Abstract
Background
Cereulide is a cyclic dodecadepsipeptide ionophore, produced via non-ribosomal peptide synthetases (NRPS), which in rare cases can lead to human death. Early studies had shown that emetic toxin formation belongs to a homogeneous group of Bacillus cereus sensu stricto and the genetic determinants of cereulide (a 24-kb gene cluster of cesHPTABCD) are located on a 270-kb plasmid related to the Bacillus anthracis virulence plasmid pXO1.
Results
The whole genome sequences from seven emetic isolates, including two B. cereus sensu stricto and five Bacillus weihenstephanensis strains, were compared, and their inside and adjacent DNA sequences of the cereulide biosynthesis gene clusters were analyzed. The sequence diversity was observed, which classified the seven emetic isolates into three clades. Different genomic locations of the cereulide biosynthesis gene clusters, plasmid-borne and chromosome-borne, were also found. Potential mobile genetic elements (MGEs) were identified in the flanking sequences of the ces gene cluster in all three types. The most striking observation was the identification of a putative composite transposon, Tnces, consisting of two copies of ISces element (belonging to IS6 family) in opposite orientations flanking the ces gene cluster in emetic B. weihenstephanensis. The mobility of this element was tested by replacing the ces gene cluster by a KmR gene marker and performing mating-out transposition assays in Escherichia coli. The results showed that Tnces::km transposes efficiently (1.04 × 10-3 T/R) and produces 8-bp direct repeat (DR) at the insertion sites.
Conclusions
Cereulide biosynthesis gene clusters display sequence diversity, different genomic locations and association with MGEs, in which the transposition capacity of a resistant derivative of the composite transposon Tnces in E. coli was demonstrated. Further study is needed to look for appropriate genetic tools to analysis the transposition of Tnces in Bacillus spp. and the dynamics of other MGEs flanking the ces gene clusters.
Keywords: Cereulide, Bacillus cereus, Bacillus weihenstephanensis, Transposon, Plasmid
Background
The Bacillus cereus group consists of B. cereus sensu stricto, Bacillus thuringiensis, Bacillus anthracis, Bacillus weihenstephanensis, Bacillus mycoides, Bacillus pseudomycoides and Bacillus cytotoxicus, which share close genetic and biochemical relatedness. They have traditionally been classified as different species based on their distinct virulence characteristics or phenotypes [1,2], the formers are mostly directly associated with large plasmids. B. anthracis causes the fatal animal and human disease anthrax, genetically determined by its pXO1 and pXO2 plasmids [3]. Similarly, the biopesticidal properties of B. thuringiensis, which distinguish it from B. cereus, are due to large plasmids encoding cry genes [4]. Ubiquitous in natural environment and best known as an opportunistic pathogen and food contaminant, B. cereus sensu stricto can cause two distinct forms of food poisoning with symptoms of diarrhea or vomiting. The diarrheal type, generally mild and mostly self-healed, is caused by several potential heat-labile enterotoxins, e.g. Hbl, Nhe, and CytK, whereas the emetic type, which represents the most serious food safety risk linked to B. cereus, is associated with a heat stable peptide toxin named cereulide. Most virulence genes of B. cereus are located on the chromosome [5,6] with the exception of the cereulide genetic determinants [7,8]. B. cytotoxicus is a recently described thermotolerant member of the B. cereus group [1]. The remaining members of the group, B. mycoides, B. pseudomycoides and B. weihenstephanensis, are mainly distinguished on the basis of their morphology (rhizoidal growth) and physiology (psychrotolerance), respectively [9,10], but may also have enteropathogenic potential [11,12]. In this respect, two B. weihenstephanensis isolates were found to produce a higher amount of cereulide than the reference B. cereus AH187 quantified by liquid chromatography mass spectrometry [13,14].
Cereulide ((D-O-Leu-D-Ala-L-O-Val-L-Val)3) is a small, heat and acid stable cyclic dodecadepsipeptide with a molecular weight of 1.2 kDa [15,16] and presents similar characteristics to valinomycin, i.e. chemical structure and toxicology [17,18]. Like valinomycin, cereulide is synthesized enzymatically via non-ribosomal peptide synthetases (NRPS), and is toxic to mitochondria by acting as a potassium ionophore [19]. It has been reported to inhibit human natural killer cells [20]. Indeed, severe and even lethal cases have been reported after the ingestion of food contaminated with high amounts of cereulide [21-24].
The cereulide genetic determinants correspond to a cluster of seven NRPS genes (cesA, B, C, D, H, P and T), which was originally found residing on a large plasmid [8]. This 270 kb element, pCER270, displays similarity to the anthrax virulence pXO1 from B. anthracis[7,25]. It is a member of pXO1-like plasmids, including pCER270, pPER272, pBC10987 and pBCXO1, which share a highly conserved core region containing genes involved in plasmid replication and its maintenance, sporulation and germination, and a formaldehyde-detoxification locus [25,26].
Previous studies have shown that enterotoxin production is broadly distributed among different members of the B. cereus group [6,27] and also found in other Bacillus spp. [28,29], whereas emetic toxin formation has been reported to be restricted to a homogeneous group of B. cereus sensu strict[30]. Although seldom, cereulide-producing B. weihenstephanensis strains have also recently been isolated [14]. In order to explore the phylogenetic relationship of the emetic isolates between B. cereus sensu stricto and B. weihenstephanensis, and to analyze the potential mode of genomic transfer of the cereulide genetic determinants, the genetic diversity between B. cereus sensu stricto and B. weihenstephanensis were analyzed in detail.
Results
Genome sequences comparison of emetic isolates
The comparison of 10 genome sequences including seven emetic (Table 1) and three non-emetic B. cereus group isolates was performed by Gegenees [31]. According to the heatmap (Figure 1A), the two emetic B. cereus sensu stricto isolates IS075 and AH187 show a similarity of more than 99%; and the five emetic B. weihenstephanensis isolates show similarities ranging from 86% to 100%, in which the similarity between MC67 and MC118, or between CER057, CER074 and BtB2-4, respectively, is 100%, whereas between MC67/MC118 and CER057/BtB2-4/CER074 is ca. 86%. Thus IS075 and AH187 share very similar gene content to form a clade in the phylogenetic tree, so do MC67 and MC118, and CER057 and CER074 and BtB2-4, respectively. CER057/BtB2-4/CER074 is more similar to B. weihenstephanensis KBAB4 than MC67/MC118, with similarities 94% vs. 86%.
Table 1.
Emetic strains used in this study
|
Strain |
Relevant characteristics |
Reference |
Genome accession no. in GenBank |
Contig containing
ces
gene cluster |
|
|---|---|---|---|---|---|
| Accession no. in GenBank | Length (bp) | ||||
| AH187 |
B. cereus, reference strain, containing pCER270 with the ces gene cluster |
(7) |
NC_010924 |
NC_010924 |
270,082 |
| IS075 |
B. cereus, isolated from mammal in Poland |
(13) |
AHCH01000000 |
AHCH02000031 |
180,702 |
| BtB2-4 |
B. weihenstephanensis, isolated from soil in Belgium |
(13) |
AHDR01000000 |
AHDR01000022 |
286,458 |
| CER057 |
B. weihenstephanensis, isolated from parsley in Belgium |
(13) |
AHDS01000000 |
AHDS01000024 |
245,438 |
| CER074 |
B. weihenstephanensis, isolated from milk in Belgium |
(13) |
AHDT01000000 |
AHDT01000022 |
288,640 |
| MC67 |
B. weihenstephanensis, isolated from soil in Denmark |
(14) |
AHEN01000000 |
AHEN01000048 |
56,684 |
| MC118 | B. weihenstephanensis, isolated from soil in Denmark | (14) | AHEM01000000 | AHEM01000066 | 26,595 |
Figure 1.
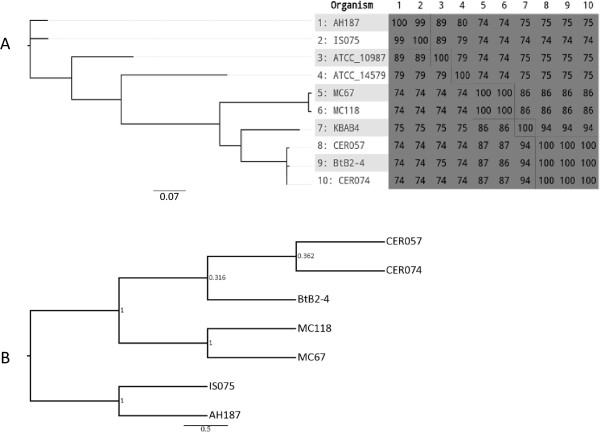
Phylogenetic analysis based on the sequences of genomes and ces genes of B. cereus group strains. (A) Phylogenetic overview in Gegenees of the genomes. The scale bar represents a 7% difference in average BLASTN score similarity. The heat-map is asymmetric because the variable contents of genomes differ in sizes and a similarity is calculated as a fraction of similar sequences in each genome. (B) Dendrogram based on the seven concatenated ces gene sequences by an NJ phylogenetic tree with a bootstrap of 1,000.
Sequence diversity of the ces gene cluster
All the emetic strains harbor the seven ces genes with the same sizes. The two "cereus" isolates, IS075 and AH187, only share three nucleotide variances for their cesB gene. For the five "weihenstephanensis" isolates, MC67 and MC118 from Denmark display only one synonymous mutation, in cesA and in cesT, respectively, and CER057, CER074 and BtB2-4 from Belgium are 100% identical. Each ces gene displays 90 ~ 95% identity between B. cereus and B. weihenstephanensis, and 95 ~ 100% identity within B. weihenstephanensis isolates. Similar but slightly lower identity levels were observed for the corresponding proteins. Thus, based on the concatenated ces genes and protein sequences, two main clusters, namely "cereus" and "weihenstephanensis", could be distinguished, and within "weihenstephanensis" cluster, two subsequent clades were identified (Figure 1B).
Genomic location of the ces gene clusters
IS075 harbors a larger plasmid pool than AH187. The cereulide gene cluster of IS075 was observed to be located on a large plasmid with a size similar to that of pCER270 (270 kb) in AH187 (Figure 2A). Like pCER270, IS075 was PCR-positive to the pXO1 backbone genes pXO1-11, pXO1-14, pXO1-45, pXO1-50 and pXO1-55, which all encode hypothetical proteins (data not shown). It was also observed that the IS075 contig containing the ces gene cluster is ca. 180.7 kb with 146 predicted CDSs, of which 85.6% matched to those of pCER270, with a good synteny (Figure 2B). This indicated that the emetic plasmid in IS075 is pXO1-like with high similarity to pCER270. The deduced proteins from 21 predicted CDSs not matching those of pCER270 were blasted with databases (Nr and Swissprot). The result showed that two matched putative transposases, one was related to putative DNA topoisomerases I, one to putative transcriptional repressors, and the others to hypothetical proteins, all with homologs in other B. cereus group plasmids.
Figure 2.
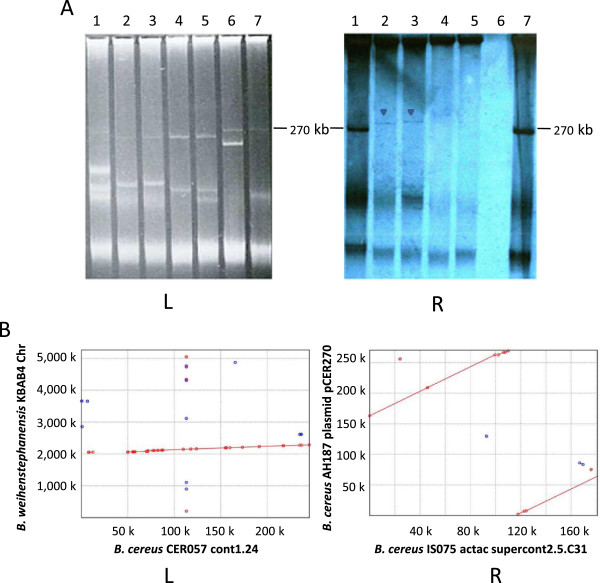
Genomic location of the cereulide gene cluster. (A) Genomic location of the cereulide gene cluster of emetic B. cereus group isolates determined by plasmid profiling (L) and hybridization (R). Lane 1: IS075, lane 2: MC118, lane 3: MC67, lane 4: CER057, lane 5: BtB2-4, lane 6: non cereulide-producing B. cereus isolate CER071, lane 7: AH187. The probe used was cesB internal fragment amplified with EmF and EmR primers from the reference strain AH187. pMC118 and pMC67, displaying a larger size than pCER270, are indicated by a dark triangle. (B) Linear arrangement of the contig containing the ces gene cluster of (L) CER057 with the chromosome of KBAB4 and (R) IS075 with the plasmid pCER270. Aligned segments are represented as dots (20 ~ 65 bp) and lines (>65 bp), with red and blue colors refer to forward and reverse matching substrings, respectively.
For BtB2-4 and CER057, although large plasmid with smaller size to pCER270 was observed in the profile, no hybridization signal was detected (Figure 2A). It was observed that the contig containing the ces gene cluster in CER057 is about 245.4 kb with 215 predicted CDSs, of which 80% and 85% matched those of the chromosomes of AH187 and KBAB4, respectively. Except for the ces genes, the deduced proteins of 25 predicted CDSs not matching the chromosome of KBAB4 were compared to protein databases (Nr and Swissprot). It was found that four CDSs encode putative transposase, acetyltransferase, phage integrase, and phosphoglycolate phosphatase, 17 encode hypothetical proteins with chromosomal homologs among B. cereus group strains and four had no hit. The linear alignment showed that the main matches were located in chromosome positions 2.15 M ~ 2.34 M for AH187, and 2.05 M ~ 2.28 M for KBAB4 (Figure 2B). Thus, it is most likely that the ces gene cluster in CER057 has a chromosomal location.
The hybridization bands of MC118 and MC67 are larger than that of pCER270, although the corresponding plasmid bands are rather weak (Figure 2A). This strongly suggests that the cereulide genetic determinants of both MC118 and MC67 (named pMC118 and pMC67) are located on plasmids larger than pCER270, which were PCR-negative to pXO1 backbone genes. Unfortunately, the contigs containing the ces gene clusters in MC67 and MC118 were very short, ca. 56.7 and 26.6 kb, respectively. Besides the seven ces genes, 30 putative CDSs were predicted in the larger contig of MC67, of which 9 had no hit, and the other 21 had homologs in the plasmids or chromosomes of other B. cereus group strains, including putative transposases, spore germination proteins, thiol-activated cytolysin, dehydratase and hypothetical proteins. However, although the gapped genome of MC67 was tentatively aligned with all the published plasmid sequences of the B. cereus group using the MAUVE contig aligner, no obvious colinear match was observed to large fragment (data not shown).
Identification of putative mobile genetic elements (MGEs) flanking the cereulide genetic determinants
About 5 kb DNA sequences upstream of cesH and downstream of cesD from the "ces" contigs were used for detailed analysis. In the case of MC67 and MC118, because the available flanking sequences were shorter they were obtained by primer walking.
Three types of flanking sequences could be observed (Figure 3). A potential group II intron, carrying an ncRNA and reverse endonuclease gene, is located 2.4 kb downstream of cesD in the plasmid of both AH187 and IS075, while an integrase/recombinase gene is located 1.1 kb downstream of cesD in chromosome of BtB2-4, CER057 and CER074. No other potential MGEs were observed in the flanking sequences of cesH of these strains. Strikingly, the ces gene cluster of pMC67 and pMC118 was found to be flanked by two copies of an IS element at each end, in opposite orientation (located ca. 2 kb from cesH and 800 bp from cesD), reminiscent of a typical class I composite transposon (designated Tnces). This IS element (named ISces) is 853 bp, contains a transposase gene and 16 bp terminal invert repeats (IR) and belongs to the IS6 family. In addition, an NERD domain or topoisomerase domains, belonging to DNA-breaking-rejoining enzyme superfamily, were also observed located between ISces and cesH and downstream of cesD and ISces on pMC67 and pMC118, respectively. Downstream of the Tnces, there is another transposase-encoding ORF showing high identity with the upstream ones, but with a shorter size. It is also flanked by the 16 bp IR (Figure 3).
Figure 3.
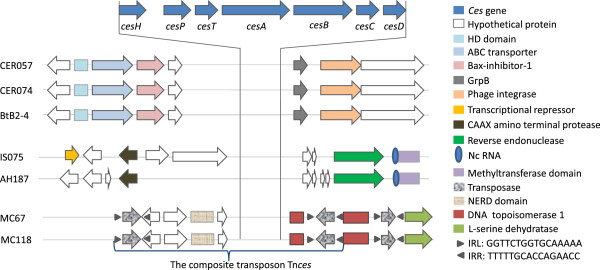
Physical map of the sequences flanking the emetic gene clusters. About 5 kb DNA sequences upstream of cesH and downstream of cesD were analyzed for CER057, CER074, BtB2-4, IS075 and F4810/75, respectively, and due to the available sequences are shorter, about 5 kb DNA sequences upstream of cesH and 2.2 kb downstream of cesD were analyzed for MC67 and MC118. The composite transposon Tnces in emetic B. weihenstephanensis MC67 and MC118 is indicated by black triangles. The Tnces consists of ces gene cluster flanked by two copies of IS element at each end in the opposite direction, containing a transposase gene and 16 bp invert repeats (IRL and IRR) at both ends. Sign and color codes are indicated on the right hand side. Physical map is not at scale.
Transposition of ISces-based composite transposon
In order to test the potential "transposability" of Tnces, the ces gene cluster was replaced by a KmR gene marker and a recombinant plasmid pTnkm was created and used for the transposition assay using a well-developed mating-out assay [32,33]. Conjugation between the donor strain E. coli JM109 (R388, pTnkm) and the recipient strain HB101 (SmR) was performed. The average transposition frequency of Tnces::km onto R388 in three independent experiments was estimated as 2.31 × 10-3 (number of KmRTpRSmR transconjugants per TpRSmR transconjugants). The final transfer frequency, which is equal to the actual transposition frequency multiplied by the conjugation frequency, was calculated as 1.04 × 10-3 KmRSmR transconjugants per SmR recipient. 60 transconjugants were randomly screened for Ampicilin resistance by disk diffusion assays and all displayed a positive result, indicating the formation of a cointegrate between the host chromosome and pTnkm.
In order to distinguish whether the KmRSmR transconjugants were achieved by transposition or other recombination events leading to plasmid integration, and whether the transposition happened randomly, a Southern-blot analysis was performed on nine transconjugants from two independent conjugation experiments that were randomly selected according to the resistance screening and the PCR validation. The hybridization was conducted on the transconjugants NdeI-digested genomic DNA using an internal bla fragment (pUC18), ISces and km as probes (Figure 4). Both hybridizations with the bla and km probes produced a single signal band, the former confirming the formation of a cointegrate of the whole pTnkm into the recipient chromosome. Using the ISces probes, besides the expected 1 and 3.1 kb bands observed in all the transconjugants, at least one extra band with variable sizes was observed in the nine tested transconjugants, indicating that independent multi-events had occurred at distinct genomic sites (Figure 5).
Figure 4.
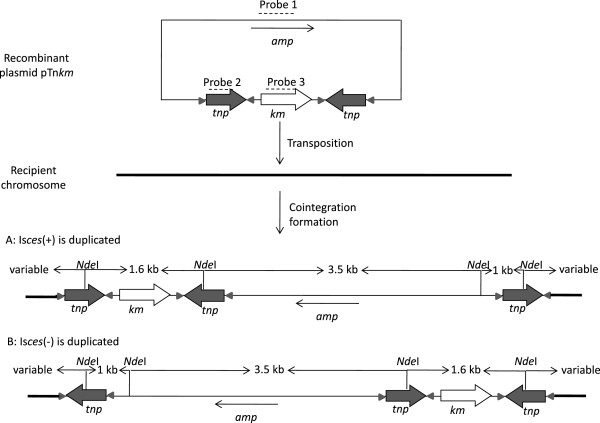
Sketch drawing of the replicative transposition of Tnces::km into recipient chromosome and the strategy of hybridization. The transposase-mediated fusion of pTnkm and the target molecules generate a third copy of ISces. There are two theoretically possible results of transposition, depending on which ISces is duplicated. Three probes 1, 2, and 3, indicated by dotted lines, represent an internal fragment of bla in cloning vector pUC18, ISces, and Km, respectively, were used for the survey of the transposition. The NdeI sites in kmRsmR transconjugants were indicated. No matter which ISces was duplicated, hybridization with probe 1 and 3, a 3.5 kb band and a 1.6 kb band is expected, respectively; with probe 2, besides the 1 kb and 3.5 kb expected bands, extra bands with variable sizes in each independent transconjugant are probably detected due to multi-transpositions. Although there is also a (remote) possibility for the duplication of the whole Tnces::km element, the result will be similar except that more bands with probe 2 are expected.
Figure 5.
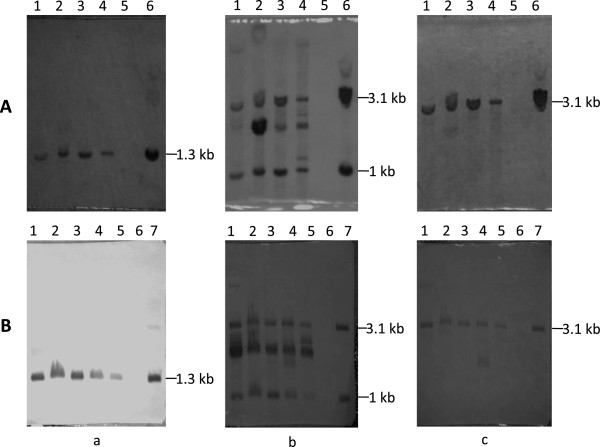
Southern blot hybridization analysis of the transconjugants of Tn ces ::km transposition in E. coli HB101. Two independent hybridizations were performed. A: lane 1–4, independent KmRSmR transconjugants, lane 5, HB101, lane 6, JM109 (pTnkm); and B: lane 1–5, independent KmRSmR transconjugants, lane 6, HB101, lane 7, JM109 (pTnkm). Three probes of Km (a), ISces (b) and blapuc18 (c), respectively were used for hybridization as illustrated in Figure 4.
To detect if the transposition of Tnces::Km displayed target site biases, the flanking sequences of insertion sites of the transconjugants used in hybridization were determined by primer walking. For three transconjugants, it was found that Tnces::Km insertions occurred in three distinct sites on plasmid R388 and that an 8-bp direct repeat (DR) was produced after transposition (Table 2), which is a typical feature of IS6 family members (see the ISfinder database, http://www-is.biotoul.fr) [34]. For the other six transconjugants, although repeated several times, it is difficult to get the flanking sequences of insertion sites by primer walking, probably due to sequence complexity caused by multiple transposition events of ISces.
Table 2.
DNA sequences flanking the insertion sites after random transposition of IS ces based transposon Tn ces :: km onto R388
| Transconjugants | Sequence of insertion sites (5’ to 3’) |
|---|---|
| Tn02 |
GCCAACTTCCAAAGGAAAGAAGCCGCATAACC-ISces-GCATAACCTGCCCTCCCCCGCTCCGGCGGGGG |
| Tn04 |
GAAGGCCAACGGTGGCGCCCAAGAAGGATTTC-ISces-AGGATTTCCGCGACACCGAGACCAATAGCGGAA |
| Tn05 | GAGCGGGCTTTTTTATCCCCGGAAGCCTGTGGA-ISces-CCTGTGGATAGAGGGTAGTTATCCACGTGAAAC |
The underlined sequences refer to the duplicated target sequences (DR).
Discussion
The taxonomy of B. cereus group has long been controversial, since many of the species are genetically heterogenous, with the exception of B. anthracis, which is essentially a clone in nature [35]. One of the reasons of this difficulty is that many toxins used for classification are encoded on MGEs that have HGT potential, e.g. plasmids or transposons [3,36,37]. Cereulide may cause severe and potential lethal infection during an "emetic" form of B. cereus food poisoning. Most emetic B. cereus strains belong to a homogeneous group of B. cereus sensu stricto. Although rare, the emetic B. weihenstephanensis strains were recently isolated in nature [13]. Furthermore, a heat stable toxin, structural related to cereulide, has also been found in Paenibacillus tundra strain [38]. As a consequence, the intra- and inter-species diversity and potential transmission of the cereulide biosynthetic gene cluster is therefore thought provoking.
In this study, the sequence diversity of emetic B. cereus sensu stricto and B. weihenstephanensis was analyzed. Since emetic B. cereus sensu stricto had been found to be restricted to a homogeneous group [30], only two B. cereus sensu stricto isolates were analyzed and compared the other five known B. weihenstephanensis. Except for AH187, the unfinished gapped genome sequences of the other emetic isolates were recently submitted [39]. As expected, the two emetic B. cereus sensu stricto isolates share very similar gene content in genome level. Furthermore, their "ces" plasmids are quite coherent in terms of synteny, protein similarity and gene content. Compared to AH187, IS075 has a larger plasmid pool, of which the "ces" plasmid is pXO1-like, but the presence of a pXO2-like plasmid was also indicated [40].
Sequence diversity between B. cereus sensu stricto and B. weihenstephanensis or within B. weihenstephanensis was observed. It was also evidenced that the ces cluster had undergone horizontal gene transfer (HGT). This could be clued by the fact that the cluster is present in different hosts (B. cereus sensu stricto vs. B. weihenstephanensis), which have different chromosomal background, and displays different genomic locations (plasmids vs. chromosome). Moreover, another striking indication for HGT was the presence of putative MGEs in all tested emetic strains.
The composite transposon, Tnces, located on large plasmids (pMC67/pMC118) in two B. weihenstephanensis strains isolated from soil in Denmark was identified. The mobility of Tnces was also proved by transposition experiments performed on a Tnces-derived element, indicating a HGT potential of the cereulide gene cluster in pMC67/pMC118. Although the ces gene cluster is not flanked by IS elements in the other two types of emetic isolates, a Group II intron carrying an endonuclease gene in AH187 and IS075, and a putative integrase/recombinase gene in CER057, CER074 and BtB2-4 were also observed downstream of cesD. Both Group II intron and recombinase can potentially be involved in genome dynamics. Group II introns are self-splicing mobile retroelements, some of which have been shown experimentally to be able to invade new DNA sites and transfer between species, sometimes accompanied by adjacent sequence deletion or rearrangement [41-43]. This also relates to previous observations that bacterial group II introns tend to be located within mobile DNA elements such as plasmids, IS elements, transposons or pathogenicity islands (PAI), which could account for their spread among bacteria [44-46].
Based on our results, it is reasonable to suggest that MGEs have played a key role in the transmission of the cereulide gene cluster. In many cases, plasmids encode passenger genes originated via HGT that generally confer adaptive functions to the host cell, the classic example being antibiotic resistance genes. For instance, the NRPS gene cluster responsible for the production of β-lactam antibiotics (e.g. penicillins and cephalosporins) was proved to be transmitted by HGT from bacteria to bacteria and from bacteria to fungi [47,48]. This is also the general mode for toxin evolution [49,50]. In contrast, as a natural analog, a recent study reported that a vertical transmission (VT) origin rather than a HGT for the vlm gene cluster in Streptomyces spp. Although there is a significant structure and toxicology similarity between valinomycin and cereulide and an organizational similarity between the vlm gene cluster and the ces gene cluster, they are highly divergent from each other at the DNA level [51]. They may also have quite different evolution history.
The conjugative and transfer promoting capacities of the emetic plasmids were also assessed by bi- and tri-parental matings, respectively. None were indicative of self-conjugative or mobilizable activities, at least under the conditions used in the assay (detection limit of 10-7 T/R) (data not shown). Yet, the emetic strains can host the conjugative plasmid pXO16, which could be transferred from its native B. thuringiensis sv. israelensis to the emetic strains and, subsequently from the emetic strains to the original B. thuringiensis sv. israelensis host [52].
An important concern arising from this study is that the cereulide gene cluster may have the potential to be transmitted by transposition and, therefore, if the emetic strain can randomly encounter the conjugative plasmid pXO16 in nature, transposition of the cereulide gene cluster into pXO16 might happen at a low frequency, and as a consequence the resulting emetic pXO16, crossing boundaries within the B. cereus group by conjugation, could pose a serious public health issue.
Conclusion
Emetic B. cereus group isolates display more variations than originally thought. The cereulide biosynthesis gene cluster was present in different hosts (B. cereus sensu stricto and B. weihenstephanensis), which have different chromosomal background and display different genomic locations (plasmids vs. chromosome). The sequences of cereulide genetic determinants are diverse and coevolved with the host. Three types of MGEs were identified in the flanking sequences of the cereulide biosynthesis gene cluster, of which the transposition capacity of a resistant derivative of the composite transposon Tnces in E. coli was demonstrated. Further study is needed to look for appropriate genetic tools to analysis the transposition of Tnces in Bacillus spp. and the dynamics of other MGEs flanking the ces gene clusters.
Methods
Strains and plasmids
Emetic strains used in this study are listed in Table 1. A non cereulide-producing B. cereus isolate CER071 was used as negative control. E. coli DH5α and JM109 were used as bacterial hosts in electroporation experiments. Plasmid R388 (Trimethoprim resistant) [53], a conjugative plasmid devoid of transposon, was used for transposition assay. E. coli was routinely cultivated at 37°C in Luria-Bertani (LB) media. B. cereus group strains were grown at 30°C. Antibiotics were used at the following concentrations: Kanamycin (Km), 50 μg/ml; Ampicilin (Amp), 50 μg/ml and Trimethoprim (Tp), 50 μg/ml.
Insertion site determination of the cereulide gene cluster and Tnces::Km
Regions flanking the cereulide gene cluster sites of the emetic B. cereus isolates and the target site and flanking sequences of the composite transposon were obtained by the method of genome walking (Takara genome walking kit), using the primer walking sets listed in Table 3. All the sequences obtained by this method were validated by PCR and subsequent sequencing.
Table 3.
Primers used in this study
| Primers | Target | Sequences (5’ → 3’) |
|---|---|---|
| EmF |
cesB |
GACAAGAGAAATTTCTACGAGCAAGTACAAT |
| EmR |
|
GCAGCCTTCCAATTACTCCTTCTGCCACAGT |
| 14 F |
pXO1-14 |
GGTAAAGAGTGCGGAAAATGA |
| 14R |
|
AATACGCCAACGCCAACTTA |
| 45 F |
pXO1-45 |
TGCAGCTCGTAATCCACAG |
| 45R |
|
TGCTAATGATAAAACGCCTGG |
| 50 F |
pXO1-50 |
TTCGTACAGATGAAACACAGG |
| 50R |
|
GTGCCTCAAGATGAACCTTC |
| 55 F |
pXO1-55 |
GATAGAGACTGCTCTTGGGAA |
| 55R |
|
GGTCTTAGCCATGAGAGTAAAAACA |
| 58 F |
pXO1-58 |
TGTGATGGACCTTTGTATTAATTTGT |
| 58R |
|
ATACCCCGCATGGAGCTTAG |
| ISF_SacI |
ISces |
GCAGAGCTCGGTTCTGGTGCAAAAACTTCAGGACA |
| ISR_XbaI |
|
GCATCTAGAGGTTCTGGTGCAAAAAGATAATAAAG |
| ISF_HindIII |
ISces |
GCAAAGCTTGGTTCTGGTGCAAAAACTTCAGGACA |
| ISR_BamHI |
|
GCAGGATCCGGTTCTGGTGCAAAAAGATAATAAAG |
| KmF_XbaI |
Km |
TCATCTAGATAAACCCAGCGAACCATTTG |
| KmR_BamHI |
|
TCAGGATCCTCTAGGTACTAAAACAATTCATCCAG |
| ISF3 |
ISces |
TCTGGTGCAAAAACTTCAGG |
| ISR3 |
|
AAGTCGCATACGACCAGGTA |
| kmF3 |
Km |
GAGGATGAGGAGGCAGATTG |
| KmR3 |
|
CGGCCAGATCGTTATTCAGT |
| APF1 |
bla |
TTTGCCTTCCTGTTTTTGCT |
| APR1 |
|
TTGCCGGGAAGCTAGAGTAA |
| ISL-SP1 |
CTTCATCCTCTTCGTCTTGGTAGC |
|
| ISL-SP2 |
GGTTCGCTGGGTTTATCTAGAGGT |
|
| ISL-SP3 |
GACAGACTGGTCCCGTAAATCAAC |
|
| ISR-SP1 |
ATATCGGGGAAGAACAGTATGTCG |
|
| ISR-SP2 |
GTACCTAGAGGATCCGGTTCTGGT |
|
| ISR-SP3 |
GACAGACTGGTCCCGTAAATCAAC |
|
| IS-LR |
CTTTCGAATCAACAGCACGA |
|
| CesD-SP1 |
GGCCTATTGTATAATGACAACG |
|
| CesD-SP2 |
GGTGTATTATTTATCTTCGCCTG |
|
| CesD-SP3 |
GGTATTTTAGGGGCGAAGGTTC |
|
| MH-SP1 |
CACTCTTGCGTTTTTGCGTATC |
|
| MH-SP2 |
AAACAATGAGCCCACCCCGAAA |
|
| MH-SP3 | CGCTTTTCCACATTCTTTACGG | |
DNA manipulation and plasmid construction
Plasmid and genomic DNA were isolated using Plasmid Mini-Midi kits and Bacterial genome extraction kit (QIAGEN), respectively. Primers (Table 3) were designed to amplify and sequence ces gene fragments based on pCER270 sequence [GenBank: NC_010924]. Standard PCR amplification experiments were performed with primers listed in Table 3. In order to evaluate the possible transposition capacity of the composite transposon containing the cereulide gene cluster of MC118, a composite transposon Tnces::Km was constructed by the replacement of the cereulide gene cluster with the KmR marker as follows. A 1.3 kb fragment containing the KmR gene was amplified with the primer pair KmF_XbaI/KmR_BamHI. Two 853 bp ISces elements (see below) containing a transposase gene, flanked by the left- and right IR, were amplified with the primer pairs ISF_ SacI/ ISR_XbaI and ISF_ HindIII/ ISR_BamHI. Products were digested with the appropriate enzymes, and mixed in a four-way ligation with BamHI-XbaI-cleaved KmR fragment, and SacI-HindIII-cleaved pUC18 vector, pTnKm was created to carry Tnces::km with two copies of ISces element in opposite orientations flanking the KmR marker. The electroporation of recombinant plasmid into E. coli DH5a and JM109 was as described by Sambrook and coll. [54].
Plasmid profiling and hybridization
Plasmid profiling of the emetic isolates was performed according to Andrup et al. [55]. Genomic DNA from E. coli strains HB101, JM109 (pTnKm), JM109 (R388, pTnKm) and transconjugants were digested with NdeI and run in a 0.8% agarose gel electrophoresis before the separated DNA fragments were transferred from agarose gels to a positively charged nylon membrane (Boehringer Mannheim, Germany). DIG-labeled probes were designed by using the "PCR DIG Probe Synthesis Kit" from Roche. Probe Pces, consisting of an internal fragment of cesB using EmF and EmR primers, was used for the location of cereulide gene cluster. Probes 1, 2, and 3, which consisted of an internal fragment of bla pUC18 using APF1 and APR1 primers, an internal fragment of IS using ISF3 and ISR3 primers, and an internal fragment of km using kmF3 and KmR3 primers, were used for transposition survey. After transfer and fixation of the DNA on the membrane, the hybridization was performed with the "DIG High Prime DNA Labeling and Detection Starter Kit I" (Roche Diagnostic, Mannheim, Germany), according to the manufacturer’s instructions.
Transposition experiments
The transposition of the pTnKm was examined using a mating-out experiment, as previously described [32,33]. For this purpose, E. coli JM109 harboring pTnKm and plasmid R388 (TpR) was used as the donor to mate with E. coli HB101 (SmR) on a membrane filter. The transposition frequency was expressed as the number of KmRSmR transconjugants per SmR recipients (T/R) and the plasmids in the transconjugants were further characterized by PCR and restriction digestion.
Sequence analysis
The complete genome sequence of AH187 and the gapped genome sequences of the other six emetic strains were obtained from NCBI (Table 1). A fragmented all-against-all comparison analysis was performed using Gegenees (version 1.1.5) software [31] by fragmenting genomes and comparing all pieces with all emetic genomes and B. cereus ATCC 10987 [GenBank: NC_ 003909], ATCC 14579 [GenBank: NC_ 004722] and B. weihenstephanensis KBAB4 [GenBank: NC_010184]. The heat-plot is based on a fragmented alignment using BLASTN made with settings 200/100. The cutoff threshold for non-conserved material was 30%. Based on this all-against-all approach, a corresponding phylogenetic dataset can be extracted and then a tree was constructed using neighbor joining method by splitstree4 (version 4.12.8) with this dataset.
Each ces gene and the concatenated sequences, as well as the deduced amino acid sequences, were aligned by MEGA version 5.2 software. A neighbor-joining (NJ) phylogenetic tree based on the concatenated gene sequences was constructed with a bootstrap of 1,000.
The contigs containing the ces gene cluster were compared with the genomes of AH187 and B. weihenstephanensis KBAB4 by BLASTN with an e-value cutoff of 1e-5. Linear alignment was finished by MUMmer software package (release 3.23) [56]. The sequences upstream of cesH and downstream of cesD were obtained from the complete genome sequence of AH187 and the contigs with the ces gene cluster located within the gapped genome sequences of the emetic strains (NCBI - Table 1), except that MC67 and MC118 by primer walking [GenBank: KF554002, KF554003, KF554006, KF554007].
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
XM carried out the mating-out and transposition experiments, and wrote the paper; KX performed the bioinformatics analysis; LY carried out primer walking and partial sequencing; ZY revised the paper; XH designed the study, constructed the recombinant plasmid, analyzed the data and wrote the paper; JM designed the study, analyzed data and revised the paper. All authors read and approved the final manuscript.
Contributor Information
Xiaofen Mei, Email: wanfangcongdan@163.com.
Kai Xu, Email: xukai915@qq.com.
Lingling Yang, Email: 393761856@qq.com.
Zhiming Yuan, Email: yzm@wh.iov.cn.
Jacques Mahillon, Email: jacques.mahillon@uclouvain.be.
Xiaomin Hu, Email: huxm@wh.iov.cn.
Acknowledgement
We are grateful to Professor Ningyi Zhou for kindly providing us with plasmid R388. We also like to gratefully acknowledge Mrs. Annika Gillis for her careful reading of the manuscript and her helpful comments. This work was supported by an NFSC grant 31170006.
References
- Guinebretière M-H, Auger S, Galleron N, Contzen M, De Sarrau B, De Buyser M-L, Lamberet G, Fagerlund A, Granum PE, Lereclus D. Bacillus cytotoxicus sp. nov. is a novel thermotolerant species of the Bacillus cereus group occasionally associated with food poisoning. Int J Syst Evol Microbiol. 2013;63(Pt 1):31–40. doi: 10.1099/ijs.0.030627-0. [DOI] [PubMed] [Google Scholar]
- Helgason E, Tourasse NJ, Meisal R, Caugant DA, Kolstø A-B. Multilocus sequence typing scheme for bacteria of the Bacillus cereus group. Appl Environ Microbiol. 2004;70(1):191–201. doi: 10.1128/AEM.70.1.191-201.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okinaka RT, Cloud K, Hampton O, Hoffmaster AR, Hill KK, Keim P, Koehler TM, Lamke G, Kumano S, Mahillon J, Manter D, Martinez Y, Ricke D, Svensson R, Jackson PJ. Sequence and organization of pXO1, the large Bacillus anthracis plasmid harboring the anthrax toxin genes. J Bacteriol. 1999;181(20):6509–6515. doi: 10.1128/jb.181.20.6509-6515.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum JA, Chu CR, Rupar M, Brown GR, Donovan WP, Huesing JE, Ilagan O, Malvar TM, Pleau M, Walters M, Vaughn T. Binary toxins from Bacillus thuringiensis active against the western corn rootworm, Diabrotica virgifera virgifera LeConte. Appl Environ Microbiol. 2004;70(8):4889–4898. doi: 10.1128/AEM.70.8.4889-4898.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova N, Sorokin A, Anderson I, Galleron N, Candelon B, Kapatral V, Bhattacharyya A, Reznik G, Mikhailova N, Lapidus A. Genome sequence of Bacillus cereus and comparative analysis with Bacillus anthracis. Nature. 2003;423(6935):87–91. doi: 10.1038/nature01582. [DOI] [PubMed] [Google Scholar]
- Prüss BM, Dietrich R, Nibler B, Märtlbauer E, Scherer S. The hemolytic enterotoxin HBL is broadly distributed among species of the Bacillus cereus group. Appl Environ Microbiol. 1999;65(12):5436–5442. doi: 10.1128/aem.65.12.5436-5442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehling-Schulz M, Fricker M, Grallert H, Rieck P, Wagner M, Scherer S. Cereulide synthetase gene cluster from emetic Bacillus cereus: Structure and location on a mega virulence plasmid related to Bacillus anthracis toxin plasmid pXO1. BMC Microbiol. 2006;6 doi: 10.1186/1471-2180-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoton FM, Andrup L, Swiecicka I, Mahillon J. The cereulide genetic determinants of emetic Bacillus cereus are plasmid-borne. Microbiology. 2005;151:2121–2124. doi: 10.1099/mic.0.28069-0. [DOI] [PubMed] [Google Scholar]
- Lechner S, Mayr R, Francis KP, Prüß BM, Kaplan T, Wießner-Gunkel E, Stewartz G, Scherer S. Bacillus weihenstephanensis sp. nov. is a new psychrotolerant species of the Bacillus cereus group. Int J Syst Bacteriol. 1998;48:1373–1382. doi: 10.1099/00207713-48-4-1373. [DOI] [PubMed] [Google Scholar]
- Nakamura LK. Bacillus pseudomycoides sp. nov. Int J Syst Bacteriol. 1998;48:1031–1035. doi: 10.1099/00207713-48-3-1031. [DOI] [PubMed] [Google Scholar]
- Stenfors LP, Mayr R, Scherer S, Granum PE. Pathogenic potential of fifty Bacillus weihenstephanensis strains. FEMS Microbiol Lett. 2002;215(1):47–51. doi: 10.1111/j.1574-6968.2002.tb11368.x. [DOI] [PubMed] [Google Scholar]
- Swiecicka I, Van der Auwera GA, Mahillon J. Hemolytic and nonhemolytic enterotoxin genes are broadly distributed among Bacillus thuringiensis isolated from wild mammals. Microb Ecol. 2006;52(3):544–551. doi: 10.1007/s00248-006-9122-0. [DOI] [PubMed] [Google Scholar]
- Hoton FM, Fornelos N, N'Guessan E, Hu XM, Swiecicka I, Dierick K, Jaaskelainen E, Salkinoja-Salonen M, Mahillon J. Family portrait of Bacillus cereus and Bacillus weihenstephanensis cereulide-producing strains. Environ Microbiol Rep. 2009;1(3):177–183. doi: 10.1111/j.1758-2229.2009.00028.x. [DOI] [PubMed] [Google Scholar]
- Thorsen L, Hansen BM, Nielsen KF, Hendriksen NB, Phipps RK, Budde BB. Characterization of emetic Bacillus weihenstephanensis, a new cereulide-producing bacterium. Appl Environ Microbiol. 2006;72(7):5118–5121. doi: 10.1128/AEM.00170-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agata N, Ohta M, Mori M, Isobe M. A novel dodecadepsipeptide, cereulide, is an emetic toxin of Bacillus cereus. FEMS Microbiol Lett. 1995;129(1):17–19. doi: 10.1016/0378-1097(95)00119-P. [DOI] [PubMed] [Google Scholar]
- Mikkola R, Saris NEL, Grigoriev PA, Andersson MA, Salkinoja-Salonen MS. Ionophoretic properties and mitochondrial effects of cereulide - the emetic toxin of B. cereus. Eur J Biochem. 1999;263(1):112–117. doi: 10.1046/j.1432-1327.1999.00476.x. [DOI] [PubMed] [Google Scholar]
- Agata N, Mori M, Ohta M, Suwan S, Ohtani I, Isobe M. A novel dodecadepsipeptide, cereulide, isolated from Bacillus cereus causes vacuole formation in HEp-2 cells. FEMS Microbiol Lett. 1994;121(1):31–34. doi: 10.1111/j.1574-6968.1994.tb07071.x. [DOI] [PubMed] [Google Scholar]
- Ladeuze S, Lentz N, Delbrassinne L, Hu X, Mahillon J. Antifungal activity displayed by cereulide, the emetic toxin produced by Bacillus cereus. Appl Environ Microbiol. 2011;77(7):2555–2558. doi: 10.1128/AEM.02519-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarvey NA, Ehling-Schulz M, Walsh CT. Characterization of the cereulide NRPS alpha-hydroxy acid specifying modules: Activation of alpha-keto acids and chiral reduction on the assembly line. J Am Chem Soc. 2006;128(33):10698–10699. doi: 10.1021/ja0640187. [DOI] [PubMed] [Google Scholar]
- Paananen A, Mikkola R, Sareneva T, Matikainen S, Hess M, Andersson M, Julkunen I, Salkinoja-Salonen MS, Timonen T. Inhibition of human natural killer cell activity by cereulide, an emetic toxin from Bacillus cereus. Clin Exp Immunol. 2002;129(3):420–428. doi: 10.1046/j.1365-2249.2002.01898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierick K, Van Coillie E, Swiecicka I, Meyfroidt G, Devlieger H, Meulemans A, Hoedemaekers G, Fourie L, Heyndrickx M, Mahillon J. Fatal family outbreak of Bacillus cereus-associated food poisoning. J Clin Microbiol. 2005;43(8):4277–4279. doi: 10.1128/JCM.43.8.4277-4279.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler H, Pasi A, Kramer JM, Schulte P, Scoging AC, Bär W, Krähenbühl S. Fulminant liver failure in association with the emetic toxin of Bacillus cereus. N Engl J Med. 1997;336(16):1142–1148. doi: 10.1056/NEJM199704173361604. [DOI] [PubMed] [Google Scholar]
- Naranjo M, Denayer S, Botteldoorn N, Delbrassinne L, Veys J, Waegenaere J, Sirtaine N, Driesen RB, Sipido KR, Mahillon J, Dierick K. Sudden death of a young adult associated with Bacillus cereus food poisoning. J Clin Microbiol. 2011;49(12):4379–4381. doi: 10.1128/JCM.05129-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pósfay-Barbe KM, Schrenzel J, Frey J, Studer R, Kroff C, Belli DC, Parvex P, Rimensberger PC, Schäppi MG. Food poisoning as a cause of acute liver failure. Pediatr Infect Dis J. 2008;27(9):846–847. doi: 10.1097/INF.0b013e318170f2ae. [DOI] [PubMed] [Google Scholar]
- Rasko DA, Rosovitz MJ, Økstad OA, Fouts DE, Jiang LX, Cer RZ, Kolstø A-B, Gill SR, Ravel J. Complete sequence analysis of novel plasmids from emetic and periodontal Bacillus cereus isolates reveals a common evolutionary history among the B. cereus group plasmids, including Bacillus anthracis pXO1. J Bacteriol. 2007;189(1):52–64. doi: 10.1128/JB.01313-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XM, Swiecicka I, Timmery S, Mahillon J. Sympatric soil communities of Bacillus cereus sensu lato: population structure and potential plasmid dynamics of pXO1-and pXO2-like elements. FEMS Microbiol Ecol. 2009;70(3):344–355. doi: 10.1111/j.1574-6941.2009.00771.x. [DOI] [PubMed] [Google Scholar]
- Hansen BM, Hendriksen NB. Detection of enterotoxic Bacillus cereus and Bacillus thuringiensis strains by PCR analysis. Appl Environ Microbiol. 2001;67(1):185–189. doi: 10.1128/AEM.67.1.185-189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan NJ, Caldow G, Gemmell CG, Hunter IS. Production of diarrheal enterotoxins and other potential virulence factors by veterinary isolates of Bacillus species associated with nongastrointestinal infections. Appl Environ Microbiol. 2003;69(4):2372–2376. doi: 10.1128/AEM.69.4.2372-2376.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan NJ, Deans K, Anderson JG, Gemmell CG, Hunter IS, Chaithong T. Putative virulence factor expression by clinical and food isolates of Bacillus spp. after growth in reconstituted infant milk formulae. Appl Environ Microbiol. 2001;67(9):3873–3881. doi: 10.1128/AEM.67.9.3873-3881.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehling-Schulz M, Svensson B, Guinebretiere MH, Lindbäck T, Andersson M, Schulz A, Fricker M, Christiansson A, Granum PE, Märtlbauer E, Nguyen-The C, Salkinoja-Salonen M, Scherer S. Emetic toxin formation of Bacillus cereus is restricted to a single evolutionary lineage of closely related strains. Microbiology. 2005;151:183–197. doi: 10.1099/mic.0.27607-0. [DOI] [PubMed] [Google Scholar]
- Ågren J, Sundström A, Håfström T, Segerman B. Gegenees: fragmented alignment of multiple genomes for determining phylogenetic distances and genetic signatures unique for specified target groups. PLoS One. 2012;7(6):e39107. doi: 10.1371/journal.pone.0039107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sota M, Endo M, Nitta K, Kawasaki H, Tsuda M. Characterization of a class II defective transposon carrying two haloacetate dehalogenase genes from Delftia acidovorans plasmid pUO1. Appl Environ Microbiol. 2002;68(5):2307–2315. doi: 10.1128/AEM.68.5.2307-2315.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Iino T. Genetic-analysis of a transposon carrying toluene degrading genes on a TOL plasmid pWWO. Mol Gen Genet. 1987;210(2):270–276. doi: 10.1007/BF00325693. [DOI] [PubMed] [Google Scholar]
- Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006;34(Database issue):D32–D36. doi: 10.1093/nar/gkj014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didelot X, Barker M, Falush D, Priest FG. Evolution of pathogenicity in the Bacillus cereus group. Syst Appl Microbiol. 2009;32(2):81–90. doi: 10.1016/j.syapm.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Hu X, Hansen BM, Yuan Z, Johansen JE, Eilenberg J, Hendriksen NB, Smidt L, Jensen GB. Transfer and expression of the mosquitocidal plasmid pBtoxis in Bacillus cereus group strains. FEMS Microbiol Lett. 2005;245(2):239–247. doi: 10.1016/j.femsle.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Zheng D, Hu X, Cai Q, Yuan Z. Conjugative transfer of insecticidal plasmid pHT73 from Bacillus thuringiensis to B. anthracis and compatibility of this plasmid with pXO1 and pXO2. Appl Environ Microbiol. 2010;76(2):468–473. doi: 10.1128/AEM.01984-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasimus S, Mikkola R, Andersson MA, Teplova VV, Venediktova N, Ek-Kommonen C, Salkinoja-Salonen M. Psychrotolerant Paenibacillus tundrae isolates from barley grains produce new cereulide-like depsipeptides (paenilide and homopaenilide) that are highly toxic to mammalian cells. Appl Environ Microbiol. 2012;78(10):3732–3743. doi: 10.1128/AEM.00049-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Auwera GA, Feldgarden M, Kolter R, Mahillon J. Whole-genome sequences of 94 environmental isolates of Bacillus cereus sensu lato. Genome Announc. 2013;1(5) doi: 10.1128/genomeA.00380-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XM, Van der Auwera G, Timmery S, Zhu L, Mahillon J. Distribution, diversity, and potential mobility of extrachromosomal elements related to the Bacillus anthracis pXO1 and pXO2 virulence plasmids. Appl Environ Microbiol. 2009;75(10):3016–3028. doi: 10.1128/AEM.02709-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickbush TH. Mobile introns: Retrohoming by complete reverse splicing. Curr Biol. 1999;9(1):R11–R14. doi: 10.1016/S0960-9822(99)80034-7. [DOI] [PubMed] [Google Scholar]
- Ferat JL, Michel F. Group II self-splicing introns in bacteria. Nature. 1993;364(6435):358–361. doi: 10.1038/364358a0. [DOI] [PubMed] [Google Scholar]
- Jia KZ, Zhu Y, Zhang YP, Li Y. Group II intron-anchored gene deletion in Clostridium. PLoS One. 2011;6(1) doi: 10.1371/journal.pone.0016693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belhocine K, Yam KK, Cousineau B. Conjugative transfer of the Lactococcus lactis chromosomal sex factor promotes dissemination of the Ll.LtrB group II intron. J Bacteriol. 2005;187(3):930–939. doi: 10.1128/JB.187.3.930-939.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai LX, Zimmerly S. Compilation and analysis of group II intron insertions in bacterial genomes: evidence for retroelement behavior. Nucleic Acids Res. 2002;30(5):1091–1102. doi: 10.1093/nar/30.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JR, Dunny GM. Bacterial group II introns and their association with mobile genetic elements. Front Biosci. 2002;7:D1843–D1856. doi: 10.2741/klein1. [DOI] [PubMed] [Google Scholar]
- Brakhage AA, Al-Abdallah Q, Tüncher A, Spröte P. Evolution of beta-lactam biosynthesis genes and recruitment of trans-acting factors. Phytochemistry. 2005;66(11):1200–1210. doi: 10.1016/j.phytochem.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Liras P, Rodríguez-García A, Martín J. Evolution of the clusters of genes for beta-lactam antibiotics: a model for evolutive combinatorial assembly of new beta-lactams. Int Microbiol. 1998;1(4):271–278. [PubMed] [Google Scholar]
- Hacker J, Hochhut B, Middendorf B, Schneider G, Buchrieser C, Gottschalk G, Dobrindt U. Pathogenomics of mobile genetic elements of toxigenic bacteria. Int J Med Microbiol. 2004;293(7–8):453–461. doi: 10.1078/1438-4221-00290. [DOI] [PubMed] [Google Scholar]
- Martínez JL. Bacterial pathogens: from natural ecosystems to human hosts. Environ Microbiol. 2013;15(2):325–333. doi: 10.1111/j.1462-2920.2012.02837.x. [DOI] [PubMed] [Google Scholar]
- Matter AM, Hoot SB, Anderson PD, Neves SS, Cheng YQ. Valinomycin biosynthetic gene cluster in Streptomyces: conservation, ecology and evolution. Plos One. 2009;4(9) doi: 10.1371/journal.pone.0007194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Auwera GA, Timmery S, Hoton F, Mahillon J. Plasmid exchanges among members of the Bacillus cereus group in foodstuffs. Int J Food Microbiol. 2007;113(2):164–172. doi: 10.1016/j.ijfoodmicro.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Ward JM, Grinsted J. Physical and genetic analysis of the Inc-W group plasmids R388, Sa, and R7K. Plasmid. 1982;7(3):239–250. doi: 10.1016/0147-619X(82)90005-1. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch E, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Andrup L, Barfod KK, Jensen GB, Smidt L. Detection of large plasmids from the Bacillus cereus group. Plasmid. 2008;59(2):139–143. doi: 10.1016/j.plasmid.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, Salzberg SL. Versatile and open software for comparing large genomes. Genome Biol. 2004;5(2) doi: 10.1186/gb-2004-5-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]


