Abstract
Background
We retrospectively evaluated the relationship between cytokine gene polymorphisms and development of postoperative pneumonia after esophagectomy.
Methods
In 120 patients who underwent esophagectomy, serum samples were obtained to measure levels of serum interleukin (IL)-6 and IL-10 at four time points (preoperatively, postoperative day (POD)0, POD1, and POD3). DNA extracted from peripheral blood in all patients was analyzed to determine polymorphisms of cytokines such as tumor necrosis factor-α -1031 T/C, IL-1β -511C/T, IL-6 -634C/G, and IL-10 -819 T/C.
Results
Postoperative pneumonia arose in 34 patients (28.3 %). Perioperative serum IL-10 levels were significantly higher for IL-10 -819 C/T + C/C genotypes than for T/T genotypes (POD0 16.7 ± 2.84 vs. 8.54 ± 0.87 pg/ml, p = 0.0002; POD1 14.0 ± 2.64 vs. 8.8 ± 0.87 pg/ml, p = 0.0143; POD3 8.9 ± 2.67 vs. 4.4 ± 0.52 pg/ml, p = 0.0076). The frequency of the IL-10 -819 T/T genotype was significantly higher in patients with postoperative pneumonia than in patients without pneumonia (p = 0.0323). Multivariate analysis of factors such as sex, smoking, length of operation, field of lymph node dissection, and IL-10 polymorphism identified IL-10 polymorphism as independent predictor of postoperative pneumonia.
Conclusions
Patients with IL-10 -819 T/T genotype may be at high risk for postoperative pneumonia after esophagectomy.
Keywords: IL-10 polymorphism, Postoperative pneumonia, Esophagectomy
Introduction
Surgery for esophageal cancer is one of the most invasive gastrointestinal surgeries,1, 2 and perioperative mortality rates remain in the range of 3–10 %.3 – 5 In the case of esophageal cancer, marked elevations in cytokine levels are observed perioperatively, inducing a systemic inflammatory response syndrome (SIRS).6, 7 Such hypercytokinemia produces excessive stress and may trigger postoperative complications.8 The lungs is one of the main organs of neutrophil sequestration under conditions of SIRS.9 – 12 Abe et al. reported that transthoracic esophagectomy causes an increase in interleukin (IL)-6 production from bronchial and alveolar epithelial cells lining the airway, and local response of lung tissue may be one source of increased serum IL-6 after esophagectomy in humans.13 The clinical effects of an immune response are orchestrated by numerous pro-inflammatory, inflammatory, and anti-inflammatory cytokines. Tumor necrosis factor (TNF)-α is a major mediator and amplifier of immune responses to infectious challenges. Azim et al. recently reported that the TNF-α -308 polymorphism contributes to infectious complications after esophagectomy.14 Moreover, associations between infection and genetic polymorphisms have been reported for other cytokines, including IL-1β, IL-6, and IL-10.15 – 18 These findings suggest that patient genotype may influence susceptibility to postoperative infection.
We have a considerable interest in whether factors such as these polymorphisms might affect the postoperative infection, particularly in terms of the effects on postoperative pneumonia. We selected TNF-α, IL-1β, IL-6, and IL-10 as representative pro-inflammatory, inflammatory, and anti-inflammatory cytokines and investigated the gene promoter polymorphisms of TNF-α -1031 T > C (rs1799964), IL-1β -511 T > C (rs3087258), IL-6 -634G > C (rs1800796), and IL-10 -819 T > C (rs1800871); all of which have been reported to influence cytokine production.18 – 22 The purpose of this study was to assess whether cytokine promoter gene polymorphisms are associated with the following: (1) perioperative cytokine production and (2) postoperative pneumonia following esophagectomy.
Methods
Patients
This was a retrospective cohort study that included patients treated for esophageal cancer between 1997 and 2011. Participants comprised 120 consecutive Japanese patients (105 men and 15 women) with thoracic esophageal cancer who underwent curative esophagectomy and reconstruction with gastric mobilization via a posterior mediastinal or retrosternal route by right posterolateral thoracotomy and laparotomy consecutively in the Department of Digestive Surgery and Surgical Oncology at Yamaguchi University Graduate School of Medicine. These patients underwent dissection of two (mediastinal and abdominal) or three (bilateral neck, mediastinal, and abdominal) lymph node fields. Patients who received neoadjuvant treatment or underwent reconstruction with small intestine or colon mobilization were excluded. We introduced thoracoscopic procedures in a prone position from 2008 and excluded these procedures from the present study. In all cases, diagnoses of esophageal squamous cell cancer were confirmed preoperatively on the basis of histopathological reports. The clinicopathological definition of thoracic esophageal cancer was based on the International Union against Cancer tumor-node-metastasis (TNM) classification of malignant tumors (6th edition). Written informed consent was obtained from all study patients. The study protocol was approved by the Institutional Review Board for the Use of Human Subjects at Yamaguchi University Graduate School of Medicine.
Perioperative Management
We performed perioperative management as follows. At the first visit to our hospital, all patients were instructed to quit smoking and respiratory rehabilitation was started at the same time. As prophylactic antibiotics, patients received cefazolin 1 g by intravenous infusion for 30 min before the operation. An additional dose was administered if the operation was prolonged beyond 3 h. Patients received the same antibiotics plus further treatment at 12-h intervals, for a total of 5 days. For nutritional support, enteral nutrition was provided via jejunostomy from 6 h postoperatively, and the dose was increased step-by-step to 2,000 kcal/day up to postoperative day (POD) 7. All patients were admitted to the intensive care unit and placed on prophylactic mechanical ventilation. The timing of extubation was decided according to the results of bronchoscopy, blood gas analysis, and chest radiography. The criteria for extubation were as follows: (1) arterial oxygen pressure >100 mmHg with inspired fraction of oxygen (FiO2) 0.4 and (2) absence of atelectasis on bronchoscopic view or radiography.
Postoperative Complications
Postoperative complications were defined as follows. Postoperative pneumonia was diagnosed by the presence of pyrexia >38 °C within 2 weeks postoperatively and either positive sputum cultures or clear clinical or radiological evidence of consolidation. Anastomotic leakage was diagnosed by gastrography and clinical features. Postoperative wound infection was defined as a systemic response to the presence of an infectious agent supported by clinical and laboratory evidence, where laboratory evidence means positive culture results. Cardiac complications including arrhythmia, angina, or heart failure were diagnosed by a cardiologist.
Serum Cytokines
Serum samples were obtained at four time points (preoperatively, end of the operation (POD0), POD1, and POD3) and stored at −80 °C until use. Serum IL-6 and IL-10 levels were measured using commercially available enzyme-linked immunosorbent assay kits (BioSource International, Camarillo, CA). Samples were prepared and tested in duplicate according to the protocol recommended by the manufacturer.
DNA Specimens and Genotyping
For DNA analysis, 7 ml of peripheral blood was obtained from all patients. DNA was isolated by a conventional NaI method and stored at 4 °C.23 All polymorphisms were identified with the tetra-primer amplification refractory mutation system-polymerase chain reaction (ARMS-PCR), and details of primers and PCR conditions have been described in the literature.24 – 26
Statistical Analysis
Data are expressed as mean values±standard error and were analyzed using the Mann-Whitney U test. Categorical data were analyzed using the χ2 test or Fisher’s exact test. Differences in genotype frequency were analyzed using the χ2 test or Fisher’s exact test of independence, with which, each of the genotype frequencies was evaluated to determine whether it was consistent with expected Hardy-Weinberg proportions. Because homozygotes for the rare allele were too few to perform a 2 × 3 χ2 test or Fisher’s exact test, homozygotes of the dominant alleles and variant carriers were compared by 2 × 2 test. Variables with a p < 0.2 in the univariate analysis that were potentially predictive of postoperative pneumonia were then entered into the multivariate logistic regression model. Odds ratio (OR) and 95 % confidence interval (CI) were also calculated. A value of p < 0.05 was considered statistically significant. All analyses were performed using StatView version 5.0 statistical software (SAS Institute, Cary, NC).
Results
In all 120 patients studied, the frequency of postoperative pneumonia was 28.3 % (34 of 120 patients). In terms of other complications, 14 patients (11.7 %) developed wound infection, 8 patients (6.7 %) developed anastomotic leakage, and 25 patients (20.8 %) developed cardiac complications. Perioperative patient- and tumor-related factors and other complications were compared between groups with and without postoperative pneumonia (Table 1). The proportion of males was significantly higher among patients with pneumonia (97.1 %) than among patients without pneumonia (82.6 %; p = 0.0384). The proportions of smoking, 3-field lymph node dissection, and the length of operation tended to be higher among patients with pneumonia than among patients without pneumonia (p = 0.147, p = 0.102, and p = 0.0679, respectively).
Table 1.
Patient characteristics in the two groups
| Pneumonia + (n = 34) | Pneumonia − (n = 86) | p value | |
|---|---|---|---|
| Age | 63.7 ± 8.9 | 63.0 ± 9.2 | 0.337 |
| Gender | |||
| Male | 33 | 71 | |
| Female | 01 | 15 | 0.0384 |
| Body mass index (kg/m2) | 21.0 ± 3.0 | 21.1 ± 3.0 | 0.954 |
| Smoking | |||
| Yes | 32 | 71 | |
| No | 02 | 15 | 0.147 |
| FEV1.0 % | 73.2 ± 12.2 | 73.1 ± 8.8 | 0.592 |
| Preoperative CRP (mg/dl) | 0.82 ± 2.4 | 0.32 ± 0.47 | 0.765 |
| Tumor locations | |||
| Upper | 04 | 09 | |
| Middle | 21 | 44 | |
| Lower | 09 | 33 | 0.465 |
| UICC stage (pathological) | |||
| I and II | 21 | 50 | |
| III and IV | 13 | 36 | 0.716 |
| Length of operation (min) | 463 ± 100 | 433 ± 99 | 0.0679 |
| Blood loss (g) | 820 ± 913 | 548 ± 267 | 0.283 |
| Field of lymph node dissection | |||
| 2 (mediastinum and abdomen) | 22 | 68 | |
| 3 (bilateral neck, mediastinum, and abdomen) | 12 | 18 | 0.102 |
| Wound infection | |||
| Yes | 5 | 09 | |
| No | 29 | 77 | 0.536 |
| Anastomotic leakage | |||
| Yes | 03 | 05 | |
| No | 31 | 81 | 0.686 |
| Cardiac complications | |||
| Yes | 09 | 16 | |
| No | 25 | 70 | 0.339 |
Data are presented as mean+standard error or absolute numbers
FEV forced expiratory volume, CRP C-reactive protein, UICC International Union Against Cancer
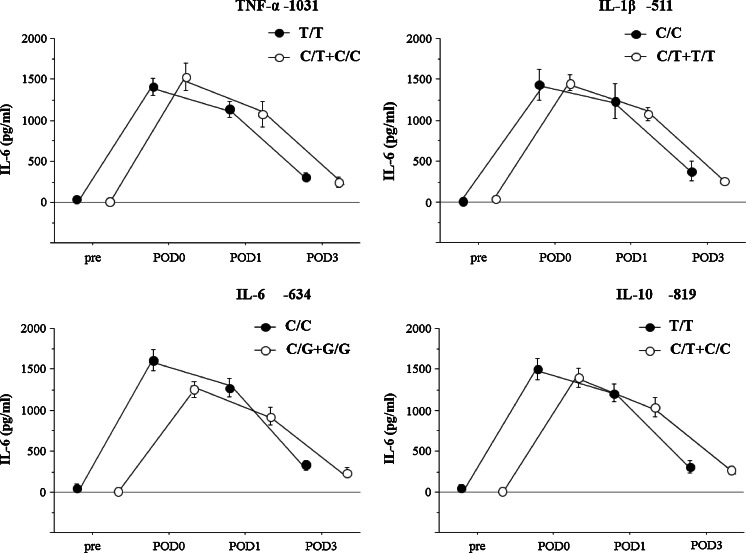
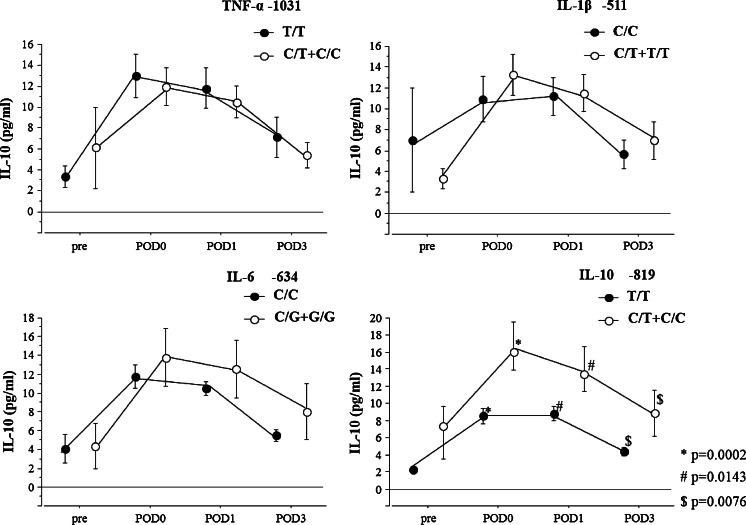
No relationship was seen between perioperative serum IL-6 levels and polymorphisms (Fig. 1). Perioperative serum IL-10 levels were significantly higher for IL-10 -819 C/T+C/C genotypes than for T/T genotypes (POD0 16.7 ± 2.84 vs. 8.54 ± 0.87 pg/ml, p = 0.0002; POD1 14.0 ± 2.64 vs. 8.8 ± 0.87 pg/ml, p = 0.0143; POD3 8.9 ± 2.69 vs. 4.4 ± 0.52 pg/ml, p = 0.0076) (Fig. 2).
Fig 1.
Pre- and postoperative serum levels of interleukin (IL)-6. The relationship between polymorphism of the cytokine genes (tumor necrosis factor (TNF)-α -1031 T/C, IL-1β -511C/T, IL-6 -634C/G, IL-10 -819 T/C) and IL-6 level in 120 patients after esophagectomy. Solid circles indicate wild-type polymorphism, open circles indicate variant-type polymorphism. Values are expressed as mean±standard error. Statistically significant according to the Mann-Whitney U test. IL interleukin, TNF tumor necrosis factor, POD postoperative day
Fig 2.
Pre- and postoperative serum levels of interleukin (IL)-10. The relationship between polymorphism of cytokine genes (tumor necrosis factor (TNF)-α -1031 T/C, IL-1β -511C/T, IL-6 -634C/G, IL-10 -819 T/C) and IL-10 level in 120 patients after esophagectomy. Solid circles indicate wild-type polymorphism, open circles indicate variant-type polymorphism. Values are expressed as mean±standard error. Statistically significant according to the Mann-Whitney U test. * p = 0.0002; # p = 0.0143; $ p = 0.0076. IL interleukin, TNF tumor necrosis factor, POD postoperative day
Among the cytokine promoter gene polymorphisms analyzed, a significant association with pneumonia was found for IL-10 -819 T/C. The frequency of the IL-10 -819 T/T genotype was significantly higher in patients with pneumonia than in those without pneumonia (p = 0.0323) (Table 2).
Table 2.
Genotype distribution in the postoperative pneumonia
| Cytokine polymorphism | Pneumonia + (n = 34) | Pneumonia − (n = 86) | p value | |
|---|---|---|---|---|
| TNF-α -1031 | T/T | 21 | 62 | 0.27 |
| T/C + C/C | 13 | 24 | ||
| IL-1β -511 | C/C | 08 | 26 | 0.463 |
| C/T+T/T | 26 | 60 | ||
| IL-6 -634 | G/G | 20 | 47 | 0.678 |
| G/C+C/C | 14 | 39 | ||
| IL-10 -819 | T/T | 22 | 37 | 0.0323 |
| T/C+C/C | 12 | 49 | ||
TNF tumor necrosis factor, IL interleukin
Although no relationship was seen between postoperative serum IL-10 levels and Il-10 polymorphisms in patients with pneumonia, these levels were significantly higher for IL-10 -819 T/C + C/C genotypes than for T/T genotypes in patients without pneumonia (Table 3).
Table 3.
Trend in serum IL-10 levels according to IL-10 -819 polymorphism with and without pneumonia
| Pneumonia ± (n = 34) | p value | Pneumonia − (n = 86) | p value | |||
|---|---|---|---|---|---|---|
| T/T (n = 22) | T/C±C/C (n = 12) | T/T (n = 37) | T/C±C/C (n = 49) | |||
| Serum IL-10 level (pg/ml) | ||||||
| POD0 | 9.5 ± 1.6 | 17.4 ± 5.3 | 0.65 | 8.0 ± 1.0 | 16.5 ± 3.3 | <0.01 |
| POD1 | 10.1 ± 1.8 | 10.9 ± 4.0 | 0.62 | 8.0 ± 0.9 | 14.8 ± 3.2 | <0.01 |
| POD3 | 5.3 ± 0.9 | 6.2 ± 3.0 | 0.77 | 3.8 ± 0.6 | 9.6 ± 3.3 | <0.01 |
Data are presented as mean±standard error
IL interleukin, POD postoperative day
Clinicopathological variables, including sex (male vs. female), smoking (yes vs. no), length of operation, field of lymph node dissection (three vs. two), and IL-10 -819 polymorphism (T/T vs. C/T + C/C) were entered into a multivariate logistic regression model to identify factors influencing pneumonia (Table 4). Only IL-10 -819 polymorphism was significantly associated with pneumonia (p = 0.0334, OR = 2.68, 95 % CI = 1.08–6.67).
Table 4.
Logistic regression predicting development of postoperative pneumonia
| OR | 95 % CI | p value | |
|---|---|---|---|
| Gender (male vs. female) | 4.64 | 0.491–43.9 | 0.18 |
| Smoking (yes vs. no) | 1.35 | 0.235–7.72 | 0.738 |
| Length of operation (min) | 1.002 | 0.998–1.007 | 0.276 |
| Field of lymph node dissection (3 vs. 2) | 1.43 | 0.516–3.97 | 0.491 |
| IL-10 Genotype (T/T vs. C/T+C/C) | 2.68 | 1.08–6.67 | 0.0334 |
OR odds ratios, CI confidence interval, IL interleukin
Discussion
This study revealed that IL-10 -819 polymorphism is associated with postoperative pneumonia in Japanese patients with esophageal cancer. The IL-10 -819 TT genotype is related to lower perioperative production of serum IL-10 level. No associations with any other cytokine polymorphism for TNF-α, IL-1β, or IL-6 were evident. Presence of the IL-10 -819 TT genotype seems to confer lower IL-10 release in response to surgical stress and an increased risk of postoperative pneumonia.
Under highly inflammatory conditions such as following esophagectomy, a genotype associated with high anti-inflammatory response may be protective against the development of postoperative respiratory inflammation. IL-10 is a potent endogenous anti-inflammatory cytokine that decreases lung inflammation, partly on the basis of TNF-α and IL-1β.27 Alveolar macrophages produce significant amounts of IL-10.28, 29 Morita reported that IL-10 production after rat thoracotomy was higher from the collapsed lung than from the uncollapsed lung, possibly reflecting suppression of IL-6 production from the collapsed lung.30 These findings are supported by the clinical observation that decreased IL-10 concentration in bronchoalveolar lavage fluid is associated with worse outcomes in patients with the acute respiratory distress syndrome.31 IL-10 therapy increased survival both in a rabbit Pseudomonas aeruginosa pneumonia model and in a murine pneumococcal pneumonia model.32 Those results indicate that IL-10 is a key regulator of the degree of inflammation in the setting of acute lung infection or inflammation.
The current finding that the high IL-10-producing -819C allele is protective against postoperative respiratory inflammation following esophagectomy is consistent with the role of intense inflammation. Lowe et al. reported that the IL-10 -592 CC polymorphism was associated with higher IL-10 release under lipopolysaccharide stimulation and lower mortality in critically ill patients.20 Similar frequencies were obtained for -819 T/C and -592 A/C in all types of participants, indicating complete linkage.33 Those findings are consistent with our own results. However, in critically ill patients with sepsis, the mortality rate was significantly higher in patients with the -592 CC genotype than in those with the -592 AA genotype.33 The overall balance between pro- and anti-inflammatory responses is important in response to injury or surgery. In patients with sepsis, a genotype associated with high IL-10 production may lead to greater immunosuppression, resulting in more severe disease burden and outcomes. In contrast, the present findings were obtained following esophagectomy. In such cases, a genotype associated with high IL-10 may have beneficial modulating effects. The notion that a high IL-10-producing -819 C allele may not be universally detrimental appears reasonable.
Azim et al. reported that not IL-10 -819 T/C but TNF-α-308G/A polymorphism contributes to infectious complications after esophagectomy.14 That result differs from the findings of the present study. However, the frequency of the -308 A allele for TNF-α is much lower in Japanese populations (1.1–1.3 %) than in Caucasians (10–18 %).33 – 36 Furthermore, the frequency of the -819 C allele of IL-10 in Japanese (30.5–33.6 %) was lower than in Caucasians (79–82 %).20, 33, 37 These results suggest that considering the influence of ethnic differences in gene polymorphism may be important. Further studies on gene polymorphisms in different ethnicities are needed to allow tailoring of specific therapies. Identifying IL-10 polymorphisms in patients prior to esophagectomy may influence management decisions where controversy currently exists, such as the use of other therapeutic strategies. For example, whether steroids should be used to treat patients perioperatively remains unresolved.38 Some patients may benefit, while others may not, depending on individual genotypes. Genotyping this IL-10 polymorphism in patients following esophagectomy may allow better risk stratification and tailoring of specific therapies to different risk groups.
Acknowledgments
Conflict of Interest
There are no conflicts of interest.
References
- 1.Haga Y, Beppu T, Doi K, et al. Systemic inflammatory response syndrome and organ dysfunction following gastrointestinal surgery. Critical care medicine. 1997;25(12):1994–2000. doi: 10.1097/00003246-199712000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Ono S, Aosasa S, Mochizuki H. Effects of a protease inhibitor on reduction of surgical stress in esophagectomy. Am J Surg. 1999;177(1):78–82. doi: 10.1016/S0002-9610(98)00300-6. [DOI] [PubMed] [Google Scholar]
- 3.Poon RT, Law SY, Chu KM, Branicki FJ, Wong J. Esophagectomy for carcinoma of the esophagus in the elderly: results of current surgical management. Annals of surgery. 1998;227(3):357–64. doi: 10.1097/00000658-199803000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey SH, Bull DA, Harpole DH, et al. Outcomes after esophagectomy: a ten-year prospective cohort. Ann Thorac Surg. 2003;75(1):217–22. doi: 10.1016/S0003-4975(02)04368-0. [DOI] [PubMed] [Google Scholar]
- 5.Atkins BZ, Shah AS, Hutcheson KA, et al. Reducing hospital morbidity and mortality following esophagectomy. Ann Thorac Surg. 2004;78(4):1170–6. doi: 10.1016/j.athoracsur.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 6.Maeba T, Maeta H, Usuki H, et al. Increase in portal blood interleukin-6 soon after the commencement of digestive surgery. Surg Today. 1996;26(11):890–4. doi: 10.1007/BF00311790. [DOI] [PubMed] [Google Scholar]
- 7.Hisano S, Sakamoto K, Ishiko T, Kamohara H, Ogawa M. IL-6 and soluble IL-6 receptor levels change differently after surgery both in the blood and in the operative field. Cytokine. 1997;9(6):447–52. doi: 10.1006/cyto.1996.0187. [DOI] [PubMed] [Google Scholar]
- 8.Roumen RM, Hendriks T, van der Ven-Jongekrijg J, et al. Cytokine patterns in patients after major vascular surgery, hemorrhagic shock, and severe blunt trauma. Relation with subsequent adult respiratory distress syndrome and multiple organ failure. Annals of surgery. 1993;218(6):769–76. doi: 10.1097/00000658-199312000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitagawa Y, Van Eeden SF, Redenbach DM, et al. Effect of mechanical deformation on structure and function of polymorphonuclear leukocytes. J Appl Physiol. 1997;82(5):1397–405. doi: 10.1152/jappl.1997.82.5.1397. [DOI] [PubMed] [Google Scholar]
- 10.Lee WL, Downey GP. Neutrophil activation and acute lung injury. Curr Opin Crit Care. 2001;7(1):1–7. doi: 10.1097/00075198-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Razavi HM, Wang le F, Weicker S, et al. Pulmonary neutrophil infiltration in murine sepsis: role of inducible nitric oxide synthase. American journal of respiratory and critical care medicine 2004; 170(3): 227-33. [DOI] [PubMed]
- 12.Lin X, Yang H, Sakuragi T, et al. Alpha-chemokine receptor blockade reduces high mobility group box 1 protein-induced lung inflammation and injury and improves survival in sepsis. Am J Physiol Lung Cell Mol Physiol. 2005;289(4):L583–90. doi: 10.1152/ajplung.00091.2005. [DOI] [PubMed] [Google Scholar]
- 13.Abe T, Oka M, Tangoku A, et al. Interleukin-6 production in lung tissue after transthoracic esophagectomy. J Am Coll Surg. 2001;192(3):322–9. doi: 10.1016/S1072-7515(00)00805-X. [DOI] [PubMed] [Google Scholar]
- 14.Azim K, McManus R, Brophy K, et al. Genetic polymorphisms and the risk of infection following esophagectomy. positive association with TNF-alpha gene -308 genotype. Annals of surgery. 2007;246(1):122–8. doi: 10.1097/01.sla.0000259389.09161.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang YH, Lee DS, Jo HS, et al. Tumor necrosis factor alpha promoter polymorphism associated with increased susceptibility to secondary hemophagocytic lymphohistiocytosis in the Korean population. Cytokine 2006; 36(1-2): 45-50. [DOI] [PubMed]
- 16.Allen ML, Hoschtitzky JA, Peters MJ, et al. Interleukin-10 and its role in clinical immunoparalysis following pediatric cardiac surgery. Critical care medicine. 2006;34(10):2658–65. doi: 10.1097/01.CCM.0000240243.28129.36. [DOI] [PubMed] [Google Scholar]
- 17.Sainz J, Perez E, Gomez-Lopera S, et al. Genetic variants of IL6 gene promoter influence on C-reactive protein levels but are not associated with susceptibility to invasive pulmonary aspergillosis in haematological patients. Cytokine. 2008;41(3):268–78. doi: 10.1016/j.cyto.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe E, Hirasawa H, Oda S, et al. Cytokine-related genotypic differences in peak interleukin-6 blood levels of patients with SIRS and septic complications. J Trauma. 2005;59(5):1181–9. doi: 10.1097/00005373-200511000-00025. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari SL, Ahn-Luong L, Garnero P, Humphries SE, Greenspan SL. Two promoter polymorphisms regulating interleukin-6 gene expression are associated with circulating levels of C-reactive protein and markers of bone resorption in postmenopausal women. J Clin Endocrinol Metab. 2003;88(1):255–9. doi: 10.1210/jc.2002-020092. [DOI] [PubMed] [Google Scholar]
- 20.Lowe PR, Galley HF, Abdel-Fattah A, Webster NR. Influence of interleukin-10 polymorphisms on interleukin-10 expression and survival in critically ill patients. Critical care medicine. 2003;31(1):34–8. doi: 10.1097/00003246-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Silkov AN, Sennikova NS, Goreva EP, Lopatnikova YA, Sennikov SV. Production of TNF-alpha and IL-1beta by peripheral blood mononuclear cells in carriers of different allele variants of the gene. Bull Exp Biol Med. 2012;153(1):68–71. doi: 10.1007/s10517-012-1646-3. [DOI] [PubMed] [Google Scholar]
- 22.Soga Y, Nishimura F, Ohyama H, et al. Tumor necrosis factor-alpha gene (TNF-alpha) -1031/-863, -857 single-nucleotide polymorphisms (SNPs) are associated with severe adult periodontitis in Japanese. J Clin Periodontol. 2003;30(6):524–31. doi: 10.1034/j.1600-051X.2003.00287.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Hirayasu K, Ishizawa M, Kobayashi Y. Purification of genomic DNA from human whole blood by isopropanol-fractionation with concentrated Nal and SDS. Nucleic Acids Res. 1994;22(9):1774–5. doi: 10.1093/nar/22.9.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamajima N, Saito T, Matsuo K, et al. Polymerase chain reaction with confronting two-pair primers for polymorphism genotyping. Jpn J Cancer Res. 2000;91(9):865–8. doi: 10.1111/j.1349-7006.2000.tb01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye S, Dhillon S, Ke X, Collins AR, Day IN. An efficient procedure for genotyping single nucleotide polymorphisms. Nucleic Acids Res. 2001;29(17):E88–8. doi: 10.1093/nar/29.17.e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okayama N, Fujimura K, Nakamura J, et al. Evaluation of a new efficient procedure for single-nucleotide polymorphism genotyping: tetra-primer amplification refractory mutation system-polymerase chain reaction. Clin Chem Lab Med. 2004;42(1):13–6. doi: 10.1515/CCLM.2004.004. [DOI] [PubMed] [Google Scholar]
- 27.Thomassen MJ, Divis LT, Fisher CJ. Regulation of human alveolar macrophage inflammatory cytokine production by interleukin-10. Clin Immunol Immunopathol. 1996;80(3 Pt 1):321–4. doi: 10.1006/clin.1996.0130. [DOI] [PubMed] [Google Scholar]
- 28.Wilkes DS, Neimeier M, Mathur PN, et al. Effect of human lung allograft alveolar macrophages on IgG production: immunoregulatory role of interleukin-10, transforming growth factor-beta, and interleukin-6. Am J Respir Cell Mol Biol. 1995;13(5):621–8. doi: 10.1165/ajrcmb.13.5.7576699. [DOI] [PubMed] [Google Scholar]
- 29.Toossi Z, Hirsch CS, Hamilton BD, et al. Decreased production of TGF-beta 1 by human alveolar macrophages compared with blood monocytes. J Immunol. 1996;156(9):3461–8. [PubMed] [Google Scholar]
- 30.Morita K. The Effects of One-Lung Ventilation on Cytokine Production from Lung Tissue of Rat Is One-Lung Ventilation Reasonable for Thoracic Surgery? Bull Yamaguchi Med Sch. 2003;50:65–75. [Google Scholar]
- 31.Donnelly SC, Strieter RM, Reid PT, et al. The association between mortality rates and decreased concentrations of interleukin-10 and interleukin-1 receptor antagonist in the lung fluids of patients with the adult respiratory distress syndrome. Ann Intern Med. 1996;125(3):191–6. doi: 10.7326/0003-4819-125-3-199608010-00005. [DOI] [PubMed] [Google Scholar]
- 32.Wang E, Simard M, Ouellet N, et al. Modulation of cytokines and chemokines, limited pulmonary vascular bed permeability, and prevention of septicemia and death with ceftriaxone and interleukin-10 in pneumococcal pneumonia. J Infect Dis. 2000;182(4):1255–9. doi: 10.1086/315811. [DOI] [PubMed] [Google Scholar]
- 33.Nakada TA, Hirasawa H, Oda S, et al. Influence of toll-like receptor 4, CD14, tumor necrosis factor, and interleukine-10 gene polymorphisms on clinical outcome in Japanese critically ill patients. J Surg Res. 2005;129(2):322–8. doi: 10.1016/j.jss.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 34.Mira JP, Cariou A, Grall F, et al. Association of TNF2, a TNF-alpha promoter polymorphism, with septic shock susceptibility and mortality: a multicenter study. Jama. 1999;282(6):561–8. doi: 10.1001/jama.282.6.561. [DOI] [PubMed] [Google Scholar]
- 35.Waterer GW, Quasney MW, Cantor RM, Wunderink RG. Septic shock and respiratory failure in community-acquired pneumonia have different TNF polymorphism associations. American journal of respiratory and critical care medicine. 2001;163(7):1599–604. doi: 10.1164/ajrccm.163.7.2011088. [DOI] [PubMed] [Google Scholar]
- 36.Furuta M, Yano Y, Ito K, et al. Relationship of the tumor necrosis factor-alpha -308 A/G promoter polymorphism with insulin sensitivity and abdominal fat distribution in Japanese patients with type 2 diabetes mellitus. Diabetes research and clinical practice. 2002;56(2):141–5. doi: 10.1016/S0168-8227(01)00358-8. [DOI] [PubMed] [Google Scholar]
- 37.Turner DM, Williams DM, Sankaran D, et al. An investigation of polymorphism in the interleukin-10 gene promoter. Eur J Immunogenet. 1997;24(1):1–8. doi: 10.1111/j.1365-2370.1997.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 38.Shimada H, Ochiai T, Okazumi S, et al. Clinical benefits of steroid therapy on surgical stress in patients with esophageal cancer. Surgery. 2000;128(5):791–8. doi: 10.1067/msy.2000.108614. [DOI] [PubMed] [Google Scholar]