Abstract
Introduction
Fingolimod is an immunomodulating agent used in multiple sclerosis (MS). It is a sphingosine-1-phosphate (S1P) receptor agonist prescribed for relapsing forms of MS to delay onset of physical disability. As fingolimod is known to cause first-dose bradycardia, telemetry is recommended for the first 6 h post-dose. We present the first reported case of deliberate fingolimod overdose requiring atropine administration for bradycardia and hemodynamic instability.
Case report
A 33-year-old woman ingested 14 mg of fingolimod and 2 g of phenoxymethylpenicillin. After presenting to the emergency department 19 h later, she was initially hemodynamically stable (heart rate (HR) 60, blood pressure (BP) 113/89 mmHg). Two hours later, she then developed bradycardia (HR 48) and hypotension (87/57 mmHg). Despite intravenous fluids, stabilisation was only achieved after administration of atropine (300 μg). She was then admitted to the intensive care unit (ICU) for further monitoring where another episode of bradycardia and hypotension required atropine. She was monitored in the ICU for 48 h and then discharged on day 5 with no further episodes.
Discussion
Fingolimod is known to cause bradycardia in the first 6 h post first therapeutic dose. Following intentional overdose, onset of bradycardia occurred at 21 h post-ingestion and was associated with hypotension. Atropine was successful in treating bradycardia and associated hypotension.
Keywords: Fingolimod, Bradycardia, Hypotension, Atropine, Poisoning
Introduction
Fingolimod is an oral sphingosine-1-phosphate (S1P) receptor modulator used in the treatment of relapsing forms of multiple sclerosis (MS). Fingolimod prevents lymphocyte egress from lymph nodes leading to a reduction in infiltration of auto-reactive lymphocytes into the central nervous system [1]. Fingolimod is known to cause first-dose bradycardia. Experimental studies indicate that the mechanism of reduced heart rate occurs via activation of G-protein-regulated, inward-rectifying potassium (GIRK) channels in atrial myocytes, most likely via sphingosine-1-phosphate receptors [2].
There have been two case reports on single therapeutic ingestion of fingolimod causing bradycardia at 21 and 39 h post-ingestion with the former also having an episode of asystole [3, 4]. We have described the first case of deliberate overdose with fingolimod and associated cardiovascular instability.
Case Report
A 33-year-old female with a 10-year history of MS and a 2-year history of depression presented voluntarily to the emergency department (ED) 19 h post deliberate ingestion of 28 × 0.5 mg fingolimod tablets and 4 × 500 mg phenoxymethylpenicillin tablets. The antibiotics were not her medication, and she was on no other regular medication. An ambulance was called by the ex-boyfriend, who had found the empty packets of tablets, and the history was confirmed with the patient that the overdose was taken the night before.
She was prescribed fingolimod 0.5 mg daily for her MS and citalopram 20 mg daily for her depression. She was commenced on fingolimod 16 months prior to this presentation and did not experience first-dose bradycardia or hypotension at that time. The patient voluntarily discontinued both of these medications 4 months prior to her presentation. At presentation, she was experiencing an exacerbation of MS and depression.
Examination revealed a normal conscious state, heart rate of 60 beats per minute (bpm) and blood pressure (BP) of 113/89 mmHg. She was euvolemic and had no signs of infection or intercurrent illness. ECG confirmed sinus rhythm with no conduction abnormalities.
Initial pathology results revealed the following: haemoglobin 126 g/L (12.6 g/dL), white cell count 2.7 × 109/L (ref 4.0–11.0) (2.7 × 103/μL), neutrophils 2.1 × 109/L (ref 2.0–7.5) (2.1 × 103/μL), lymphocytes 0.2 × 109/L (ref 1.0–4.0) (0.2 × 103/μL), and platelets 234 × 109/L (234 × 103/μL). Electrolytes, renal function, and arterial blood gas values were within normal range. Lactate was 0.7 mmol/L (ref 0.5–1.6).
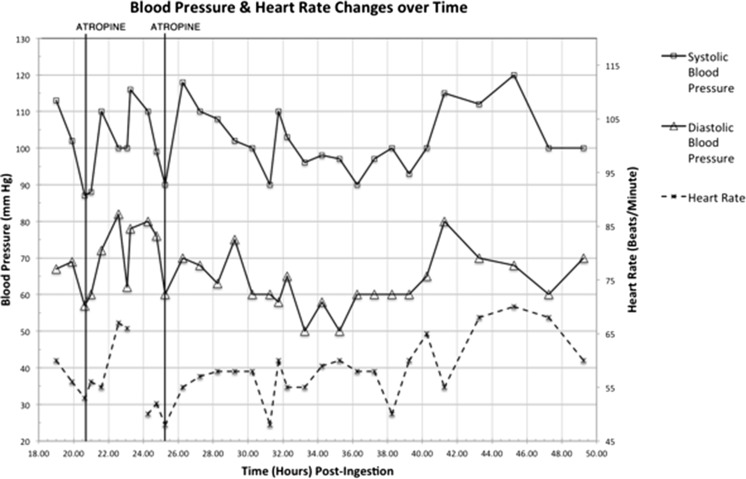
Two hours post-arrival in the ED (21 h post-ingestion), her heart rate decreased to 48 bpm and her BP dropped to 87/57 mmHg. She was given intravenously 1,000 mL of compound sodium lactate over 30 min. Her heart rate after the infusion was 56 bpm (Fig. 1), and her BP was 88/60 mmHg. She was then given intravenously a bolus of 300 μg of atropine (Fig. 2). Her BP improved to 110/72 mmHg with a heart rate of 67 bpm. She was admitted to the intensive care unit (ICU) for further monitoring. She had another episode of bradycardia with hypotension (heart rate 48 bpm, BP 90/60 mmHg) 25 h post-ingestion for which she received atropine (300 μg) with good effect. Thirty-two hours post-ingestion, she had another episode of bradycardia (heart rate 48 bpm) and associated hypotension 90/60 mmHg. This resolved spontaneously without requiring further atropine. Her neutrophils remained low (1.4 × 109/L) (1.4 × 103/μL), and lymphocytes increased to 0.4 × 109/L (0.4 × 103/μL) 48 h after presentation. She was discharged from the ICU after 48 h. She remained in the hospital for five more days and was evaluated by neurology and psychiatry services.
Fig. 1.
Twelve-lead ECG of first episode of bradycardia (56 bpm) and hypotension
Fig. 2.
Observations (blood pressure and heart rate) vs time post-ingestion. Time when atropine has been given is marked on the graph
Discussion
Fingolimod is known to elicit a negative chronotropic effect on heart rate in both nonhuman trials and healthy adults but attenuates over time with continued administration [5]. Therefore, cardiac monitoring is recommended for both the first dose of fingolimod and any re-introductory dose after a medication break. Our patient had stopped taking fingolimod 4 months before her overdose. Despite not experiencing bradycardia with her first dose when originally commencing fingolimod, she had bradycardia with associated hypotension at 21 and 32 h post-exposure on this presentation. Delayed onset bradycardia has been reported with first therapeutic dosing [3, 4]. However, multiple hypotensive episodes have not been observed. These case reports did not report on blood pressure or stated it was stable. Neutropenia and lymphopenia are associated with therapeutic fingolimod administration and may be observed within days of the first dose [6]. The most commonly reported side effects are nasopharyngitis, GI symptoms (nausea, vomiting, diarrhoea), and headache. Our patient had both neutropenia and lymphopenia at presentation, which stabilised after during her admission, with a neutrophil count of 1.4 × 109/L and lymphocyte count of 0.4 × 109/L at time of discharge.
Fingolimod is efficiently absorbed with an oral bioavailability of >90 %, and absorption is unaffected by meals with dose-proportional exposure between 0.125 and 5 mg in single and multi-dose studies [6]. Blood drug concentrations rise slowly to reach broad plateau 8 to 36 h post-administration with absolute cmax reaching generally 12–16 h post-dose and may occur over a long length of the gastrointestinal tract [7]. Fingolimod is reversibly phosphorylated by sphingosine kinase to form the active moiety fingolimod-phosphate and is dephosphorylated back by sphingosine lyase and/or sphingosine phosphatase [7]. During maintenance dosing, there is a stable blood level ratio of both moieties. Volume of distribution is 1,200–1,700 L, with high plasma protein binding and slow clearance (6–8 L/h) that are both independent of dose [7]. The elimination half-life of fingolimod averages 6–9 days.
Fingolimod via the S1P receptors decreases heart rate via activation of G-protein-regulated, inward-rectifying potassium channels in atrial myocytes [5]. Fingolimod has also been shown to stimulate endothelial production of nitric oxide via the S1P3 receptor in mice and human endothelial cells and is a possible explanation for the patient’s hypotension [8]. Atropine has been used in previous cases of fingolimod-associated bradycardia and suggests a vagal rate-related mechanism leading to bradycardia [4]. There have been no previous case reports on deliberate fingolimod overdose nor have there been any reports on significant hypotension secondary to therapeutic fingolimod use.
Management in this case necessitated only modest doses of atropine. In failing response to administration of atropine for hypotension, isoproterenol has been proven to attenuate bradycardia in therapeutic doses of fingolimod [9]. We postulate that it may also be useful in overdose-related hypotension and bradycardia. Activated charcoal may be helpful, preferably if given within 1 h of ingestion. With high protein binding and a large volume of distribution, enhanced elimination modalities such as hemodialysis and multi-dose activated charcoal are likely to be ineffective. For an elimination half-life of 6–9 days, prolonged cardiac monitoring may be necessary.
In this case, the patient responded to small doses of atropine. Her response to this was adequate, and aggressive use of fluids or other modalities was unnecessary. By 43 h post-overdose, no further episodes of bradycardia was observed.
A limitation of our case report is the lack of a fingolimod level. No fingolimod concentration assays were available; however, the clinical picture scores a definite adverse drug reaction by using the Naranjo algorithm [10].
Conclusion
We report on the first case of fingolimod overdose associated with cardiovascular instability. Bradycardia occurred at 21, 25 and 32 h post-ingestion in this case and was associated with hypotension. Given the long half-life of fingolimod, cardiovascular effects may persist for greater than 24 h and require prolonged cardiac monitoring. In this case, atropine was used successfully to treat bradycardia, with concurrent resolution of hypotension.
Acknowledgments
Conflict of Interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Brinkmann V, Lynch KR. FTY720: targeting G-protein-coupled receptors for sphingosine 1-phosphate in transplantation and autoimmunity. Current Opin Immunol. 2002;14:569–575. doi: 10.1016/S0952-7915(02)00374-6. [DOI] [PubMed] [Google Scholar]
- 2.Hla T. Signaling and biological actions of sphingosine-1-phosphate. Pharmacol Res. 2003;47:401–407. doi: 10.1016/S1043-6618(03)00046-X. [DOI] [PubMed] [Google Scholar]
- 3.Espinosa PS, Berger JR. Delayed fingolimod-associated asystole. Multiple Sclerosis Journal. 2011;17(11):1387–1389. doi: 10.1177/1352458511410344. [DOI] [PubMed] [Google Scholar]
- 4.Faber H, Fischer HJ, Weber F. Prolonged and symptomatic bradycardia following a single dose of fingolimod. Mult Scler. 2013;19(1):126–128. doi: 10.1177/1352458512447596. [DOI] [PubMed] [Google Scholar]
- 5.Koyrakh L, Roman MI, Brinkmann V, Wickman K. The heart rate decrease caused by acute FTY720 administration is medicated by the G protein-gated potassium channel I. Am J Transplant. 2005;5:529–5367. doi: 10.1111/j.1600-6143.2005.00754.x. [DOI] [PubMed] [Google Scholar]
- 6.Kovarik JM, Hartmann S, Bartlett M, et al. Oral-intravenous crossover study of fingolimod pharmacokinetics, lymphocyte responses and cardiac effects. Biopharm Drug Dispos. 2007;28:97–104. doi: 10.1002/bdd.535. [DOI] [PubMed] [Google Scholar]
- 7.David OJ, Kovarik JM, Schmouder RL. Clinical pharmacokinetics of fingolimod. Clin Pharmacokinet. 2012;51(1):15–28. doi: 10.2165/11596550-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Tolle M, Levkau B, Keul P, et al. Immunomodulator FTY720 induces eNOS-dependent arterial vasodilation via the lysophospholipid receptor S1P3. Circ Res. 2005;96(8):913–920. doi: 10.1161/01.RES.0000164321.91452.00. [DOI] [PubMed] [Google Scholar]
- 9.Kovarik JM, Riviere GJ, Neddermann D, et al. A mechanistic study to assess whether isoproterenol can reverse the negative chronotropic effect of fingolimod. J Clin Pharmacol. 2008;48(3):303–310. doi: 10.1177/0091270007312903. [DOI] [PubMed] [Google Scholar]
- 10.Naranjo CA, Busto U, Seller EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol. 1981;30(2):239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]