Abstract
Background
The geographic distribution of canine infection with vector-borne disease agents in the United States appears to be expanding.
Methods
To provide an updated assessment of geographic trends in canine infection with Dirofilaria immitis, Borrelia burgdorferi, Ehrlichia spp., and Anaplasma spp., we evaluated results from an average of 3,588,477 dogs tested annually by veterinarians throughout the United States from 2010 – 2012.
Results
As in an earlier summary report, the percent positive test results varied by agent and region, with antigen of D. immitis and antibody to Ehrlichia spp. most commonly identified in the Southeast (2.9% and 3.2%, respectively) and antibody to both B. burgdorferi and Anaplasma spp. most commonly identified in the Northeast (13.3% and 7.1%, respectively) and upper Midwest (4.4% and 3.9%, respectively). Percent positive test results for D. immitis antigen were lower in every region considered, including in the Southeast, than previously reported. Percent positive test results for antibodies to B. burgdorferi and Ehrlichia spp. were higher nationally than previously reported, and, for antibodies to Anaplasma spp., were higher in the Northeast but lower in the Midwest and West, than in the initial report. Annual reports of human cases of Lyme disease, ehrlichiosis, and anaplasmosis were associated with percent positive canine test results by state for each respective tick-borne disease agent (R2 = 0.701, 0.457, and 0.314, respectively). Within endemic areas, percent positive test results for all three tick-borne agents demonstrated evidence of geographic expansion.
Conclusions
Continued national monitoring of canine test results for vector-borne zoonotic agents is an important tool for accurately mapping the geographic distribution of these agents, and greatly aids our understanding of the veterinary and public health threats they pose.
Keywords: Anaplasma, Borrelia burgdorferi, Canine, Dirofilaria immitis, Ehrlichia
Background
In 2009, we reported results of a national veterinary clinic based survey of dogs in the United States for antigen to heartworm and antibody to tick-borne disease agents [1]. Based on reported results from testing over 3 million dogs from 2001 to 2007, this study was the first to document and map percent positive test results on a national level to four vector-borne disease agents, namely, Dirofilaria immitis, Borrelia burgdorferi, Anaplasma spp., and Ehrlichia spp. [1]. Dirofilaria immitis, the causative agent of heartworm disease, is transmitted to dogs by a number of different mosquito species and is present throughout much of the United States [2]. Borrelia burgdorferi and Anaplasma phagocytophilum are transmitted by Ixodes spp. ticks, while Ehrlichia spp. are known to be vectored by Rhipicephalus sanguineus sensu lato as well as ticks in the genera Amblyomma, Dermacentor, and Ixodes[3,4].
Veterinarians and pet owners are aware of these infections, and canine preventive medicine protocols commonly include recommendations for administering routine heartworm prevention and tick control to dogs as well as vaccination to prevent transmission of B. burgdorferi, the agent of Lyme disease, in areas where transmission occurs [5]. However, not all dogs receive adequate veterinary care, prevention recommendations are not consistently followed, and even when implemented, these strategies are not 100% effective at preventing infection [2,6,7].
Because vector-borne infections can have serious implications for canine health, annual testing of dogs for these infections is recommended and commonly performed [2,5,8]. A number of studies have confirmed the utility of dogs as sentinels for tick-borne diseases [9-12]. Indeed, our original report has been widely cited and the data repurposed by other research groups [13], and subsequent work has shown that canine infection with some tick-borne disease agents correlates on a state-wide basis with reports of human disease [14]. Here, we provide an update to our original report by summarizing the percent positive test results of dogs tested by veterinarians in the United States from 2010 – 2012.
Methods
Source of data
Testing results included in the 2010–2012 summary and analysis were obtained from 5 different USDA licensed test kits manufactured by IDEXX Laboratories, Inc: PetChek® Heartworm PF Test, a microtiter plate ELISA for use in-clinic or at a reference laboratory for the detection of D. immitis antigen in canine serum or plasma; SNAP® HW RT Test kit, an in-clinic ELISA for the detection of D. immitis antigen in canine serum, plasma, or whole blood; SNAP® 4Dx® Test kit, an in-clinic ELISA for simultaneous detection of canine antibodies to E. canis, B. burgdorferi and A. phagocytophilum, and to D. immitis antigen; and SNAP® 4Dx® Plus Test kit, which was released in 2012 to replace SNAP 4Dx and is an in-clinic ELISA for simultaneous detection of canine antibodies to E. canis, E. ewingii, B. burgdorferi, A. phagocytophilum, and A. platys, and to D. immitis antigen.
Results of testing on these various test kits were obtained from two primary sources: the IDEXX Reference Laboratories network (PetChek® Heartworm PF, SNAP® 4Dx®, and SNAP® 4Dx Plus®), and those results generated by veterinarians using all five assays and recorded in IDEXX VetLab® Instrumentation and Software. For the latter results, information was obtained for both results entered manually by the clinic staff and those automatically recorded by IDEXX SnapShot Dx® instrumentation. For reasons of privacy, patient results were obtained in the absence of owner information or unique identification. Because of this, it was not possible to exclude repeat testing events either within a practice or between the practice and the reference laboratory.
Performance of test kits
Performance of the PetChek® Heartworm PF Test, SNAP® HW RT Test kit, SNAP® 3Dx® Test kit, and SNAP® 4Dx® Test kit has been reported previously [1,15]. The SNAP® 4Dx Plus® Test uses a peptide from a major outer surface protein (p28) of E. ewingii on the Ehrlichia portion of the test and has 96.5% sensitivity and 93.9% specificity for the detection of E. ewingii antibodies. The Anaplasma portion of the SNAP® 4Dx Plus® Test uses a synthetic peptide from the major surface protein of A. phagocytophilum (MSP2/p44) and has 89.1% sensitivity and 99.8% specificity for the detection of A. platys antibodies [16].
Data and statistical analysis
Test results were compiled by county based on the associated postal zip code of the veterinary hospital submitting the sample or providing the test result. Data were assembled into state and regional groups as previously described [1,17]. Four primary regional groups (Midwest, Northeast, Southeast, and West) were considered. Percent positive results were calculated by dividing the number of tests reported as positive for each agent by the total number of testing events recorded in a given county, state, or region. For state-wide summary tables and comparison to human disease reports, all results collected from 2010 – 2012 were included. For construction of county-based prevalence maps, individual counties in which fewer than 30 test results were available from a single year were excluded. Differences in the frequency of reported positive test results between counties, states, and regions, as well as differences in frequency of reported positive test results in the present survey and in our earlier report [1], were evaluated for significance with Chi-square test using SAS (Windows 9.1) (SAS Institute Inc., Cary, NC) with significance assigned at p < 0.0001.
Human cases of Lyme disease, ehrlichiosis, and anaplasmosis reported to the Centers for Disease Control and Prevention in 2010 and 2011 [18] were adjusted to reflect reported cases per 100,000 using average state population data based on intercensal estimates from the United States Census Bureau [19]; summaries of reported human cases from 2012 are not yet available. Comparison of population adjusted reports of human disease to canine seroprevalence rates for each respective agent (B. burgdorferi, Ehrlichia spp., and Anaplasma spp.) were performed using a linear regression and the coefficient of determination (R2) calculated as previously described [14]. Analyses were performed using GraphPad Prism v.5 (GraphPad Software, La Jolla, CA).
Results
Summary
A total of 30,917,280 data points were available from dogs tested in 1,778 counties and in all of the 50 states in the United States over the three year period summarized in the present paper (Table 1); evidence of at least one agent was found in dogs from every state considered. Antigen to heartworm was identified in dogs in every state, and antibodies to the tick-borne agents were identified in dogs in every state except Alaska, where test results were not available, and, for B. burgdorferi and Ehrlichia spp., Montana. Distribution of positive tests and relative percent positive values by county and state are shown in Figures 1, 2, 3 and 4.
Table 1.
Percent positive test results (number positive/number tested) by region for dogs tested from 2010 – 2012 in the United States for antigen of Dirofliaria immitis and antibody to Borrelia burgdorferi, Ehrlichia spp., and Anaplasma spp.
| State | Dirofilaria immitis | Borrelia burgdorferi | Ehrlichia spp. | Anaplasma spp. |
|---|---|---|---|---|
| Northeast |
0.4% (11,675/3,175,080) |
13.3% (373,212/2,806,273) |
0.9% (24,011/2,806,112) |
7.1% (189,486/2,652,801) |
| Midwest |
0.7% (20,014/2,817,915) |
4.4% (76,025/1,720,510) |
1.0% (17,337/1,720,168) |
3.9% (59,580/1,548,686) |
| Southeast |
2.9% (101,850/3,562,190) |
2.5% (51,232/2,058,574) |
3.2% (65,191/2.057,984) |
0.9% (14,046/1,631,332) |
| West |
0.8% (8,887/1,178,947) |
1.4% (5,726/410,840) |
1.3% (5,134/410,419) |
2.0% (7,056/359,449) |
| Overall mean | 1.3% (142,426/10,734,132) | 7.2% (509,195/6,996,197) | 1.6% (111,673/6,994,683) | 4.4% (270,168/6,192,268) |
Figure 1.
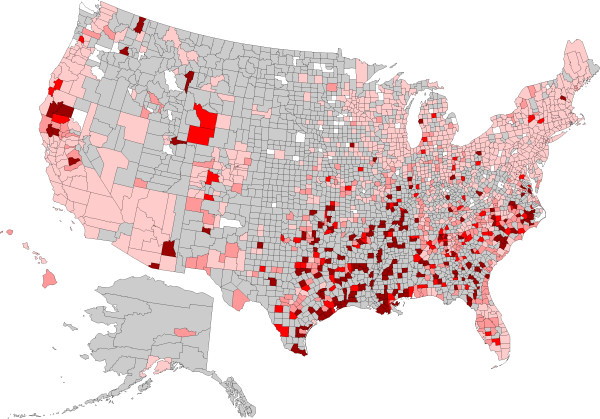
Evidence of antigen to Dirofilaria immitis in dogs by county, grouped according to percent positive tests. No results (<30) were received from counties shaded gray, precluding interpretation of the presence of antigen in dogs from these areas. Counties depicted in white had no dogs reported as positive (0%). Remaining counties were coded as follows: 0.1-2.0% (light pink), 2.1-4.0% (pink), 4.1-6.0% (red), and > 6.0% (dark red).
Figure 2.
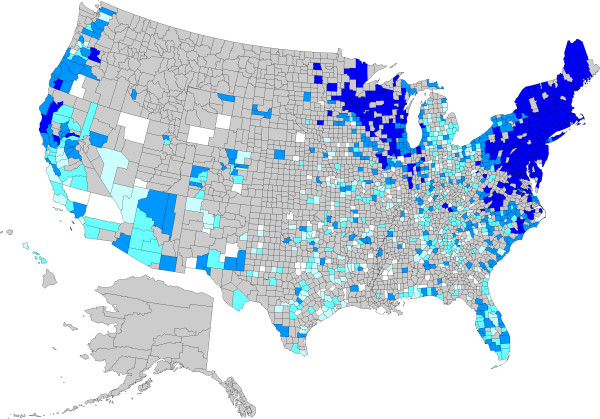
Evidence of antibody to Borrelia burgdorferi in dogs by county, grouped according to percent positive tests. No results (<30) were received from counties shaded gray, precluding interpretation of the presence of antibody in dogs from these areas. Counties depicted in white had no dogs reported as positive (0%). Remaining counties were coded as follows: 0.1-0.5% (light blue), 0.5-1.0% (blue), 1.1-5.0% (dark blue), and > 5.0% (very dark blue).
Figure 3.
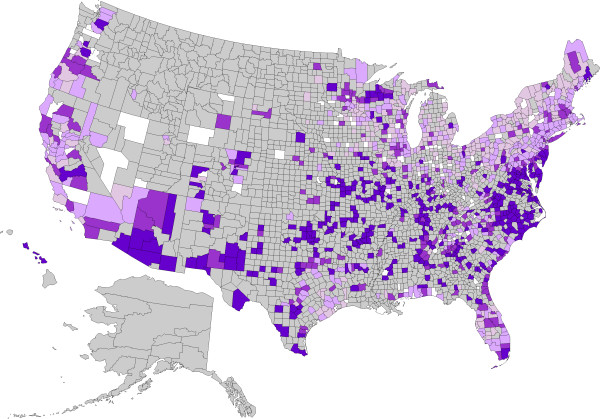
Evidence of antibody to Ehrlichia spp. in dogs by county, grouped according to percent positive tests. No results (<30) were received from counties shaded gray, precluding interpretation of the presence of antibody in dogs from these areas. Counties depicted in white had no dogs reported as positive (0%). Remaining counties were coded as follows: 0.1-0.5% (light purple), 0.5-1.0% (purple), 1.1-2.0% (dark purple), and > 2.0% (very dark purple).
Figure 4.
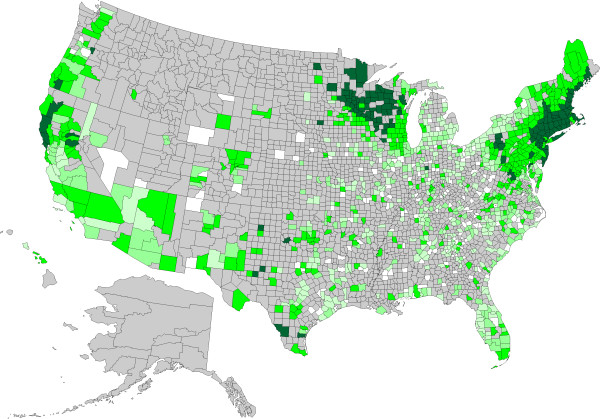
Evidence of antibody to Anaplasma spp. in dogs by county, grouped according to percent positive tests. No results (<30) were received from counties shaded gray, precluding interpretation of the presence of antibody in dogs from these areas. Counties depicted in white had no dogs reported as positive (0%). Remaining counties were coded as follows: 0.1-0.5% (light green), 0.5-1.0% (green), 1.1-5.0% (dark green), and > 5.0% (very dark green).
Heartworm
Percent positive test results for antigen of D. immitis were higher in the Southeast than in the other three regions, and were higher in the West and Midwest than in the Northeast (Table 1). Within areas of relatively low percent positive test results, certain counties had unexpectedly high results, including Belknap County, New Hampshire; Summit County, Utah; Walla Walla and Stevens Counties in Washington; and Shasta and Trinity Counties in California (Figure 1). Regional prevalence of percent positive test results for D. immitis antigen was lower in each of the four regions considered, and dramatically so in the Southeast, as compared to our previous report.
Lyme disease
Percent positive test results for antibody to B. burgdorferi were higher in the Northeast than in the other three regions, and were higher in the Midwest than in the Southeast or West (Table 1). Omitting Virginia and West Virginia (9.7% and 3.5%, respectively), where Lyme borreliosis is known to be endemic, from the Southeast resulted in a percent positive test result of 0.92% (15,086/1,631,562) in the remainder of the region. Within areas of relatively low percent positive test results, certain counties had unexpectedly high results, including Pulaski and Whitley Counties in Kentucky; Monroe County in Indiana; and Clark, Crawford, and Lawrence Counties in Illinois (Figure 2). Regional prevalence of percent positive test results for B. burgdorferi antibody was higher in the Northeast, Southeast, and Midwest, but unchanged in the West, compared to our previous report.
Ehrlichiosis
Percent positive test results for antibody to Ehrlichia spp. were higher in the Southeast than in the other three regions, and were higher in the West than in the Northeast or Midwest (Table 1). Within areas of relatively low percent positive test results, certain counties had unexpectedly high results, including Douglas and La Plata Counties in Colorado; Hancock County, Maine; Clackamas County, Oregon; Lamoille County, Vermont; and Clark and King Counties in Washington (Figure 3). Percent positive test results for Ehrlichia spp. antibody were higher nationally and in every region considered compared to our previous report.
Anaplasmosis
Percent positive test results for antibody to Anaplasma spp. were highest in the Northeast and Midwest, and were higher in the West than in the Southeast (Table 1). Within areas of relatively low percent positive test results, certain counties had unexpectedly high results, including Jackson County, Oklahoma; Howard and Potter Counties in Texas; and Minnehaha County, South Dakota (Figure 4). Percent positive test results for Anaplasma spp. antibody were lower nationally and in the Midwest and West, but higher in the Northeast, compared to our previous report.
Comparison to human disease reports
Percent positive test results for antibodies to B. burgdorferi in dogs and reported human cases of Lyme borreliosis by state were positively associated (R2 = 0.701, F = 110.0), although for some states reported human cases were higher (Delaware and New Hampshire) or lower (Rhode Island and South Dakota) than expected based on canine testing. Percent positive test results for Ehrlichia spp. in dogs and human case reports of ehrlichiosis by state were positively associated (R2 = 0.457, F = 34.5), although for some states reported human cases were higher (Oklahoma, Missouri, and Delaware) or lower (Arizona, Mississippi, and Washington) than expected based on canine testing. Percent positive test results for Anaplasma spp. in dogs and human case reports of anaplasmosis by state were positively associated (R2 = 0.314, F = 18.8), although for some states reported human cases were higher (Minnesota and Wisconsin) or lower (Connecticut and South Dakota) than expected based on canine testing.
Discussion
Vector-borne infections are increasingly important to the health of people and other animals worldwide [20-22]. The data reported in the present paper update our previous study and comprise the most comprehensive effort to date to document the distribution and prevalence of infection with these agents using canine samples [1]. Annual testing for heartworm and tick-borne infections is recommended and commonly performed by veterinarians [2,5]. We are not able to identify individual dogs or eliminate test results from the same dogs with the reporting system used, and some of the results undoubtedly reflect repeated testing of the same dogs. However, even if the results reported here represent repeated testing of 3 – 4 million pet dogs, we have likely documented the past or current infection status of approximately 5% of the estimated 70 million pet dogs in the United States, and perhaps as many as 10 – 15% [23].
Test results were available from at least 30 dogs from 1,420 counties, constituting 45.2% of the 3,144 counties in the United States. Because dogs cluster in human population centers, the present paper is biased towards urban areas, an approach which may underestimate true infection risk in some locales, particularly for tick-borne infections. Although urban transmission patterns have been described, the risk of tick-borne disease is generally greater in areas of lower housing density with more supportive habitat for the vector ticks, [24-27]. Moreover, an adequate number of test results were not available from many sparsely populated regions and thus we cannot determine the likelihood for these infections to be present across the entire nation.
As expected, heartworm infection was most commonly identified in dogs in the southeastern United States although the percent positive test results in this region were lower than previously reported [1]. While we cannot fully explain this reduction, the decrease may have been due to differences in data capture strategies, which resulted in inclusion of a higher frequency of routine heartworm tests in the data set. Strains of D. immitis resistant to heartworm preventives have recently been described from the southern United States, and veterinarians may be responding with greater vigilance [28-30]. Repeatedly testing dogs on heartworm preventive, and thus at low risk of infection, would be expected to decrease the percent positive test results overall. However, several states in the area of the country most affected by reports of resistance, including Alabama, Georgia, Louisiana, and Mississippi, actually have higher percent positive test results in the present paper than were reported previously [1], (Table 1).
Although the national prevalence of positive tests for heartworm in the present study was lower, the overall geographic distribution of positive test results was similar to that described in the previous report. Within this expected pattern, a number of counties with unexpectedly high percent positive test results were identified. Some of these unexpected results can be explained by the relatively low number (n = 30–45, data not shown) of test results available. Testing only a small number of dogs has been shown to result in an inaccurate impression of the presence of vector-borne transmission cycles due to translocation of infected pets [31]. In contrast, the counties identified with high percent positive test results in northern California were based on testing thousands of dogs and are consistent with our current understanding of D. immitis prevalence in domestic dogs and wild canids in that region [32,33].
In contrast to heartworm, percent positive results for antibody to agents of tick-borne diseases, particularly Lyme disease and ehrlichiosis, were higher nationally in the present study than in the previous report [1], a finding consistent with increased reports of these diseases in people in recent years [34,35]. Increases in percent positive test results were also seen regionally with the most striking change in the Southeast, where a nearly 1.5 fold increase in prevalence of B. burgdorferi was seen, largely due to increases in the percent positive test results of dogs in the states of Virginia, West Virginia, and Kentucky. A similar increased geographic distribution of the risk of B. burgdorferi infection has been described in public health reports although autochthonous transmission of this infection in people appears to remain focused in clear endemic and hyperendemic regions [34,36].
An increase in percent positive test results to Ehrlichia spp. was also evident in each of the four regions (Table 1). This increase was not attributable to recent modifications in the assay to include detection of antibodies to E. ewingii[14,37] because it was evident in the data in 2010 and 2011 (data not shown), before any platform changes were instituted. Moreover, the more widespread geographic distribution parallels reports from human surveillance and suggests the distribution of autochthonous transmission has increased, consistent with other data documenting continued geographic expansion of A. americanum ticks [35,38-41]. However, we suspect infection with a novel Ehrlichia sp. transmitted by ticks other than A. americanum are responsible for the positive test results that continue to be identified in Minnesota and Wisconsin, as has been previously described [1,42,43].
The percent positive test results for Anaplasma spp. were lower overall, although higher in the Northeast, than in the previous report, although large geographic variation was seen within the regions considered. Reports of human anaplasmosis cases have also increased in the Northeast in recent years [35,44]. The foci of elevated percent positive test results identified in dogs in western Texas and Oklahoma are mostly likely due to R. sanguineus transmitted infection with A. platys[14,45]. Brown dog ticks are common in this region, remarkably tolerant of arid conditions, and thrive during the 2011–2012 drought in the southcentral United States [46], Little, unpubl. data.
For each of the tick-borne agents, a few counties were identified with unexpectedly high percent positive test results given their geographic location within areas largely considered low or non endemic for those diseases. Some of these unexpected results are likely due to the low number of test results available (<300), while others may be due to the presence of novel agents. While the overall pattern in the maps provided is of great value in understanding distribution of disease, results for individual counties, particularly in areas not likely to support autochthonous transmission based on known vector phenology, should be interpreted with caution [1]. In addition, some of the assays used may detect novel agents as previously described [1,42,43]. Indeed, infection with an Ehrlichia sp. other than E. canis, E. chaffeensis, or E. ewingii may account for the higher than expected percent positive test results identified Clark and King Counties in Washington, where results from more than 2,000 dogs were available (Figure 3).
The canine serology results for the tick-borne disease agents in the present study largely agreed with reports of human disease for each respective type of disease. However, the association was strongest for canine antibodies to Borrelia burgdorferi and human Lyme disease, a finding which is not surprising as the assay used in dogs is known to be exquisitely specific and canine serology has been shown previously to correspond with reports of Lyme borreliosis in people [1,13,47]. The associations between canine serology and reports of human ehrlichiosis and anaplasmosis, while significant, were admittedly weak. The assays used in the present study detect multiple Ehrlichia spp. and Anaplasma spp., including some agents thought unlikely to cause disease in people, such as E. canis and A. platys, suggesting canine seroprevalence data may be of limited value in understanding the distribution of and transmission risk for those zoonotic agents [47-49]. Previous studies which showed strong agreement between canine seroprevalence to Ehrlichia spp. and reports of human ehrlichiosis employed species-specific serologic assays, a strategy which may be necessary to generate accurate predictive models for zoonotic infection risk [14]. Nonetheless, there were geographies with discordant results for human ehrlichiosis even using specific canine assays. This discrepancy may be, in part, due to the lack of species-specific diagnostic tools for use in human medicine when detecting antibodies to E. chaffeensis and A. phagocytophilum[50,51].
Annual canine preventive medicine protocols include testing for evidence of vector-borne infections in addition to implementing preventive strategies like routine acaricide use and vaccination. The canine serology results help veterinarians to assess the efficacy of the prevention protocol for that particular patient and modify it accordingly if evidence of transmission is identified [52]. By testing all dogs within a practice, it is possible to gain a broader perspective on the risks of transmission for recognized disease agents as well as identify the emergence of a previously uncommon pathogen. Translocation of infected dogs also occurs [1,31], complicating interpretation, although identifying infection with disease agents outside the range where they are known to be transmitted can be important to the health of individual patients. Finally, by understanding the relative importance of different vector-borne diseases within their practice area, veterinarians provide a public health service to the community by educating pet owners not only on the risks for their dog but the potential risk that these vector-borne infections may have for public health.
Conclusions
In this study, we have provided a comprehensive update on the frequency of positive test results for the most common vector-borne disease agents in the dog in the United States. While the broad geographical trends remained consistent with the previous report, several important differences were noted. Positive test results for D. immitis antigen were significantly lower in every region considered, while percent positive test results for antibodies to B. burgdorferi and Ehrlichia spp. were higher nationally than previously reported, and antibodies to Anaplasma spp. were higher in the Northeast but lower in the Midwest and West. Canine serology and the frequency of positive test results is an important tool for accurately mapping the geographic distribution of these agents. Recognizing geographic variations in percent positive test results also raises opportunities for future investigations on the distribution of tick species and the risk of human tick-borne disease in both endemic and non-endemic areas.
Competing interests
In the past five years, SL and DB have received reimbursement, speaking fees, or research support from IDEXX Laboratories, manufacturer of diagnostic tests for heartworm and tick-borne disease agents. In addition, MB, RC, and JS are employees of IDEXX Laboratories.
Authors’ contributions
SL, DD, and MB conceived of the study, SL and MB coordinated its design and execution and drafted the manuscript, and DD, RC, and JS reviewed and validated the data and the manuscript. All authors read and approved the final version of the manuscript.
Contributor Information
Susan E Little, Email: susan.little@okstate.edu.
Melissa J Beall, Email: Melissa-beall@idexx.com.
Dwight D Bowman, Email: ddb3@cornell.edu.
Ramaswamy Chandrashekar, Email: Ramaswamy-Chandrashekar@idexx.com.
John Stamaris, Email: john-stamaris@idexx.com.
Acknowledgements
We are grateful to the thousands of veterinarians throughout the United States who are monitoring the health of their patients by testing dogs for vector-borne disease agents. The veterinary profession’s commitment to both canine and public health is truly admirable and their dedication made this work possible. Funding to support the data analyses and creation of the maps was provided by the Krull-Ewing Endowment at Oklahoma State University. Outstanding technical support was provided by a number of individuals at IDEXX Laboratories, Inc. (Jessica Lachtara, Roger Boivin, and Kelly Cochrane) and Oklahoma State University (Jeff Gruntmeir), who assisted in validating the data and assembling the spread sheets for analysis.
References
- Bowman D, Little SE, Lorentzen L, Shields J, Sullivan MP, Carlin EP. Prevalence and geographic distribution of Dirofilaria immitis, Borrelia burgdorferi, Ehrlichia canis, and Anaplasma phagocytophilum in dogs in the United States: results of a national clinic-based serologic survey. Vet Parasitol. 2009;160:138–148. doi: 10.1016/j.vetpar.2008.10.093. [DOI] [PubMed] [Google Scholar]
- Graham W, Rubin SB, Boeckh A, Burhardt LF, Jones S, Miller M, Payne P, Rehm C, Smith-Blackmore M, Stannard R, Nelson CT, Atkins C, Carithers D, McCall J, von Simson C. Current canine guidelines for the diagnosis, prevention, and management of heartworm (Dirofilaria immitis) infection in cats (revised January 2012) http://www.heartwormsociety.org, Accessed October 1, 2013.
- Little SE, Heise SR, Blagburn BL, Callister SM, Mead PS. Lyme borreliosis in dogs and people in the USA. Trends Parasitol. 2010;26:213–218. doi: 10.1016/j.pt.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Nicholson WL, Allen KE, McQuiston JH, Breitschwerdt EB, Little SE. The increasing recognition of rickettsial pathogens in dogs and people. Trends Parasitol. 2010;26:205–212. doi: 10.1016/j.pt.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Companion Animal Parasite Council. CAPC General Guidelines, 2013. http://www.capcvet.org/capc-recommendations/capc-general-guidelines Accessed November 1, 2013.
- Hebert D, Eschner A. Seroprevalence of Borrelia burgdorferi-specific C6 antibody in dogs before and after implementation of a nonadjuvanted recombinant outer surface protein A vaccine in a Rhode Island small animal clinic. Vet Ther. 2010;11:E1–E9. [PubMed] [Google Scholar]
- Gates MC, Nolan TJ. Factors influencing heartworm, flea, and tick preventative use in patients presenting to a veterinary teaching hospital. Prev Vet Med. 2010;93:193–200. doi: 10.1016/j.prevetmed.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Animal Hospital Association (AAHA) AAHA-AVMA canine preventive healthcare guidelines. JAAHA. 2011;47:5. [Google Scholar]
- Duncan AW, Correa MT, Levine JF, Breitschwerdt EB. The dog as a sentinel for human infection: prevalence of Borrelia burgdorferi C6 antibodies in dogs from southeastern and mid-Atlantic States. Vector Borne Zoonotic Dis. 2005;5:101–109. doi: 10.1089/vbz.2005.5.101. [DOI] [PubMed] [Google Scholar]
- Cardosa L, Mendao C, de Carvalho LM. Prevalence of Dirofilaria immitis, Ehrlichia canis, Borrelia burgdorferi sensu lato, Anaplasma spp. and Leishmania infantum in apparently healthy and CVBD-suspect dogs in Portugal – a national serological study. Parasit Vector. 2012;5:62. doi: 10.1186/1756-3305-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer SA, Tsao JI, Walker ED, Mansfield LS, Foster ES, Hickling GJ. Use of tick surveys and serosurveys to evaluate pet dogs as a sentinel species for emerging Lyme disease. Am J Vet Res. 2009;70:49–56. doi: 10.2460/ajvr.70.1.49. [DOI] [PubMed] [Google Scholar]
- Smith FD, Ballantyne R, Morgan ER, Wall R. Estimating Lyme disease risk using pet dogs as sentinels. Comp Immunol Microbiol Infect Dis. 2012;35:163–167. doi: 10.1016/j.cimid.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Mead P, Goel R, Kugeler K. Canine serology as adjunct to human Lyme disease surveillance. Emerg Infect Dis. 2011;17:1710–1712. doi: 10.3201/1709.110210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall MJ, Alleman AR, Breitschwerdt EB, Cohn LA, Couto CG, Dryden MW, Guptill LC, Iazbik C, Kania SA, Lathan P, Little SE, Roy A, Sayler KA, Stillman BA, Welles EG, Wolfson W, Yabsley MJ. Seroprevalence of Ehrlichia canis, Ehrlichia chaffeensis and Ehrlichia ewingii in dogs in North America. Parasit Vectors. 2012;5:29. doi: 10.1186/1756-3305-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar R, Mainville CA, Beall MJ, O’Connor T, Eberts MD, Alleman AR, Gaunt SD, Breitschwerdt EB. Performance of a commercially available in-clinic ELISA for the detection of antibodies against Anaplasma phagocytophilum, Ehrlichia canis, and Borrelia burgdorferi and Dirofilaria immitis antigen in dogs. Am J Vet Res. 2010;71:1443–1450. doi: 10.2460/ajvr.71.12.1443. [DOI] [PubMed] [Google Scholar]
- Stillman BA, Monn M, Liu J, Thatcher B, Foster P, Andrews B, Little S, Eberts M, Breitschwerdt E, Beall M, Chandrashekar R. Performance of a new commercially available in-clinic ELISA for the detection of antibodies to Ehrlichia ewingii (granulocytic ehrlichiosis) and Anaplasma platys (thrombocytopenic anaplasmosis) in dogs. JAVMA. 2014. in press. [DOI] [PubMed]
- Blagburn BL, Lindsay DS, Vaughn JK, Rippey NS, Wright JC, Lynn RC, Kelch WJ, Ritchie GC, Hepler DI. Prevalence of canine parasites based on fecal flotation. Comp Cont Ed Pract Vet. 1996;18:483–509. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) http://www.cdc.gov/mmwr/mmwr_nd/ Accessed November 1, 2013.
- United States Census Bureau. 2013. http://www.census.gov/popest/data/intercensal/index.html.
- Kilpatrick AM, Randolph SE. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet. 2012;380:1946–1955. doi: 10.1016/S0140-6736(12)61151-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little SE. Future challenges for parasitology: vector control and one health in the Americas. Vet Parasitol. 2013;195:249–255. doi: 10.1016/j.vetpar.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Mencke N. Future challenges for parasitology: vector control and ‘One health’ in Europe: the veterinary medicinal view on CVBDs such as tick borreliosis, rickettsiosis and canine leishmaniosis. Vet Parasitol. 2013;195:256–271. doi: 10.1016/j.vetpar.2013.04.007. [DOI] [PubMed] [Google Scholar]
- American Veterinary Medical Association (AVMA) U. S. pet ownership and demographics sourcebook, 2012. http://www.avma.org.
- Duffy DC, Clark DD, Campbell SR, Gurney S, Perello R, Simon N. Landscape patterns of abundance of Ixodes scapularis (Acari: Ixodidae) on Shelter Island, New York. J Med Entomol. 1994;31:875–879. doi: 10.1093/jmedent/31.6.875. [DOI] [PubMed] [Google Scholar]
- Standaert SM, Dawson JE, Schaffner W, Childs JE, Biggie KL, Singleton J Jr, Gerhardt RR, Knight ML, Hutcheson RH. Ehrlichiosis in a golf-oriented retirement community. N Engl J Med. 1995;333:420–425. doi: 10.1056/NEJM199508173330704. [DOI] [PubMed] [Google Scholar]
- Jobe DA, Nelson JA, Adam MD, Martin SA Jr. Lyme disease in urban areas, Chicago. Emerg Infect Dis. 2007;13:1799–1800. doi: 10.3201/eid1311.070801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer SA, Goldberg TL, Kitron UD, Brawn JD, Anderson TK, Loss SR, Walker ED, Hamer GL. Wild birds and urban ecology of ticks and tick-borne pathogens, Chicago, Illinois, USA, 2005–2010. Emerg Infect Dis. 2012;18:1589–1595. doi: 10.3201/eid1810.120511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder DE, Wiseman S, Cruthers LR, Slone RL. Ivermectin and milbemycin oxime in experimental adult heartworm (Dirofilaria immitis) infection of dogs. JVIM. 2011;25:61–64. doi: 10.1111/j.1939-1676.2010.0657.x. [DOI] [PubMed] [Google Scholar]
- Blagburn BL, Dillon AR, Arther RG, Butler JM, Newton JC. Comparative efficacy of four commercially available heartworm preventive products against the MP3 laboratory strain of Dirofilaria immitis. Vet Parasitol. 2011;176:189–194. doi: 10.1016/j.vetpar.2010.12.049. [DOI] [PubMed] [Google Scholar]
- Bowman DD. Heartworms, macrocyclic lactones, and the specter of resistance to prevention in the United States. Parasit Vectors. 2012;5:138. doi: 10.1186/1756-3305-5-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millen K, Kugeler KJ, Hinckley AF, Lawaczeck EW, Mead PS. Elevated Lyme disease seroprevalence among dogs in a nonendemic county: harbinger or artifact? Vector Borne Zoonotic Dis. 2013;13:340–341. doi: 10.1089/vbz.2012.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters LL, Lavoipierre MM. Landscape epidemiology of mosquito-borne canine heartworm (Dirofilaria immitis) in northern California, USA. I. Community-based surveys of domestic dogs in three landscapes. J Med Entomol. 1984;21:1–16. doi: 10.1093/jmedent/21.1.1. [DOI] [PubMed] [Google Scholar]
- Sacks BN, Caswell-Chen EP. Reconstructing the spread of Dirofilaria immitis in California coyotes. J Parasitol. 2003;89:319–323. doi: 10.1645/0022-3395(2003)089[0319:RTSODI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bacon RM, Kugeler KJ, Mead PS. Surveillance for Lyme disease–United States, 1992–2006. MMWR Surveill Summ. 2008;57:1–9. [PubMed] [Google Scholar]
- Dahlgren FS, Mandel EJ, Krebs JW, Massung RF, McQuiston JH. Increasing incidence of Ehrlichia chaffeensis and Anaplasma phagocytophilum in the United States, 2000–2007. Am J Trop Med Hyg. 2011;85:124–131. doi: 10.4269/ajtmh.2011.10-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diuk-Wasser MA, Hoen AG, Cislo P, Brinkerhoff R, Hamer SA, Rowland M, Cortinas R, Vourc’h G, Melton F, Hickling GJ, Tsao JI, Bunikis J, Barbour AG, Kitron U, Piesman J, Fish D. Human risk of infection with Borrelia burgdorferi, the Lyme disease agent, in eastern United States. Am J Trop Med Hyg. 2012;86:320–327. doi: 10.4269/ajtmh.2012.11-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor TP, Saucier JM, Daniluk D, Stillman BA, Krah R, Rikihisa Y, Xiong Q, Yabsley MJ, Adams DS, Diniz PP, Breitschwerdt EB, Gaunt SD, Chandrashekar R. Evaluation of peptide- and recombinant protein-based assays for detection of anti-Ehrlichia ewingii antibodies in experimentally and naturally infected dogs. Am J Vet Res. 2010;71:1195–1200. doi: 10.2460/ajvr.71.10.1195. [DOI] [PubMed] [Google Scholar]
- Childs JE, Paddock CD. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu Rev Entomol. 2003;48:307–337. doi: 10.1146/annurev.ento.48.091801.112728. [DOI] [PubMed] [Google Scholar]
- Nicholson WL, Paddock CD, Demma L, Traeger M, Johnson B, Dickson J, McQuiston J, Swerdlow D. Rocky Mountain spotted fever in Arizona: documentation of heavy environmental infestations of Rhipicephalus sanguineus at an endemic site. Ann N Y Acad Sci. 2006;1078:338–341. doi: 10.1196/annals.1374.065. [DOI] [PubMed] [Google Scholar]
- Beeler E, Abramowicz KF, Zambrano ML, Sturgeon MM, Khalaf N, Hu R, Dasch GA, Eremeeva ME. A focus of dogs and Rickettsia massiliae-infected Rhipicephalus sanguineus in California. Am J Trop Med Hyg. 2011;84:244–249. doi: 10.4269/ajtmh.2011.10-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortinas R, Spomer S. Lone star tick (Acari: Ixodidae) occurrence in Nebraska: historical and current perspectives. J Med Entomol. 2013;50:244–251. doi: 10.1603/ME12207. [DOI] [PubMed] [Google Scholar]
- Pritt BS, Sloan LM, Johnson DK, Munderloh UG, Paskewitz SM, McElroy KM, McFadden JD, Binnicker MJ, Neitzel DF, Liu G, Nicholson WL, Nelson CM, Franson JJ, Martin SA, Cunningham SA, Steward CR, Bogumill K, Bjorgaard ME, Davis JP, McQuiston JH, Warshauer DM, Wilhelm MP, Patel R, Trivedi VA, Eremeeva ME. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. NEJM. 2011;365:422–429. doi: 10.1056/NEJMoa1010493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty BC, Maggi RG, Koskinen P, Beall MJ, Eberts M, Chandrashekar R, Breitschwerdt EB. Ehrlichia muris infection in a dog from Minnesota. JVIM. 2012;26:1217–1220. doi: 10.1111/j.1939-1676.2012.00968.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Anaplasmosis and ehrlichiosis - Maine, 2008. MMWR Morb Mortal Wkly Rep. 2009;58:1033–1036. [PubMed] [Google Scholar]
- Little SE, O’Connor TP, Hempstead JE, Saucier J, Reichard MV, Meinkoth K, Meinkoth JH, Andrews B, Ullom S, Ewing SA, Chandrashekar R. Ehrlichia ewingii infection and exposure rates in dogs from the southcentral United States. Vet Parasitol. 2010;172:355–360. doi: 10.1016/j.vetpar.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol. 2008;152:173–185. doi: 10.1016/j.vetpar.2007.12.030. [DOI] [PubMed] [Google Scholar]
- O’Connor TP, Esty KJ, Machenry P, Hanscom JL, Bartol BA, Lawton T. Performance evaluation of Ehrlichia canis and Borrelia burgdorferi peptides in a new Dirofilaria immitis combination assay. American Heartworm Society Triannual Symposium. 2002. pp. 77–84.
- O’Connor TP, Esty KJ, Hanscom JL, Shields P, Philipp MT. Dogs vaccinated with common Lyme disease vaccines do not respond to IR6, the conserved immunodominant region of the VlsE surface protein of Borrelia burgdorferi. Clin Diagn Lab Immunol. 2004;11:458–462. doi: 10.1128/CDLI.11.3.458-462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little SE. Ehrlichiosis and anaplasmosis in dogs and cats. Vet Clin North Am Small Anim Pract. 2010;40:1121–1140. doi: 10.1016/j.cvsm.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Walls JJ, Aguero-Rosenfeld M, Bakken JS, Goodman JL, Hossain D, Johnson RC, Dumler JS. Inter- and intralaboratory comparison of Ehrlichia equi and human granulocytic ehrlichiosis (HGE) agent strains for serodiagnosis of HGE by the immunofluorescent-antibody test. J Clin Microbiol. 1999;37:2968–2973. doi: 10.1128/jcm.37.9.2968-2973.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumler JS, Madigan JE, Pusterla N, Bakken JS. Ehrlichioses in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2007;Suppl 1:S45–S51. doi: 10.1086/518146. [DOI] [PubMed] [Google Scholar]
- Miro G, Montyoa A, Roura X, Glavez R, Sainz A. Seropositivity rates for agents of canine vector-borne diseases in Spain: a multicenter study. Parasit Vectors. 2013;6:117. doi: 10.1186/1756-3305-6-117. [DOI] [PMC free article] [PubMed] [Google Scholar]


