Abstract
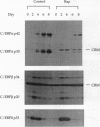
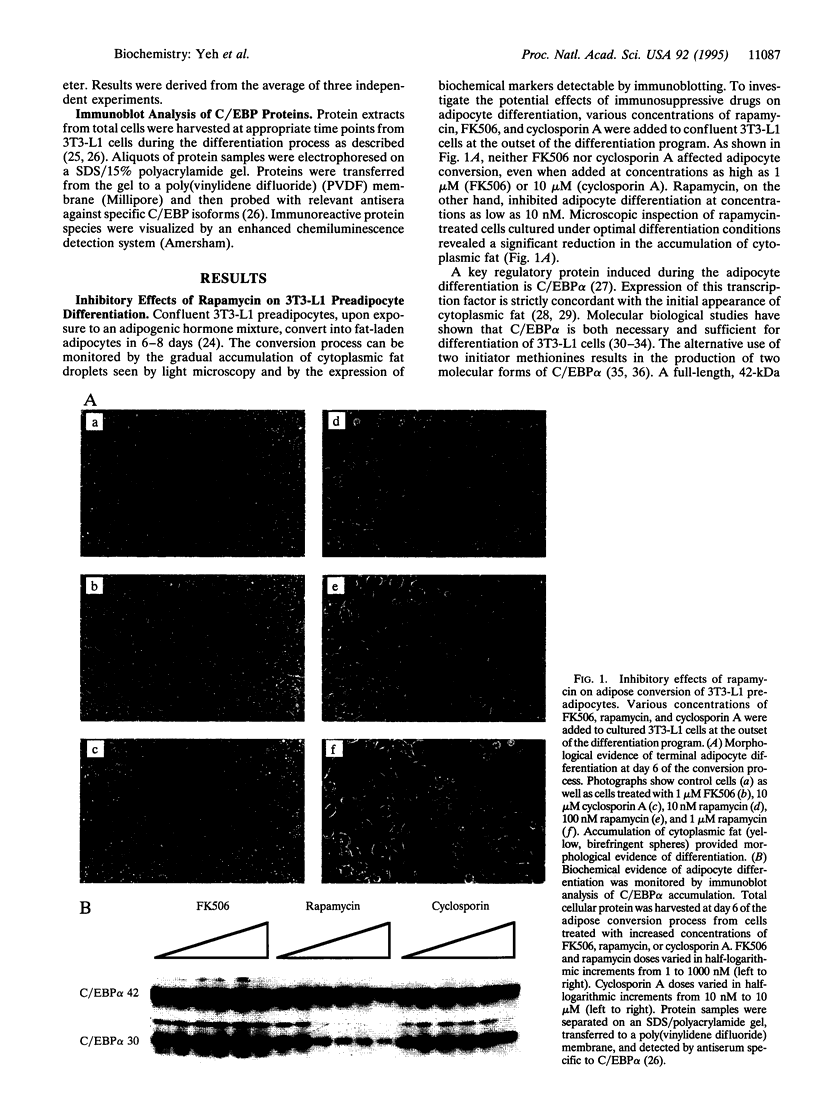
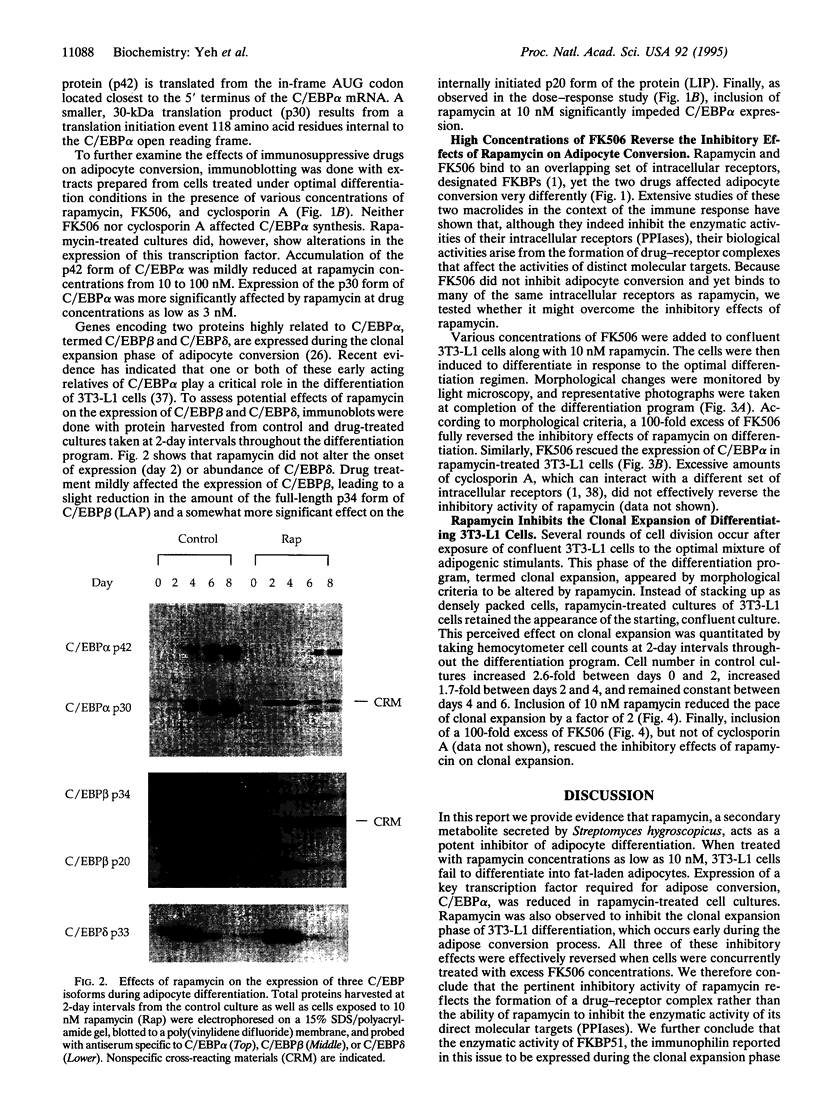
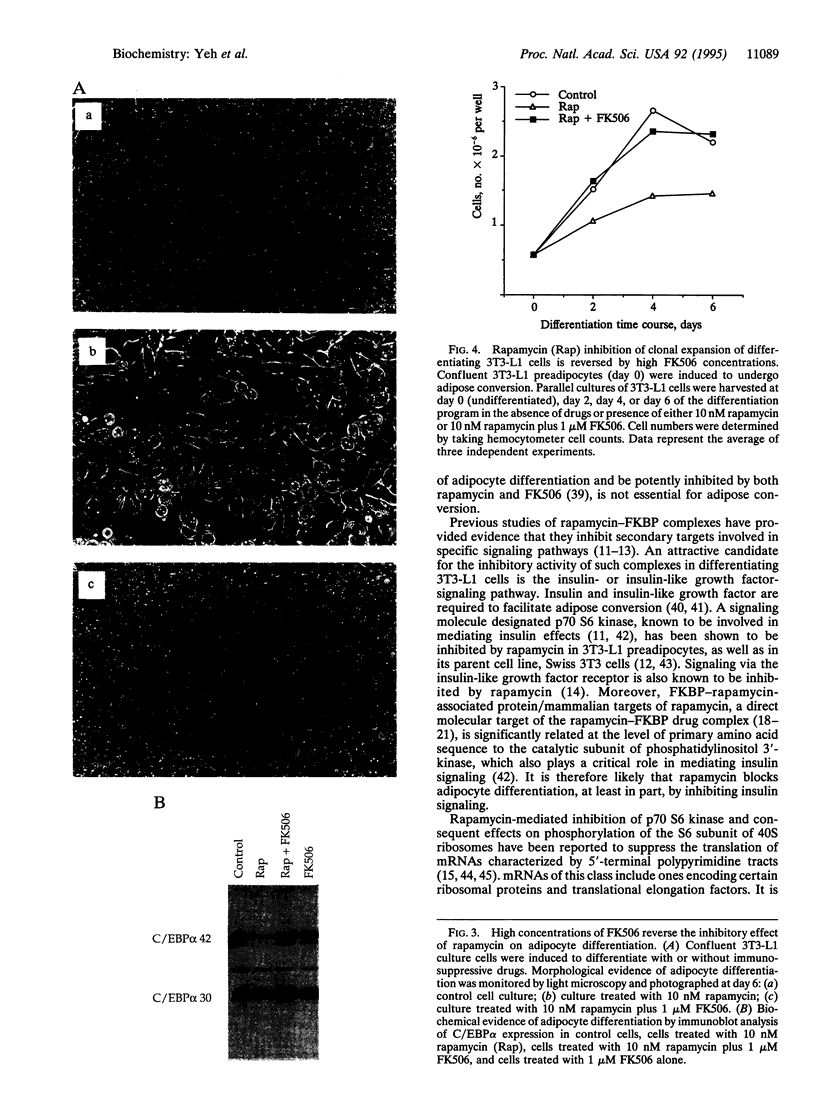
Differentiating 3T3-L1 cells express an immunophilin early during the adipocyte conversion program as described in this issue [Yeh, W.-C., Li, T.-K., Bierer, B. E. & McKnight, S. L. (1995) Proc. Natl. Acad. Sci. USA 92, 11081-11085]. The temporal expression profile of this protein, designated FK506-binding protein (FKBP) 51, is concordant with the clonal-expansion period undertaken by 3T3-L1 cells after exposure to adipogenic hormones. Having observed FKBP51 synthesis early during adipogenesis, we tested the effects of three immunosuppressive drugs--cyclosporin A, FK506, and rapamycin--on the terminal-differentiation process. Adipocyte conversion was not affected by either cyclosporin A or FK506 and yet was significantly reduced by rapamycin at drug concentrations as low as 10 nM. Clonal expansion was impeded in drug-treated cultures, as was the accumulation of cytoplasmic lipid droplets normally seen late during differentiation. Rapamycin treatment likewise inhibited the expression of CCAAT/enhancer binding protein alpha, a transcription factor required for 3T3-L1 cell differentiation. All three of these effects were reversed by high FK506 concentrations, indicating that the operative inhibitory event was mediated by an immunophilin-rapamycin complex.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aagaard-Tillery K. M., Jelinek D. F. Inhibition of human B lymphocyte cell cycle progression and differentiation by rapamycin. Cell Immunol. 1994 Jul;156(2):493–507. doi: 10.1006/cimm.1994.1193. [DOI] [PubMed] [Google Scholar]
- Albers M. W., Williams R. T., Brown E. J., Tanaka A., Hall F. L., Schreiber S. L. FKBP-rapamycin inhibits a cyclin-dependent kinase activity and a cyclin D1-Cdk association in early G1 of an osteosarcoma cell line. J Biol Chem. 1993 Oct 25;268(30):22825–22829. [PubMed] [Google Scholar]
- Bierer B. E. Cyclosporin A, FK506, and rapamycin: binding to immunophilins and biological action. Chem Immunol. 1994;59:128–155. [PubMed] [Google Scholar]
- Bierer B. E., Mattila P. S., Standaert R. F., Herzenberg L. A., Burakoff S. J., Crabtree G., Schreiber S. L. Two distinct signal transmission pathways in T lymphocytes are inhibited by complexes formed between an immunophilin and either FK506 or rapamycin. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9231–9235. doi: 10.1073/pnas.87.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer B. E., Somers P. K., Wandless T. J., Burakoff S. J., Schreiber S. L. Probing immunosuppressant action with a nonnatural immunophilin ligand. Science. 1990 Oct 26;250(4980):556–559. doi: 10.1126/science.1700475. [DOI] [PubMed] [Google Scholar]
- Birkenmeier E. H., Gwynn B., Howard S., Jerry J., Gordon J. I., Landschulz W. H., McKnight S. L. Tissue-specific expression, developmental regulation, and genetic mapping of the gene encoding CCAAT/enhancer binding protein. Genes Dev. 1989 Aug;3(8):1146–1156. doi: 10.1101/gad.3.8.1146. [DOI] [PubMed] [Google Scholar]
- Brown E. J., Albers M. W., Shin T. B., Ichikawa K., Keith C. T., Lane W. S., Schreiber S. L. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994 Jun 30;369(6483):756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- Cao Z., Umek R. M., McKnight S. L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991 Sep;5(9):1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- Chiu M. I., Katz H., Berlin V. RAPT1, a mammalian homolog of yeast Tor, interacts with the FKBP12/rapamycin complex. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12574–12578. doi: 10.1073/pnas.91.26.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J., Kuo C. J., Crabtree G. R., Blenis J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell. 1992 Jun 26;69(7):1227–1236. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- Cooper M. H., Gregory S. H., Starzl T. E., Wing E. J. Rapamycin but not FK506 inhibits the proliferation of mononuclear phagocytes induced by colony-stimulating factors. Transplantation. 1994 Feb;57(3):433–439. doi: 10.1097/00007890-199402150-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descombes P., Schibler U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell. 1991 Nov 1;67(3):569–579. doi: 10.1016/0092-8674(91)90531-3. [DOI] [PubMed] [Google Scholar]
- Dilling M. B., Dias P., Shapiro D. N., Germain G. S., Johnson R. K., Houghton P. J. Rapamycin selectively inhibits the growth of childhood rhabdomyosarcoma cells through inhibition of signaling via the type I insulin-like growth factor receptor. Cancer Res. 1994 Feb 15;54(4):903–907. [PubMed] [Google Scholar]
- Dumont F. J., Melino M. R., Staruch M. J., Koprak S. L., Fischer P. A., Sigal N. H. The immunosuppressive macrolides FK-506 and rapamycin act as reciprocal antagonists in murine T cells. J Immunol. 1990 Feb 15;144(4):1418–1424. [PubMed] [Google Scholar]
- Fingar D. C., Hausdorff S. F., Blenis J., Birnbaum M. J. Dissociation of pp70 ribosomal protein S6 kinase from insulin-stimulated glucose transport in 3T3-L1 adipocytes. J Biol Chem. 1993 Feb 5;268(4):3005–3008. [PubMed] [Google Scholar]
- Freytag S. O., Geddes T. J. Reciprocal regulation of adipogenesis by Myc and C/EBP alpha. Science. 1992 Apr 17;256(5055):379–382. doi: 10.1126/science.256.5055.379. [DOI] [PubMed] [Google Scholar]
- Freytag S. O., Paielli D. L., Gilbert J. D. Ectopic expression of the CCAAT/enhancer-binding protein alpha promotes the adipogenic program in a variety of mouse fibroblastic cells. Genes Dev. 1994 Jul 15;8(14):1654–1663. doi: 10.1101/gad.8.14.1654. [DOI] [PubMed] [Google Scholar]
- Friedman A. D., Landschulz W. H., McKnight S. L. CCAAT/enhancer binding protein activates the promoter of the serum albumin gene in cultured hepatoma cells. Genes Dev. 1989 Sep;3(9):1314–1322. doi: 10.1101/gad.3.9.1314. [DOI] [PubMed] [Google Scholar]
- Fruman D. A., Burakoff S. J., Bierer B. E. Immunophilins in protein folding and immunosuppression. FASEB J. 1994 Apr 1;8(6):391–400. doi: 10.1096/fasebj.8.6.7513288. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975 May;5(1):19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- Harding M. W., Galat A., Uehling D. E., Schreiber S. L. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature. 1989 Oct 26;341(6244):758–760. doi: 10.1038/341758a0. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Bye J. M. Density and cell cycle dependence of cell surface proteins in hamster fibroblasts. Cell. 1974 Oct;3(2):113–120. doi: 10.1016/0092-8674(74)90114-7. [DOI] [PubMed] [Google Scholar]
- Jayaraman T., Marks A. R. Rapamycin-FKBP12 blocks proliferation, induces differentiation, and inhibits cdc2 kinase activity in a myogenic cell line. J Biol Chem. 1993 Dec 5;268(34):25385–25388. [PubMed] [Google Scholar]
- Jefferies H. B., Reinhard C., Kozma S. C., Thomas G. Rapamycin selectively represses translation of the "polypyrimidine tract" mRNA family. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4441–4445. doi: 10.1073/pnas.91.10.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. F., Landschulz W. H., Graves B. J., McKnight S. L. Identification of a rat liver nuclear protein that binds to the enhancer core element of three animal viruses. Genes Dev. 1987 Apr;1(2):133–146. doi: 10.1101/gad.1.2.133. [DOI] [PubMed] [Google Scholar]
- Kunz J., Henriquez R., Schneider U., Deuter-Reinhard M., Movva N. R., Hall M. N. Target of rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression. Cell. 1993 May 7;73(3):585–596. doi: 10.1016/0092-8674(93)90144-f. [DOI] [PubMed] [Google Scholar]
- Levy S., Avni D., Hariharan N., Perry R. P., Meyuhas O. Oligopyrimidine tract at the 5' end of mammalian ribosomal protein mRNAs is required for their translational control. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3319–3323. doi: 10.1073/pnas.88.8.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. T., Lane M. D. Antisense CCAAT/enhancer-binding protein RNA suppresses coordinate gene expression and triglyceride accumulation during differentiation of 3T3-L1 preadipocytes. Genes Dev. 1992 Apr;6(4):533–544. doi: 10.1101/gad.6.4.533. [DOI] [PubMed] [Google Scholar]
- Lin F. T., Lane M. D. CCAAT/enhancer binding protein alpha is sufficient to initiate the 3T3-L1 adipocyte differentiation program. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8757–8761. doi: 10.1073/pnas.91.19.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. T., MacDougald O. A., Diehl A. M., Lane M. D. A 30-kDa alternative translation product of the CCAAT/enhancer binding protein alpha message: transcriptional activator lacking antimitotic activity. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9606–9610. doi: 10.1073/pnas.90.20.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morice W. G., Brunn G. J., Wiederrecht G., Siekierka J. J., Abraham R. T. Rapamycin-induced inhibition of p34cdc2 kinase activation is associated with G1/S-phase growth arrest in T lymphocytes. J Biol Chem. 1993 Feb 15;268(5):3734–3738. [PubMed] [Google Scholar]
- Morice W. G., Wiederrecht G., Brunn G. J., Siekierka J. J., Abraham R. T. Rapamycin inhibition of interleukin-2-dependent p33cdk2 and p34cdc2 kinase activation in T lymphocytes. J Biol Chem. 1993 Oct 25;268(30):22737–22745. [PubMed] [Google Scholar]
- Ossipow V., Descombes P., Schibler U. CCAAT/enhancer-binding protein mRNA is translated into multiple proteins with different transcription activation potentials. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8219–8223. doi: 10.1073/pnas.90.17.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D. J., Grove J. R., Calvo V., Avruch J., Bierer B. E. Rapamycin-induced inhibition of the 70-kilodalton S6 protein kinase. Science. 1992 Aug 14;257(5072):973–977. doi: 10.1126/science.1380182. [DOI] [PubMed] [Google Scholar]
- Sabatini D. M., Erdjument-Bromage H., Lui M., Tempst P., Snyder S. H. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994 Jul 15;78(1):35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- Sabers C. J., Martin M. M., Brunn G. J., Williams J. M., Dumont F. J., Wiederrecht G., Abraham R. T. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. J Biol Chem. 1995 Jan 13;270(2):815–822. doi: 10.1074/jbc.270.2.815. [DOI] [PubMed] [Google Scholar]
- Samuelsson L., Strömberg K., Vikman K., Bjursell G., Enerbäck S. The CCAAT/enhancer binding protein and its role in adipocyte differentiation: evidence for direct involvement in terminal adipocyte development. EMBO J. 1991 Dec;10(12):3787–3793. doi: 10.1002/j.1460-2075.1991.tb04948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S. L. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991 Jan 18;251(4991):283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- Siekierka J. J., Hung S. H., Poe M., Lin C. S., Sigal N. H. A cytosolic binding protein for the immunosuppressant FK506 has peptidyl-prolyl isomerase activity but is distinct from cyclophilin. Nature. 1989 Oct 26;341(6244):755–757. doi: 10.1038/341755a0. [DOI] [PubMed] [Google Scholar]
- Smith P. J., Wise L. S., Berkowitz R., Wan C., Rubin C. S. Insulin-like growth factor-I is an essential regulator of the differentiation of 3T3-L1 adipocytes. J Biol Chem. 1988 Jul 5;263(19):9402–9408. [PubMed] [Google Scholar]
- Student A. K., Hsu R. Y., Lane M. D. Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J Biol Chem. 1980 May 25;255(10):4745–4750. [PubMed] [Google Scholar]
- Terada N., Patel H. R., Takase K., Kohno K., Nairn A. C., Gelfand E. W. Rapamycin selectively inhibits translation of mRNAs encoding elongation factors and ribosomal proteins. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11477–11481. doi: 10.1073/pnas.91.24.11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. F., Kahn C. R. The insulin signaling system. J Biol Chem. 1994 Jan 7;269(1):1–4. [PubMed] [Google Scholar]
- Yeh W. C., Cao Z., Classon M., McKnight S. L. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 1995 Jan 15;9(2):168–181. doi: 10.1101/gad.9.2.168. [DOI] [PubMed] [Google Scholar]
- Yeh W. C., Li T. K., Bierer B. E., McKnight S. L. Identification and characterization of an immunophilin expressed during the clonal expansion phase of adipocyte differentiation. Proc Natl Acad Sci U S A. 1995 Nov 21;92(24):11081–11085. doi: 10.1073/pnas.92.24.11081. [DOI] [PMC free article] [PubMed] [Google Scholar]