Abstract
Introduction
Intravenous lipid emulsion (ILE) resuscitation is now frequently being used for severe overdoses due to lipophilic drugs. However, the optimal dose, duration, and safety are still unclear.
Case Report
A patient with refractory cardiovascular collapse following an amitriptyline overdose was treated with ILE with initial improvement. Instability recurred after ILE discontinuation and lipid therapy was restarted, but high-dose treatment was complicated by severe lipemia. A low-dose infusion was instead used, and the patient did not experience further toxicity despite amitriptyline levels in the toxic range for 21 days. He survived to discharge without long-term sequelae.
Discussion
A low-dose infusion of ILE was well tolerated and may have successfully prevented recurrent toxicity in a case of severe tricyclic antidepressant overdose.
Keywords: Poisoning, Antidepressive agents, Tricyclic, Fat emulsions, Intravenous, Antidotes
Introduction
Intravenous lipid emulsion (ILE) is emerging as a promising therapy for severe toxicity from lipophilic drugs. While there are now reports of its use in verapamil, metoprolol, and tricyclic antidepressants overdoses [1, 2], dosing recommendations remained to be largely based on its original indication for local anesthetic systemic toxicity (LAST)—a complication of regional anesthesia. Its use in overdoses, however, represents a different pharmacologic scenario, as the quantity of drug and the elimination time are much greater. It is, therefore, possible that more ILE may need to be given and be administered for longer, but the effectiveness and safety of this approach is unknown [3]. We present a case of severe tricyclic antidepressant (TCA) overdose in which a low-dose ILE infusion was used for several days. Lipemia developed, but there were no other clear adverse reactions. The patient also survived with no lasting sequelae despite severe TCA toxicity.
Case Report
A 44-year-old, 55-kg Caucasian man was found outdoors unconscious, hypothermic, and hypotensive. He had last been seen 8 h prior. Intubated in the field, his blood pressure (BP) was initially 65/42 mmHg with a core temperature of 29 °C. Initial QRS duration was 160 ms. Warm intravenous (IV) fluids were administered, vasopressors were started, and active rewarming was initiated externally and by warm gastric, bladder, and pleural irrigation. At a temperature of 31 °C, the patient suffered a generalized tonic–clonic seizure followed by pulseless electrical activity (PEA) and subsequent ventricular fibrillation (VF). Toxicology revealed an initial total tricyclic level of 7,400 mmol/L (therapeutic range, 178–585 mmol/L) prior to treatment. He continued to have marked QRS prolongation to 200 ms, with recurrent episodes of ventricular fibrillation despite a sodium bicarbonate infusion (150 mmol/L NaHCO3 in D5W at 150 mL/h) and a total of 300 mmol of NaHCO3 delivered as 50 mmol IV boluses. This corrected the arterial pH from 7.04 initially to 7.46. The pH was then maintained above 7.4 for the remainder of his hospitalization. Continuous renal replacement therapy (CRRT) was initiated to aid in rewarming, and the patient’s core temperature reached 38 °C after 6 h of resuscitation. In spite of this, the QRS remained 200 ms, and infusions of epinephrine (0.5 μg/kg/min), dopamine (20 μg/kg/min), and norepinephrine (0.4 μg/kg/min) were required to maintain a mean arterial pressure of 60 mmHg. By this time, the patient’s mother had found a suicide note and reported missing amitriptyline tablets representing a possible ingestion of 2.25 g. With some limited guidance from our regional Poison Control Center, we decided to administer Intralipid® (Baxter, Canada). Twenty percent ILE was administered as a 250-mL bolus followed by an infusion at 100 mL/h. The QRS duration normalized within minutes from 200 to 120 ms (Fig. 1). There were no further episodes of VF, and most vasopressors were weaned off over the next 24 h. After 24 h, the ILE was discontinued, but the QRS interval lengthened to 160 ms, and the patient’s vasopressor requirements increased. ILE was reinitiated at a dose of 100 mL/h with normalization of the QRS duration. Whole bowel irrigation with polyethylene glycol (PegLyte®, Pharmascience, Canada) was initiated at 50 mL/h with resultant diarrhea. At this time, the patient’s serum became grossly lipemic. It was then decided to continue the ILE infusion at a lower rate of only 18 mL/h starting postingestion day 3. Serum TCA levels dropped to 4,995 mmol/L on day 2 but then rose again to 8,858 mmol/L by day 4, before declining thereafter. Nevertheless, TCA levels remained in the toxic range for 21 days. ILE was continued at 18 mL/h until day 19. TCA levels were still 1,617 mmol/L upon discontinuation. However, the QRS was less than 120 ms for the rest of his hospitalization. The sodium bicarbonate infusion was continued at 150 mL/h for the first 48 h and then at 75 mL/h until day 17 to maintain a normal serum bicarbonate level (Fig. 2). While serum lipid levels were not measured systematically throughout this patient’s hospitalization, the serum was characterized as grossly lipemic for the first 4 days of therapy, with resultant difficulties with laboratory testing. The patient’s course was complicated by prolonged ileus requiring total parenteral nutrition, acute renal failure requiring renal replacement therapy, respiratory failure, and critical illness myopathy. Nevertheless, he was eventually discharged home with no sequelae and remains alive to this day.
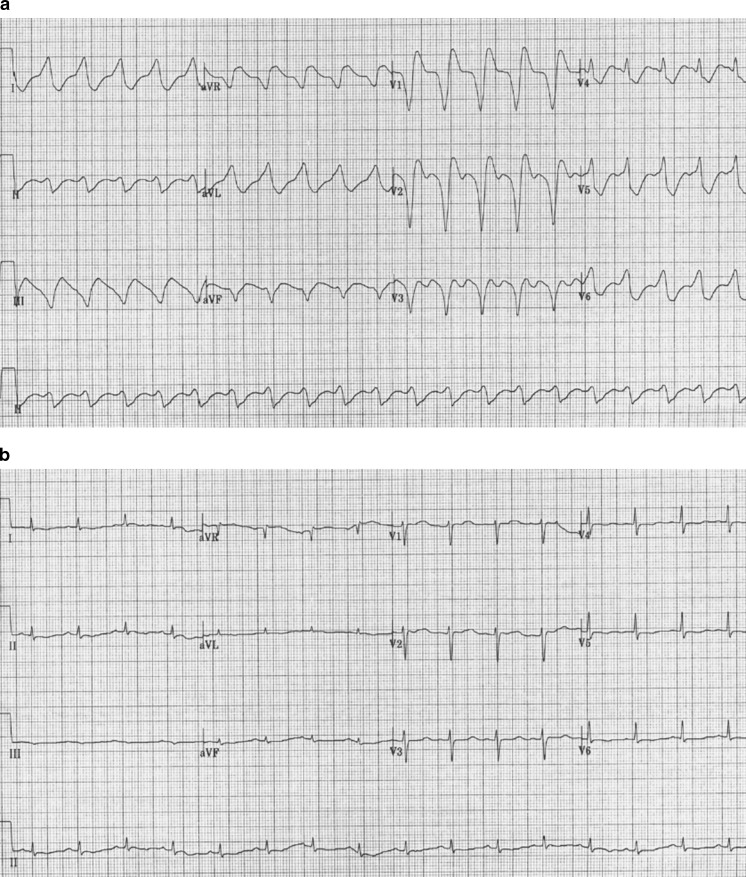
Fig. 1.
Twelve-lead electrocardiograms taken following initial resuscitation prior to (a) and after (b) bolus administration of 250 mL 20 % ILE and initiation of ILE infusion at 100 mL/h
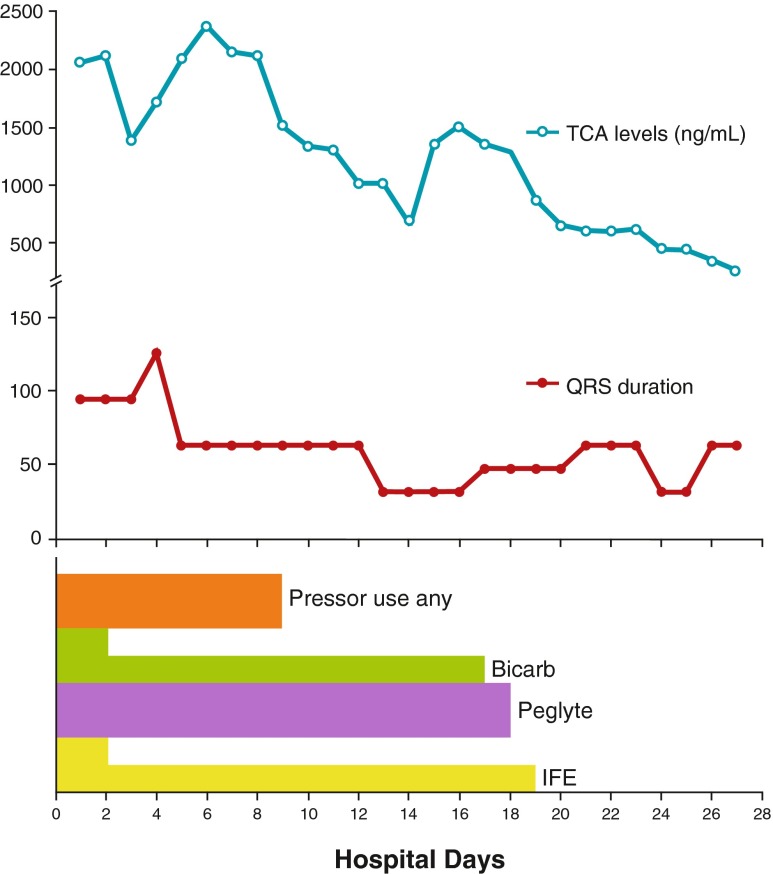
Fig. 2.
Chart summarizing therapies administered, TCA levels, and QRS duration over time after initial resuscitation. The increase in the QRS interval on day 2 followed discontinuation of the ILE infusion and prompted a rebolus of 250 mL 20 % ILE and reinitiation of an infusion at 100 mL/h for further 24 h after which the rate was decreased to 18 mL/h. Following this, the QRS duration remained within the normal range despite rising/very elevated TCA levels (half size bar indicates lower-dose bicarbonate and ILE infusions, respectively)
Discussion
This case adds to the evolving experience with the use of ILE for severe TCA intoxications. Our case is unique because of the large amount of ILE administered and the use of a prolonged infusion. The patient ultimately survived, and potential benefit is supported primarily by repeated QRS narrowing immediately following ILE administration. During his initial resuscitation, the QRS narrowed within minutes of adding lipid therapy to fairly high-dose vasopressor and reasonable bicarbonate therapy. On the 2nd day, despite having already received an extended infusion of ILE and being continued on a sodium bicarbonate infusion, the patient’s QRS widened again after the lipid therapy was stopped. When the ILE therapy was restarted, the QRS once again narrowed. TCA levels at that time remained very high, suggesting that the QRS widening was indeed due to the amitriptyline. Faced with persistently high levels, the concern to avoid recurrent toxicity while limiting potential adverse effects of ILE, we chose to maintain the ILE infusion at an arbitrarily selected low level. No further QRS widening was observed, and the ILE infusion was not again discontinued until the TCA levels were much lower several days later. Given that even very high TCA level do not generally correlate well with toxicity, the benefit of this low-level infusion is unclear, as we did not periodically interrupt the ILE infusion to demonstrate QRS widening. Thus, the ILE could potentially have been discontinued much earlier. Engels and Davidow reported a scenario similar to our own in which ILE was administered for refractory shock and cardiac arrest [4]. They also noted a prompt improvement in hemodynamics, which persisted without the need for a prolonged or repeated administration of ILE, despite a larger suspected ingestion of 4.25 g compared with 2.25 g in our patient, highlighting the individual variability in toxicological response. Indeed, we also suspect that the patient’s profound hypothermia allowed high tissue levels to accumulate before toxicity became manifest following rewarming. Recurrent toxicity following discontinuation of ILE has previously been reported by others [5], and repeated boluses have been advocated [6] and reported previously. Al-Duaij reported a case where two boluses of ILE were administered for persistent hemodynamic instability. The patient improved but continued to have periodic bursts of ventricular tachycardia for further 4–6 h [7]. This suggests that toxicity was only partially reversed and that more ILE might have been helpful. However, in a case series of ILE use for toxic ingestions in nine patients, repeated boluses were not associated with improved survival, nor was an infusion associated with a statistically significant improvement in mean arterial pressure (MAP), although MAP did appear to decline over time following bolus-only administration [2]. Thus, the optimal dosing scheme for ILE in situations of overdose remains unclear. Furthermore, our patient’s improvement cannot be solely attributed to ILE, as two important confounders exist. First, the patient continued to receive bicarbonate therapy with the ILE. Second, the severe initial hypothermia and rewarming may have contributed to the hemodynamic instability. Hypothermia may have prevented some of the initial toxicity prior to hospitalization, but this also contributed to prolonging the drug’s half-life and accounted for some of the delayed toxicity. Thus, it is impossible to say what role it ultimately played.
ILE dosing for this case was different from that reported elsewhere in the literature, as providers used a larger initial bolus and maintained the patient on an infusion for longer time. At the time of this case in 2009, a standard protocol was not available, and there was access only to remote Poison Center and Toxicology support, so dosing was done somewhat empirically. Thus, an initial high-dose infusion was administered following the bolus, which was only discontinued once the patient’s vasopressor requirements had improved substantially. Despite receiving a large amount of ILE, the patient still showed signs of recurrent toxicity upon discontinuation at 24 h. Recent guidelines for ILE rescue therapy [8] still base dosing on its initial indication for bupivacaine toxicity but do suggest that repeat dosing or an extended infusion may be appropriate for recurrent instability. Indeed, many different dosing regimens have been reported in cases of toxicity other than LAST [2, 5] and extended infusions and repeat bolus dosing have been described. There remains some concern regarding the safety of this approach. The LD 50 for ILE is unknown, but estimates based on a rat model suggest a dose limit of 10–12 mL/kg over 30 min [5]. The average dose received in the initial cases of lipid resuscitation was 3.7 mL/kg, compared to 4.5 mL/kg in our patient and 1.5 mL/kg as recommended by the American Society of Regional Anesthesia [5]. It is clear, however, that overdose intoxications represent a distinct pharmacologic scenario compared with LAST, where tissue levels are lower and drug elimination is much faster than the former. There is no clear dose–response data currently available for any drug, and thus, the optimal dosing for TCA overdoses remains unknown.
Our patient survived to discharge without any long-term sequelae. He developed persistent lipemia; however, he did not experience any other potential adverse effects from the ILE such as pancreatitis, hepatic steatosis, or venous thromboembolism that have been reported in other cases [2, 3, 6]. The patient did have prolonged respiratory failure due to ARDS. It is possible that ILE contributed to ongoing lung inflammation by providing proinflammatory fatty acids [6], but similar to the experience of others [2], our patient had reasons to develop ARDS independent of ILE.
Lipid therapy's mechanism of action remains unclear. The most widely held view is that ILE partitions out lipophilic drugs into a “lipid sink.” For many investigators though, the rapidity of action seen in overdose cases, including ours, implies that the ILE must be exerting a direct cardiotonic effect. Observations from animal work suggest that ILE may activate the cytoprotective Akt pathway within cells, increase inotropy by activating intracellular calcium currents, or compete with drugs for ion channel binding [9]. The rapid resolution of QRS widening in our patient is consistent with a direct cellular effect of ILE, and the lack of recurrent toxicity seen with the low-dose infusion may also have been due to mechanisms other than drug partitioning.
We were able to confirm extremely elevated total tricyclic levels starting at 7,400 mmol/L prior to the initiation of lipid therapy (Fig. 2). The levels actually increased transiently around day 4 before declining. Harvey and Cave also noted a transient rise in TCA levels following lipid administration without recurrent toxicity [10]. They hypothesized that this was due to an enhanced enteric absorption of TCA into lipemic serum. TCA levels remained well within the toxic range nearly 20 days. We surmise that the patient’s initial hypothermia played a significant role by contributing to protracted gut dysfunction with ongoing enteric drug absorption and by slowing drug metabolism. Also, Litonius et al. noted in a porcine model of TCA intoxication that ILE administration was associated with a slower decline in total amitriptyline levels but that the free drug fraction was significantly smaller than that in controls [11]. Renal dysfunction also likely contributed, and genetic factors (including CYP2D6) could not be ruled out. Despite these levels, the patient did not experience recurrent toxicity. This may simply reflect the fact that TCA levels do not correlate well with clinical toxicity [12]. However, this observation applies to TCA levels measured in different individuals, but whether a tighter relationship between levels and toxicity exists within a given patient is unknown. Given this possibility, it appears that once administered, ILE provides long-lasting protection from further toxicity, either by cellular mechanisms or by establishing a lipid reservoir. This may explain the apparent lack of additional benefit of prolonged infusions in some series [2]. In our patient, a low-dose infusion was maintained as part of parenteral nutrition, with the thought that it might stave off further toxicity. Because we were only able to measure total and not free TCA levels, we cannot be certain whether the amitriptyline that remained was indeed sequestered in a lipid sink. However, the serum triglycerides were measured at only 2.62 mmol/L (232 mg/dL) on day 9, so it is difficult to imagine that a significant lipid phase was available for partitioning. Thus, the benefit of the low-dose infusion may have been at the tissue level, if indeed any effect was present.
In conclusion, ILE appears to have been beneficial in the management of severe amitriptyline toxicity. Consistent with prior reports, the administration of ILE was associated with prompt QRS narrowing and improved hemodynamic stability. The extremely long drug elimination time is a unique feature of this case and serves to illustrate areas of uncertainty; optimal drug dosage in overdose situations has not yet been defined, nor is the exact mechanism of action clear. A better definition of the latter, in an animal model for instance, might potentially allow for a more rational approach to dosing; partitioning would potentially require maintaining fairly high lipid levels, whereas tissue level effects might be maintained with lower doses. Our case is, thus, hypothesis generating, and we hope that it stimulates further avenues of investigation into this evolving area in toxicology.
Acknowledgments
Conflict of Interest
None of the authors report any conflict of interest.
Footnotes
This work was previously presented in abstract at the 2012 North American Congress of Clinical Toxicology annual meeting, October 1–6, Las Vegas, NV (Clin Toxicol. 2012; 50(7):579).
References
- 1.Jamaty C, Bailey B, Larocque A, Notebaert E, Sanogo K, Chauny J-M. Lipid emulsions in the treatment of acute poisoning: a systematic review of human and animal studies. Clin Toxicol (Phila) 2010;48(1):1–27. doi: 10.3109/15563650903544124. [DOI] [PubMed] [Google Scholar]
- 2.On behalf of the Toxicology Investigators’ Consortium (ToxIC) Geib A-J, Liebelt E, Manini AF. Clinical experience with intravenous lipid emulsion for drug-induced cardiovascular collapse. J Med Toxicol. 2011;8(1):10–14. doi: 10.1007/s13181-011-0187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turner-Lawrence DE, Kerns Ii W. Intravenous fat emulsion: a potential novel antidote. J Med Toxicol. 2008;4(2):109–114. doi: 10.1007/BF03160965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engels PT, Davidow JS. Intravenous fat emulsion to reverse haemodynamic instability from intentional amitriptyline overdose. Resuscitation. 2010;81(8):1037–1039. doi: 10.1016/j.resuscitation.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Weinberg GL. Lipid emulsion infusion: resuscitation for local anesthetic and other drug overdose. Anesthesiology. 2012;117(1):180–187. doi: 10.1097/ALN.0b013e31825ad8de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothschild L, Bern S, Oswald S, Weinberg G. Intravenous lipid emulsion in clinical toxicology. Scand J Trauma Resusc Emerg Med. 2010;18:51. doi: 10.1186/1757-7241-18-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Duaji N, George M, O’Donnell K, et al. Lipid emulsion therapy in massive imipramine overdose. Clin Toxicol (Phila) 2009;47(5):436–510. doi: 10.1080/15563650902952273. [DOI] [Google Scholar]
- 8.American College of Medical Toxicology ACMT position statement: interim guidance for the use of lipid resuscitation therapy. J Med Toxicol. 2011;7(1):81–82. doi: 10.1007/s13181-010-0125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinberg GL. Lipid resuscitation. Crit Care Med. 2012;40(8):2521. doi: 10.1097/CCM.0b013e318258e930. [DOI] [PubMed] [Google Scholar]
- 10.Harvey M, Cave G. Case report: successful lipid resuscitation in multi-drug overdose with predominant tricyclic antidepressant toxidrome. Int J Emerg Med. 2012;5(1):8. doi: 10.1186/1865-1380-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Litonius E, Niiya T, Neuvonen PJ, Rosenberg PH. No antidotal effect of intravenous lipid emulsion in experimental amitriptyline intoxication despite significant entrapment of amitriptyline. Basic Clin Pharmacol Toxicol. 2011;110(4):378–383. doi: 10.1111/j.1742-7843.2011.00826.x. [DOI] [PubMed] [Google Scholar]
- 12.Bailey B, Buckley NA, Amre DK. A meta-analysis of prognostic indicators to predict seizures, arrhythmias or death after tricyclic antidepressant overdose. J Toxicol Clin Toxicol. 2004;42(6):877–888. doi: 10.1081/CLT-200035286. [DOI] [PubMed] [Google Scholar]