = 8.369, p = 0.005), and decreased aggressive (F(1,99) = 23.399, p = 0.000) behavior compared to non-defeated animals. We also found main effects of social defeat on the number of stretch attend postures (F(1,99) = 5.320 p = 0.023) and the number of attacks (F(1,99) = 31.563 p = 0.000) (Table 1). In contrast, there were no significant interactions or drug treatment main effects for the frequency of flight, stretch-attend postures, or attacks (p > 0.05, Table 1).
Figure 3.
Durations (mean ± SE) of a) submissive, b) aggressive, c) non-agonistic social, and d) nonsocial behavior are shown for a 5-minute test with a novel, non-aggressive opponent. Defeated animals received an injection of 8-OH-DPAT or vehicle 20 minutes before social defeat training. Likewise, non-defeated controls received an injection of 8-OH-DPAT or vehicle 20 minutes before exposure to a resident aggressor’s empty cage. ** Indicates a significant defeat by drug treatment interaction (p < 0.05). *Indicates a main effect of social defeat (p < 0.05).
Table 1.
The frequencies of flee, stretch attend, and attack (mean ± SE) during conditioned defeat testing are shown. Defeated (D) animals were treated with 0.0 mg/kg, 0.25 mg/kg, or 0.5 mg/kg of 8-OH-DPAT prior to social defeat. No defeat (ND) animals were treated with 0.0 mg/kg or 0.25 mg/kg of 8-OH-DPAT before exposure to an aggressor’s empty cage.
| Behavior | D 0.0 mg/kg | D 0.25 mg/kg | D 0.5 mg/kg | ND 0.0 mg/kg | ND 0.25 mg/kg |
|---|---|---|---|---|---|
| Flee | 1.032 ± .487 | .034 ± .035 | .071 ± .071 | .000 ± .000 | .000 ± .000 |
| Stretch Attend | .452 ± .166a | .517 ± .236a | .071 ± .071a | .048 ± .048b | .130 ± .072b |
| Attack | .000 ± .000b | .172 ± .141b | .071 ± .071b | 2.190 ± .575a | 1.261 ± .346a |
a > b, p < 0.05
Injection of 8-OH-DPAT prior to social defeat training appeared to increase the amount of aggression resident aggressors directed towards subjects. Vehicle controls received 373.8 s (±22.9) of aggression during social defeat training and individuals injected with 0.25 or 0.5 mg/kg of 8-OH-DPAT received 447.9 s (±19.3) and 478.4 s (±34.5) of aggression, respectively (F(2,70) = 4.802 p = 0.011). It is noteworthy that increased aggression received by 8-OH-DPAT treated animals was associated with reduced conditioned defeat. Although drug treatment increased the duration of aggression during social defeats, it did not alter the number of attacks received by subjects. Vehicle controls received 13.8 (±1) attacks during social defeat training and individuals injected with 0.25 or 0.5 mg/kg of 8-OH-DPAT received 14.6 (±0.9) and 13.1 (±1.3) attacks, respectively (F(2,70) = 0.413 p = 0.663).
3.1.2 8-OH-DPAT effects on Y-maze behavior
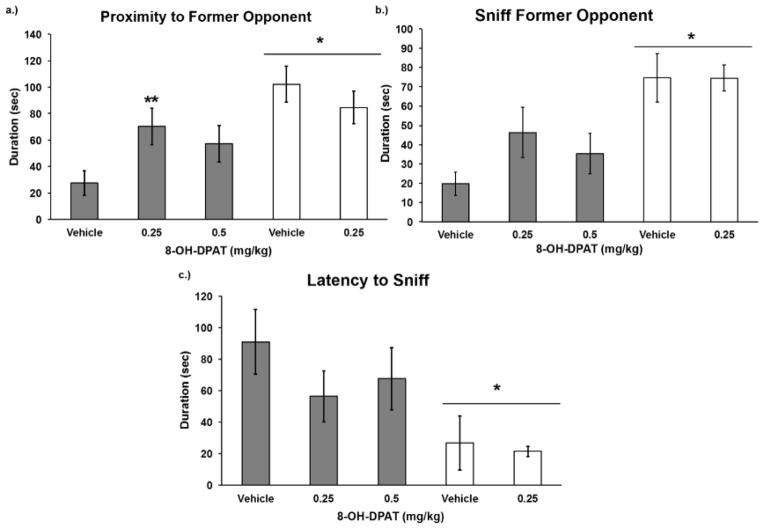
Injection of 8-OH-DPAT prior to social defeat increased the amount of time spent in the distal compartment of the Y-maze near the RA (Figure 4). We found a significant drug by defeat interaction for proximity to the RA in the Y-maze such that 8-OH-DPAT reduced social avoidance in defeated animals while it had no effect in non-defeated animals (F(1,47) = 5.916, p = 0.019). Similarly, the test for a dose-response relationship showed a significant effect of 8-OH-DPAT treatment on proximity to the RA (F(2,41) = 3.227, p = 0.050; post-hoc p = 0.017). We did not find a significant drug by defeat interaction for the amount of time spent sniffing the RA (F(1,47) = 1.940, p = 0.170) or the latency to sniff the RA (F(1,47) = 0.997, p = 0.323). Main effects of social defeat were found for the amount of time spent in proximity to the RA (F(1,47) = 12.825, p = 0.001), sniffing the RA (F(1,47) = 16.941, p = 0.000), and the latency to sniff the RA (F(1,47) = 8.957, p = 0.004). In a separate set of animals, we found that defeated hamsters did not avoid familiar RAs more than unfamiliar RAs in the Y-maze. Subjects spent 44.1 s (±9.3) in proximity to one of the RAs that defeated them, while they spent 53.1 s (±13.7) in proximity to an unfamiliar RA that defeated another animal (t(10) = −0.568, p = 0.583).
Figure 4.
Durations (mean ± SE) of time spent in a) proximity to former opponent, b) sniffing the former opponent, and c) latency to sniff the former opponent are shown for a 3-minute test in the Y-maze. Defeated animals received an injection of 8-OH-DPAT or vehicle 20 minutes before social defeat training. Likewise, non-defeated controls received an injection of 8-OH-DPAT or vehicle 20 minutes before exposure to a resident aggressor’s empty cage. ** Indicates a significant defeat by drug treatment interaction (p < 0.05). *Indicates a main effect of social defeat (p < 0.05).
3.1.3 8-OH-DPAT effects on open field behavior
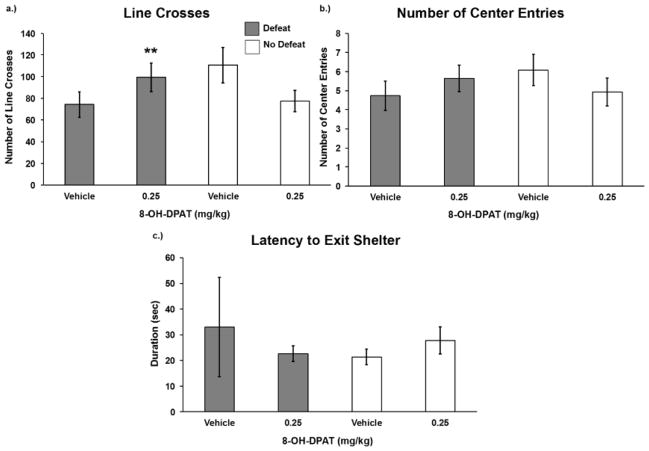
We found a significant drug by defeat interaction for the number of line crosses within the open field (Fig. 5a; F(1,50) = 4.812, p = 0.033). This result indicates that 8-OH-DPAT treatment increased locomotion in the open field for defeated animals although it decreased locomotion for non-defeated controls. The interaction of social defeat and drug treatment on the number of entries into the center of the open field approached significance (F(1,50) = 3.314 p = 0.075). There were no significant effects of social defeat or drug treatment on the latency to exit the shelter in the open field.
Figure 5.
Number (mean ± SE) of a) line crosses, b) center entries, and c) the latency to exit the shelter are shown for a 5-minute open field test. Defeated animals received an injection of 8-OH-DPAT or vehicle 20 minutes before social defeat training. Likewise, non-defeated controls received an injection of 8-OH-DPAT or vehicle 20 minutes before exposure to a resident aggressor’s empty cage. ** Indicates a significant defeat by drug treatment interaction (p < 0.05).
3.2 Experiment 2
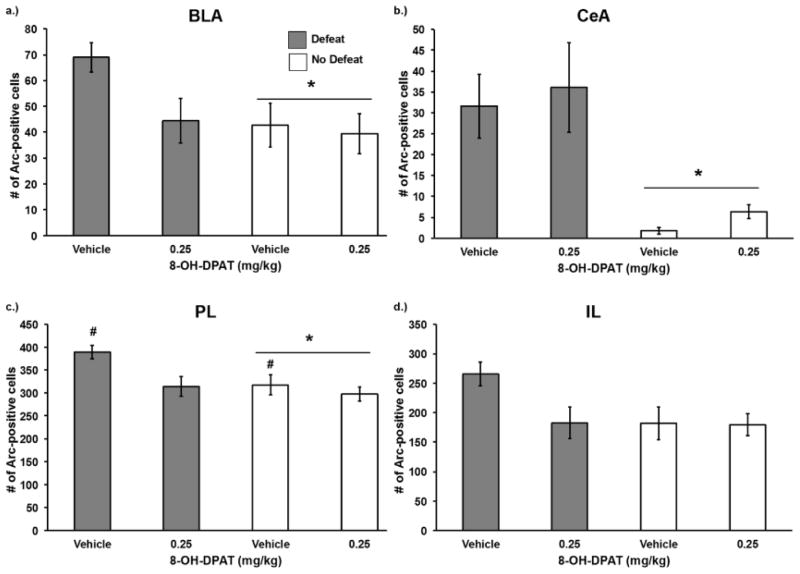
3.2.1 8-OH-DPAT effects on Arc expression
Social defeat increased the number of Arc immunopositive cells in several brain regions, while injection of 8-OH-DPAT reduced it. In the PL we found a significant main effect of social defeat (F(1,36) = 5.66, p = 0.023) and a significant main effect of 8-OH-DPAT treatment (F(1,36) = 6.38, p = 0.016; Fig. 6c). A similar pattern of Arc immunoreactivity was found in the IL and the BLA, although comparisons often failed to reach statistical significance. Social defeat increased the number of Arc immunopositive cells in the BLA (F(1,36) = 4.02, p = 0.05), although the effect of 8-OH-DPAT was non-significant (F(1,36) = 3.16, p = 0.084; Fig. 6a). In the IL the effects of both social defeat and 8-OH-DPAT treatment were not statistically significant (F(1,36) = 3.33, p = 0.076; F(1,36) = 3.24, p = 0.08; Fig. 6d). In contrast, social defeat produced a large increase in Arc immunoreactivity in the CeA (F(1,36) = 19.96, p < 0.0001), while there was no effect of 8-OH-DPAT treatment (F(1,36) = 0.46, p = 0.503; Fig. 6b).
Figure 6.
Number (mean ± SE) of Arc immunopositive cells in the a) BLA b) CeA c) PL and d) IL following social defeat training. Defeated animals received an injection of 8-OH-DPAT or vehicle 20 minutes before social defeat training. Likewise, non-defeated controls received an injection of 8-OH-DPAT or vehicle 20 minutes before exposure to a resident aggressor’s empty cage. *Indicates a main effect of social defeat (p ≤ 0.05). # indicates a main effect of drug treatment (p < 0.05).
Consistent with the behavior experiments, 8-OH-DPAT treatment significantly increased the amount of aggression subjects received. Vehicle controls received 328.2 s (±32.5) of aggression, and individuals injected with 8-OH-DPAT received 492.1 s (±41.9) of aggression (t(18) = −3.093, p = 0.006). Vehicle controls received 15.5 (±1.6) attacks during social defeat training and individuals injected with 8-OH-DPAT received 21.5 (±2.2) attacks (t(18) = −2.204, p = 0.041).
4. Discussion
We found that administration of 8-OH-DPAT, a nonselective 5-HT1A receptor agonist, prior to social defeat reduced the acquisition of conditioned defeat as indicated by a decrease in submissive and defensive behavior at testing. 8-OH-DPAT treatment did not alter agonistic behavior in non-defeated animals, which suggests that 5-HT1A receptor activation disrupts defeat-induced changes in behavior but not agonistic behavior in general. We also found that administration of 8-OH-DPAT prior to social defeat reduced avoidance of former opponents in a Y-maze. These results suggest that activation of 5-HT1A receptors impairs avoidance of familiar opponents. We also found that 8-OH-DPAT treatment increased line crosses in the open field among defeated animals, while the effects in non-defeated animals were in the opposite direction. Finally, social defeat increased Arc immunoreactivity in several brain regions and 8-OH-DPAT treatment reduced Arc immunoreactivity in the PL. Altogether, our results suggest that pharmacological activation of 5-HT1A receptors may disrupt fear-related memories critical for conditioned defeat by impairing Arc immunoreactivity in brain regions such as the PL.
Johnston and colleagues have pioneered the use of a Y-maze for testing social avoidance in hamsters and we based our procedure on their work. They have shown that following acute social defeat hamsters avoid familiar winners more than unfamiliar winners in a Y-maze test (Lai and Johnston, 2002; Lai et al., 2005). We found that hamsters avoided unfamiliar winners to the same extent as familiar winners, which may be related to the more intense social defeat experience used in our study. Thus, although hamsters are capable of selectively avoiding former opponents, it is unclear whether 8-OH-DPAT treatment reduces avoidance to former opponents only. Although we found an interaction of social defeat and 8-OH-DPAT treatment on locomotion in the open field, the interaction was partly due to drug effects in non-defeated animals. Because there was not a robust effect of 8-OH-DPAT treatment in defeated animals themselves, our findings suggest a limited effect of 5-HT1A receptor activation on anxiety-like behavior in an open field test. These results are consistent with previous research showing that chronic social subjugation in juvenile hamsters results in increased fear-related behavior in a Y-maze test but no change in anxiety-like behavior in an open field test (Bastida et al., 2009). Hamsters are capable of showing anxiety-like behavior in an open field as anxiogenic stimuli and anxiolytic drugs can alter open field locomotion (Solomon et al., 2007; Gannon et al., 2011). Also, in rats, acute social defeat can reduce locomotion in an open field (Meerlo et al., 1996c). Altogether, 8-OH-DPAT treatment appeared to more effectively reduce the acquisition of social avoidance in the Y-maze than defeat-induced anxiety-like behavior in the open field test.
We unexpectedly found that 8-OH-DPAT treatment reduced locomotion in non-defeated animals, which suggests that 8-OH-DPAT treatment enhances anxiety-like behavior 24 hours later in an open field. Uncontrollable stress has been shown to elevate 5-HT concentrations in the DRN, desensitize 5-HT1A autoreceptors, and lead to learned helplessness (Amat et al., 2005; Bambico et al., 2009; Rozeske et al., 2011). In our study, it is possible that 8-OH-DPAT treatment produced an anxiety-like response in non-defeated animals by desensitizing 5-HT1A autoreceptors, which in turn would sensitize the DRN to future mild stressors such as open field testing. Also, 8-OH-DPAT treatment may have direct effects on the expression of locomotion. For example, 8-OH-DPAT administration immediately prior to testing has been shown to reduce locomotion in an open field (Carey et al., 2004). We suspect that direct effects on locomotion may have contributed to why 8-OH-DPAT treated animals received increased aggression from RAs during social defeat. However, it is noteworthy that the amount of aggression received during social defeat was inversely related to the amount of submissive and defensive behavior produced at conditioned defeat testing.
Systemic 8-OH-DPAT treatment can target both 5-HT1A presynaptic autoreceptors and post-synaptic receptors, and activation of both pre- and post-synaptic 5-HT1A receptors can reduce conditioned defeat in hamsters (Cooper et al., 2008; Morrison and Cooper, 2012). We selected doses of 8-OH-DPAT that previous research indicates should target post-synaptic receptors (Fernandez-Guasti and Lopez-Rubalcava, 1995; Cervantes et al., 2010). However, our findings should be treated with caution as a role for 5-HT1A presynaptic receptors has not be ruled out in this study. Also, we found that 0.5 mg/kg of 8-OH-DPAT was less effective at reducing the acquisition of conditioned defeat compared to 0.25 mg/kg. It is possible that this non-linear dose response curve is due to 8-OH-DPAT binding to other post-synaptic sites such as 5-HT7 receptors. Eriksson et al. (2008) found that 8-OH-DPAT activity at 5-HT7 receptors counteracts 5-HT1A receptor-mediated impairment on a passive avoidance task.
We found that acute social defeat increased Arc immunoreactivity in several brain regions including the CeA, BLA, and PL. These findings are consistent with previous research in rats showing that two consecutive days of social defeat enhances Arc mRNA expression in the prefrontal cortex (Coppens et al. 2011). We also found that 8-OH-DPAT treatment reduced Arc immunoreactivity in the PL cortex and led to a similar, albeit non-significant, reduction in the BLA and IL. These results suggest that 5-HT1A receptor activation likely reduces Arc expression in several brain regions as well as reduces a fear-related component of conditioned defeat. The BLA and DRN are likely neural substrates mediating the cellular mechanisms by which 5-HT1A receptor activation reduces conditioned defeat. We have previously shown that pharmacological activation of 5-HT1A receptors in both the BLA and DRN reduces the acquisition of conditioned defeat (Cooper et al., 2008; Morrison et al., 2012). Interestingly, the PL cortex may also regulate the effects of 5-HT1A receptor activation on the acquisition of conditioned defeat. This role for the PL is consistent with other research showing that the PL has abundant 5-HT1A receptors (Pompeiano et al., 1992), is necessary for the expression of learned fear (Vidal-Gonzalez et al., 2006), and is a site where BDNF signaling enhances the consolidation of fear memories (Choi et al., 2010).
Conditioned defeat is a complex change in agonistic behavior that involves stress-induced changes in both fear and anxiety. Our findings suggest that 5-HT1A receptor activation disrupts Arc expression in the PL, and perhaps other brain regions, which impairs the development of a fear memory that is essential for the conditioned defeat response. These findings should be useful when considering treatment options for humans that have a stress-related mental illness with a large fear component.
Highlights.
8-OH-DPAT treatment reduces the acquisition of conditioned defeat
8-OH-DPAT treatment prior to social defeat reduces avoidance of familiar opponents
Social defeat increases Arc-IR in the amygdala and prefrontal cortex
5-HT1A receptor activation impairs Arc-IR in the PL and reduces defeat-induced fear
Acknowledgments
We thank Sonya Gross and Catie Clinard for their technical assistance. This work was supported by National Institutes of Health grant R21 MH085230.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albers HE, Huhman KL, Meisel RL. Hormonal basis of social conflict and communication. In: Plaff DW, et al., editors. Hormones, Brain and Behavior. San Diego: Academic Press; 2002. pp. 393–433. [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Anisman H, Zacharko RM. Depression as a consequence of inadequate neurochemical adaptation in response to stressors. Br J of Psychiatry Suppl. 1992;15:36–43. [PubMed] [Google Scholar]
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. JEndocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Bambico FR, Nguyen NT, Gobbi G. Decline in serotonergic firing activity and desensitization of 5-HT1A autoreceptors after chronic unpredictable stress. Eur Neuropsychopharmacol. 2009;19:215–228. doi: 10.1016/j.euroneuro.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Pederzani T, Sacerdote P, Panerai AE, Parmigiani S, Palanza P. Behavioral and physiological characterization of male mice under chronic psychosocial stress. Psychoneuroendocrinology. 2004;29:899–910. doi: 10.1016/j.psyneuen.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Bastida CC, Puga F, Delville Y. Risk assessment and avoidance in juvenile golden hamsters exposed to repeated stress. Horm Behav. 2009;55:158–162. doi: 10.1016/j.yhbeh.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Berton O, Aguerre S, Sarrieau A, Mormede P, Chaouloff F. Differential effects of social stress on central serotonergic activity and emotional reactivity in Lewis and spontaneously hypertensive rats. Neuroscience. 1998;82:147–159. doi: 10.1016/s0306-4522(97)00282-0. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen B, Sakai RR. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20:117–134. doi: 10.1016/0306-4530(94)e0045-b. [DOI] [PubMed] [Google Scholar]
- Calvo N, Cecchi M, Kabbaj M, Watson SJ, Akil H. Differential effects of social defeat in rats with high and low locomotor response to novelty. Neuroscience. 2011;183:81–89. doi: 10.1016/j.neuroscience.2011.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey RJ, Depalma G, Damianopoulos E, Muller CP, Huston JP. The 5-HT1A receptor and behavioral stimulation in the rat: effects of 8-OHDPAT on spontaneous and cocaine-induced behavior. Psychopharmacology. 2004;177:46–54. doi: 10.1007/s00213-004-1917-4. [DOI] [PubMed] [Google Scholar]
- Cervantes MC, Biggs EA, Delville Y. Differential responses to serotonin receptor ligands in an impulsive-aggressive phenotype. Behav Neurosci. 2010;124:455–469. doi: 10.1037/a0020171. [DOI] [PubMed] [Google Scholar]
- Choi DC, Maguschak KA, Ye K, Jang SW, Myers KM, Ressler KJ. Prelimbic cortical BDNF is required for memory of learned fear but not extinction or innate fear. Proc Natl Acad Sci U S A. 2010;107:2675–2680. doi: 10.1073/pnas.0909359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, McIntyre KE, Huhman KL. Activation of 5-HT1A autoreceptors in the dorsal raphe nucleus reduces the behavioral consequences of social defeat. Psychoneuroendocrinology. 2008;33:1236–1247. doi: 10.1016/j.psyneuen.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppens CM, Siripornmongcolchai T, Wibrand K, Alme MN, Buwalda B, de Boer SF, Koolhaas JM, Bramham CR. Social Defeat during Adolescence and Adulthood Differentially Induce BDNF-Regulated Immediate Early Genes. Front Behav Neurosci. 2011;5:72. doi: 10.3389/fnbeh.2011.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson JR. Treatment of posttraumatic stress disorder: the impact of paroxetine. Psychopharmacol Bull. 2003;37(Suppl 1):76–88. [PubMed] [Google Scholar]
- Day DE, Cooper MA, Markham CM, Huhman KL. NR2B subunit of the NMDA receptor in the basolateral amygdala is necessary for the acquisition of conditioned defeat in Syrian hamsters. Behav Brain Res. 2011;217:55–59. doi: 10.1016/j.bbr.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson TM, Golkar A, Ekstrom JC, Svenningsson P, Ogren SO. 5-HT7 receptor stimulation by 8-OH-DPAT counteracts the impairing effect of 5-HT(1A) receptor stimulation on contextual learning in mice. Eur J Pharmacol. 2008;596:107–110. doi: 10.1016/j.ejphar.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Flugge G. Chronic social stress: effects on limbic brain structures. Physiol Behav. 2003;79:417–427. doi: 10.1016/s0031-9384(03)00161-6. [DOI] [PubMed] [Google Scholar]
- Gannon RL, Lungwitz E, Batista N, Hester I, Huntley C, Peacock A, Delagrange P, Millan MJ. The benzodiazepine diazepam demonstrates the usefulness of Syrian hamsters as a model for anxiety testing: evaluation of other classes of anxiolytics in comparison to diazepam. Behav Brain Res. 2011;218:8–14. doi: 10.1016/j.bbr.2010.11.029. [DOI] [PubMed] [Google Scholar]
- Gross C, Santarelli L, Brunner D, Zhuang X, Hen R. Altered fear circuits in 5-HT1A receptor KO mice. Biol Psychiatry. 2000;48:1157–1163. doi: 10.1016/s0006-3223(00)01041-6. [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Kelly L, Mazur C, Lovenberg T, Sutcliffe JG, Bonaventure P. 8-OH-DPAT acts on both 5-HT1A and 5-HT7 receptors to induce hypothermia in rodents. Eur J Pharmacol. 2004;487:125–132. doi: 10.1016/j.ejphar.2004.01.031. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Pich EM, Miczek KA, Britton KT, Koob GF. Corticotropin-releasing factor antagonist reduces emotionality in socially defeated rats via direct neurotropic action. Brain Res. 1992;581:190–197. doi: 10.1016/0006-8993(92)90708-h. [DOI] [PubMed] [Google Scholar]
- Huang CH, Kuo MT, Lai WS. Characterization of behavioural responses in different test contexts after a single social defeat in male golden hamsters (Mesocricetus auratus) Behav processes. 2011;86:94–101. doi: 10.1016/j.beproc.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, Jasnow AM. Conditioned defeat in male and female Syrian hamsters. Horm Behav. 2003;44:293–299. doi: 10.1016/j.yhbeh.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Iio W, Tokutake Y, Matsukawa N, Tsukahara T, Chohnan S, Toyoda A. Anorexic behavior and elevation of hypothalamic malonyl-CoA in socially defeated rats. Biochem Biophys Res Commun. 2012;421:301–304. doi: 10.1016/j.bbrc.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Izumi T, Boku S, Shinmin W, Inoue T, Konno K, Yamaguchi T, Yoshida T, Matsumoto M, Watanabe M, Koyama T, Yoshioka M. Retrieval of conditioned fear activates the basolateral and intercalated nucleus of amygdala. J Neurosci Res. 2011;89:773–790. doi: 10.1002/jnr.22592. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Shi C, Israel JE, Davis M, Huhman KL. Memory of social defeat is facilitated by cAMP response element-binding protein overexpression in the amygdala. Behav Neurosci. 2005;119:1125–1130. doi: 10.1037/0735-7044.119.4.1125. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, Bailey MT, Sheridan JF, Padgett DA, Avitsur R. Repeated social defeat causes increased anxiety-like behavior and alters splenocyte function in C57BL/6 and CD-1 mice. Brain Behav Immun. 2007;21:458–466. doi: 10.1016/j.bbi.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusserow H, Davies B, Hortnagl H, Voigt I, Stroh T, Bert B, Deng DR, Fink H, Veh RW, Theuring F. Reduced anxiety-related behaviour in transgenic mice overexpressing serotonin 1A receptors. Brain Res Mol Brain Res. 2004;129:104–116. doi: 10.1016/j.molbrainres.2004.06.028. [DOI] [PubMed] [Google Scholar]
- Lai WS, Johnston RE. Individual recognition after fighting by golden hamsters: a new method. Physiol Behav. 2002;76:225–239. doi: 10.1016/s0031-9384(02)00721-7. [DOI] [PubMed] [Google Scholar]
- Lai WS, Ramiro LL, Yu HA, Johnston RE. Recognition of familiar individuals in golden hamsters: a new method and functional neuroanatomy. J Neurosci. 2005;25:11239–11247. doi: 10.1523/JNEUROSCI.2124-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Luo T, Jiang X, Wang J. Anxiolytic effects of 5-HT(1)A receptors and anxiogenic effects of 5-HT(2)C receptors in the amygdala of mice. Neuropharmacology. 2012;62:474–484. doi: 10.1016/j.neuropharm.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox SA, Schafe GE. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for reconsolidation of a Pavlovian fear memory. J Neurosci. 2011;31:7073–7082. doi: 10.1523/JNEUROSCI.1120-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann KE, Huhman KL. The effect of escapable versus inescapable social defeat on conditioned defeat and social recognition in Syrian hamsters. Physiol Behav. 2012;105:493–497. doi: 10.1016/j.physbeh.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerlo P, De Boer SF, Koolhaas JM, Daan S, Van den Hoofdakker RH. Changes in daily rhythms of body temperature and activity after a single social defeat in rats. Physiol Behav. 1996a;59:735–739. doi: 10.1016/0031-9384(95)02182-5. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Overkamp GJ, Benning MA, Koolhaas JM, Van den Hoofdakker RH. Long-term changes in open field behaviour following a single social defeat in rats can be reversed by sleep deprivation. Physiol Beh. 1996b;60:115–119. doi: 10.1016/0031-9384(95)02271-6. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Overkamp GJ, Daan S, Van Den Hoofdakker RH, Koolhaas JM. Changes in Behaviour and Body Weight Following a Single or Double Social Defeat in Rats. Stress. 1996c;1:21–32. doi: 10.3109/10253899609001093. [DOI] [PubMed] [Google Scholar]
- Messaoudi E, Kanhema T, Soule J, Tiron A, Dagyte G, da Silva B, Bramham CR. Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. J Neurosci. 2007;27:10445–10455. doi: 10.1523/JNEUROSCI.2883-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison KE, Cooper MA. A role for 5-HT1A receptors in the basolateral amygdala in the development of conditioned defeat in Syrian hamsters. Pharmacol Biochem Behav. 2012;100:592–600. doi: 10.1016/j.pbb.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrulis A, Weidner M, Johnston RE. Recognition of competitors by male golden hamsters. Physiol Behav. 2004;81:629–638. doi: 10.1016/j.physbeh.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Ploski JE, Pierre VJ, Smucny J, Park K, Monsey MS, Overeem KA, Schafe GE. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of pavlovian fear conditioning in the lateral amygdala. J Neurosci. 2008;28:12383–12395. doi: 10.1523/JNEUROSCI.1662-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J Neurosci. 1992;12:440–453. doi: 10.1523/JNEUROSCI.12-02-00440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potegal M, Huhman K, Moore T, Meyerhoff J. Conditioned defeat in the Syrian golden hamster (Mesocricetus auratus) Behav Neural Biol. 1993;60:93–102. doi: 10.1016/0163-1047(93)90159-f. [DOI] [PubMed] [Google Scholar]
- Raab A, Dantzer R, Michaud B, Mormede P, Taghzouti K, Simon H, Le Moal M. Behavioural, physiological and immunological consequences of social status and aggression in chronically coexisting resident-intruder dyads of male rats. Physiol Behav. 1986;36:223–228. doi: 10.1016/0031-9384(86)90007-7. [DOI] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad U S A. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, Ressler KJ. Brain-derived neurotrophic factor in amygdala-dependent learning. Neuroscientist. 2005;11:323–333. doi: 10.1177/1073858404272255. [DOI] [PubMed] [Google Scholar]
- Ricci LA, Rasakham K, Grimes JM, Melloni RH., Jr Serotonin-1A receptor activity and expression modulate adolescent anabolic/androgenic steroid-induced aggression in hamsters. Pharmacol Biochem Behav. 2006;85:1–11. doi: 10.1016/j.pbb.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE. Intra-amygdala blockade of the NR2B subunit of the NMDA receptor disrupts the acquisition but not the expression of fear conditioning. J Neurosci. 2001;21:6889–6896. doi: 10.1523/JNEUROSCI.21-17-06889.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozeske RR, Evans AK, Frank MG, Watkins LR, Lowry CA, Maier SF. Uncontrollable, but not controllable, stress desensitizes 5-HT1A receptors in the dorsal raphe nucleus. J Neurosci. 2011;31:14107–14115. doi: 10.1523/JNEUROSCI.3095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruis MA, te Brake JH, Buwalda B, De Boer SF, Meerlo P, Korte SM, Blokhuis HJ, Koolhaas JM. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology. 1999;24:285–300. doi: 10.1016/s0306-4530(98)00050-x. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Solomon MB, Karom MC, Huhman KL. Sex and estrous cycle differences in the display of conditioned defeat in Syrian hamsters. Horm Behav. 2007;52:211–219. doi: 10.1016/j.yhbeh.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Stiedl O, Misane I, Spiess J, Ogren SO. Involvement of the 5-HT1A receptors in classical fear conditioning in C57BL/6J mice. J Neurosci. 2000;20:8515–8527. doi: 10.1523/JNEUROSCI.20-22-08515.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauscher J, Bagby RM, Javanmard M, Christensen BK, Kasper S, Kapur S. Inverse relationship between serotonin 5-HT(1A) receptor binding and anxiety: a [(11)C]WAY-100635 PET investigation in healthy volunteers. Am J Psychiatry. 2001;158:1326–1328. doi: 10.1176/appi.ajp.158.8.1326. [DOI] [PubMed] [Google Scholar]
- Taylor SL, Stanek LM, Ressler KJ, Huhman KL. Differential brain-derived neurotrophic factor expression in limbic brain regions following social defeat or territorial aggression. Behav Neurosci. 2011;125:911–920. doi: 10.1037/a0026172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol Behav. 1993;53:983–993. doi: 10.1016/0031-9384(93)90278-n. [DOI] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Raunch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt MJ, Burke AR, Renner KJ, Forster GL. Adolescent male rats exposed to social defeat exhibit altered anxiety behavior and limbic monoamines as adults. Behav Neurosci. 2009;123:564–576. doi: 10.1037/a0015752. [DOI] [PMC free article] [PubMed] [Google Scholar]