Abstract
Aims
The aim of this study was to assess the relationship between sex and clinical outcomes and treatment-related complications in patients with ST-elevation or non-ST-elevation acute coronary syndromes (ACS) randomized to treatment with ticagrelor or clopidogrel in the PLATelet inhibition and patient Outcomes (PLATO) trial.
Methods
The associations between sex subgroup and the primary composite outcomes, secondary outcomes, and major bleeding endpoints as well as interaction of sex subgroup with treatment effects were analysed using Cox proportional-hazards models.
Results
Sex was not significantly associated with the probability of the primary composite endpoint [adjusted hazard ratio (HR): 1.02 (0.91−1.16)], or other adverse cardiovascular endpoints. Ticagrelor was similarly more effective than clopidogrel in reducing rates of the primary endpoint in women 11.2 vs. 13.2% [adjusted HR: 0.88 (0.74−1.06)] and men 9.4 vs. 11.1% [adjusted HR: 0.86 (0.76−0.97)] (interaction P-value 0.78), all-cause death in women 5.8 vs. 6.8% [adjusted HR: 0.90 (0.69−1.16)] and men 4.0 vs. 5.7% [adjusted HR: 0.80 (0.67−0.96)] (interaction P-value 0.49), and definite stent thrombosis in women 1.2 vs. 1.4% [adjusted HR: 0.71 (0.36−1.38)] and men 1.4 vs. 2.1% [adjusted HR: 0.63 (0.45−0.89)] (interaction P-value 0.78). The treatments did not differ for PLATO-defined overall major bleeding complications in women [adjusted HR: 1.01 (0.83−1.23)] or men [adjusted HR: 1.10 (0.98−1.24)]. Sex had no significant association with these outcomes (interactions P = 0.43−0.88).
Conclusion
Female sex is not an independent risk factor for adverse clinical outcomes in moderate-to-high risk ACS patients. Ticagrelor has a similar efficacy and safety profile in men and women.
Keywords: Acute coronary syndromes, Sex, Platelets, P2Y12 receptor, Ticagrelor, Thrombosis
Introduction
Women who present with acute coronary syndrome (ACS) are typically older than men, and have a higher frequency of concomitant hypertension or diabetes mellitus, and less often a history of myocardial infarction (MI).1–12 Studies suggest that the treatment and approach of ACS also differs between women and men, including the use of effective short-term medication (as thrombolytics), long-term medication, do-not-resuscitate orders, and invasive procedures.1,3,5–9,13
A number of reports indicated no significant differences between sexes for in-hospital or 30-day mortality, after adjustment for baseline characteristics.4–6,8–11,14 Also regarding long-term mortality rates, adjusted data in several reports suggest no difference between the sexes.1,2,6,9 However, the Register of Information and Knowledge About Swedish Heart Intensive Care Admissions (RIKS-HIA) study reported lower all-cause mortality in women at 1 year, after adjustment for age.10
Ticagrelor is a reversibly binding, oral P2Y12 receptor antagonist.15 It has been approved in >90 countries for preventing thrombotic events in patients with ACS.16,17 In the phase III PLATelet inhibition and patient Outcomes (PLATO) trial, ticagrelor significantly reduced the composite rate of death from cardiovascular causes (CV death), MI, or stroke at 12 months, without increasing the rate of PLATO-defined overall major bleeding, compared with clopidogrel in patients with ACS with or without ST-segment elevation.18
Given the potential importance of sex on treatment approach and outcome in patients with ACS, we present a pre-specified analysis of the association between sex and the effects of ticagrelor vs. clopidogrel on clinical outcomes and treatment-related complications in patients with ST-elevation or non-ST-elevation ACS in the PLATO trial.
Methods
Summary of PLATO methodology
PLATO was an international, multicentre, randomized, double-blind trial. The methodology of PLATO (including study design, inclusion and exclusion criteria, patients and outcome variables) has been published previously.18,19 In brief, 18 624 patients hospitalized for ACS, with or without ST segment elevation, were randomized to receive ticagrelor or clopidogrel in a double-blind, double-dummy manner within 24 h of the most recent cardiac ischaemic symptoms and before any planned or urgent percutaneous coronary intervention (PCI).
Ticagrelor was administered as a loading dose of 180 mg followed by 90 mg twice daily. Clopidogrel was administered as a maintenance dose of 75 mg daily, preceded by a loading dose of 300 mg in patients who had not received an open-label loading dose and had not received clopidogrel for ≥5 days before randomization. Before PCI procedures, a further 300 mg clopidogrel dose (or ticagrelor 90 mg in the case of PCI procedures later than 24 h after randomization) was permitted at the discretion of the investigator. The assigned study treatment was continued for at least 6 months and up to a maximum of 12 months. All the patients also received acetylsalicylic acid (aspirin) 75–100 mg/day unless they were known to be aspirin intolerant. For patients who previously had not received aspirin, the preferred loading dose was a maximum of 325 mg. After stent placement, an aspirin dose of up to 325 mg/day for 6 months was permitted.
The primary efficacy endpoint was the composite of time to first occurrence of MI, stroke, or CV death, and the primary safety endpoint was time to PLATO-defined and adjudicated first major bleeding event.19
The study adhered fully to the ethical principles of the Declaration of Helsinki, the research protocol was approved by all appropriate locally appointed ethics committees, and informed consent was obtained from the patients.18,19
Statistical analysis
Patient characteristics were compared according to sex using χ2 and Wilcoxon rank-sum tests. Cox proportional-hazards models were used to analyse the association between sex subgroup and the primary composite endpoint and each of the secondary endpoints and major bleeding endpoints.
Adjustment variables besides geographic region also included age, weight, height, body mass index, waist circumference, heart rate, systolic blood pressure, smoking status, white blood cell count, haemoglobin level, creatinine clearance, troponin level, time from onset of symptoms to randomization, changes in electrocardiogram (ECG) at entry (ST-elevation or ST-depression), Killip class at entry, glycoprotein inhibitors (GPIs) at entry, prior MI, prior stroke, prior transient ischaemic attack (TIA), prior PCI, prior coronary artery bypass grafting (CABG), prior gastrointestinal bleeding episodes, diabetes, peripheral arterial disease (PAD), dyslipidaemia, angina pectoris, congestive heart failure, final diagnosis, randomized treatment, and treatment approach.
The interaction of sex subgroup with treatment effects was investigated by use of Cox proportional-hazards models. For efficacy endpoints, all analyses were based on the intent-to-treat approach. Bleeding endpoints were analysed up to 7 days after permanent discontinuation, and only in patients who had received at least one dose of the study drug. Dyspnoea rates by gender and randomized treatment at 1 year were estimated using the Kaplan–Meier (KM) method. Hazard ratios (HR) comparing the randomized treatments and the treatment by gender interaction test were derived using a Cox regression model.
The proportional hazard assumption was assessed using the method proposed by Lin et al.20 based on the cumulative sum of martingale residuals. For the endpoints where the proportional hazard assumption was not met, a piecewise proportional hazard model was used. The cutpoints for the piecewise segments were selected using likelihood ratio tests.
Ventricular pauses by gender and treatment at first week and 30 days were summarized as percentages. Odds ratios comparing randomized treatments and the treatment by gender interaction were derived using a logistic regression model.
All the analyses were performed using SAS®, version 9.2 (SAS Institute, Gary, NC, USA). Comparisons for exploratory analyses used a two-sided significance level of 0.05 without correction for multiple comparisons.
Results
Baseline characteristics
Baseline characteristics of the study population are presented in Table 1, stratified by sex. Fewer women than men were randomized in the PLATO trial, with the study population comprising 28.4% women (5288/18 624); percentage of women was approximately uniform across regions (data not shown). There was no significant difference in the allocation of randomized treatments between sexes. Baseline characteristics of women randomized to the two arms of the study are presented in Supplementary material online, Table S1B.
Table 1.
Patient baseline characteristics, final diagnosis, and management
| Characteristic | Women (n = 5288) | Men (n = 13 336) | P-value |
|---|---|---|---|
| Weight and BMI | |||
| Median body weight, kg (25th–75th percentile) | 71 (62–81) | 82 (73–92) | <0.0001 |
| Body weight <60 kg, % (n) | 16.7 (878) | 3.3 (434) | <0.0001 |
| Median BMI, kg/m2 (25th–75th percentile) | 27.5 (24.4–31.1) | 27.4 (24.8–30.2) | 0.1040 |
| Age ≥75 years | 23.7 (1251) | 12.2 (1627) | <0.0001 |
| Habitual smoker | 23.1 (1221) | 41.0 (5457) | <0.0001 |
| Hypertension | 76.8 (4058) | 61.0 (8125) | <0.0001 |
| Dyslipidaemia, including hypercholesterolaemia | 48.2 (2547) | 46.1 (6142) | 0.0090 |
| Angina pectoris | 48.6 (2568) | 43.4 (5790) | <0.0001 |
| Myocardial infarction | 17.8 (940) | 21.6 (2884) | <0.0001 |
| Congestive heart failure | 8.2 (431) | 4.6 (619) | <0.0001 |
| PCI | 10.9 (575) | 14.4 (1917) | <0.0001 |
| CABG | 4.2 (221) | 6.6 (885) | <0.0001 |
| TIA | 3.2 (169) | 2.5 (330) | 0.0059 |
| Non-haemorrhagic stroke | 4.6 (243) | 3.6 (479) | 0.0014 |
| PAD | 5.4 (287) | 6.4 (857) | 0.0106 |
| Chronic renal disease | 4.7 (247) | 4.0 (538) | 0.0508 |
| Baseline laboratory values, median (25th–75th percentile) | |||
| Glucose, mmol/L | 7.0 (5.8–9.2) | 6.8 (5.7–8.7) | <0.0001 |
| HbA1c, % | 6.1 (5.7–6.9) | 5.9 (5.6–6.5) | <0.0001 |
| EGFR, mL/min/1.73 m2 | 74 (59–89) | 86 (71–01) | <0.0001 |
| Final diagnosis, % (n) | |||
| STEMI | 30.6 (1613) | 40.7 (5413) | <0.0001 |
| NSTEMI | 43.0 (2266) | 42.8 (5689) | |
| Unstable angina | 22.8 (1202) | 14.3 (1910) | |
| Other | 3.6 (192) | 2.2 (297) | |
| Pre-randomization management strategy | |||
| Planned invasive, % (n) | 64.0 (3382) | 75.2 (10 026) | <0.0001 |
| Randomized to ticagrelor, % (n) | 50.2 (2655) | 50.1 (6678) | 0.8699 |
| Procedures during study | |||
| PCI before discharge | 51.0 (2698) | 65.0 (8665) | <0.0001 |
| PCI during study | 54.1 (2859) | 68.4 (9119) | <0.0001 |
| Coronary angiography before discharge | 74.4 (3935) | 84.3 (11 235) | <0.0001 |
| Coronary angiography during study | 79.2 (4187) | 88.4 (11 793) | <0.0001 |
| CABG before discharge | 4.0 (211) | 5.7 (759) | <0.0001 |
| CABG during study | 7.8 (411) | 11.2 (1488) | <0.0001 |
| Concomitant medication taken during study, % (n) | |||
| Aspirin | 95.8 (5062) | 96.5 (12 852) | 0.0317 |
| β-Blockers | 85.3 (4505) | 85.5 (11 390) | 0.6918 |
| ACE and/or angiotensin II inhibitors | 87.1 (4598) | 85.3 (11 363) | 0.0022 |
| Statins | 91.6 (4838) | 94.8 (12 622) | <0.0001 |
| Calcium channel inhibitors | 29.6 (1565) | 21.7 (2893) | <0.0001 |
| Diuretics | 49.4 (2607) | 38.3 (5100) | <0.0001 |
χ2 and Wilcoxon rank-sum tests were used for comparisons between sexes. No adjustments were made for multiple testing.
ACE, angiotensin-converting enzyme; BMI, body mass index; CABG, coronary artery bypass grafting; EGFR, estimated glomerular filtration rate; HbA1c, glycosylated haemoglobin; NSTEMI, non-ST-elevation myocardial infarction; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction; TIA, transient ischaemic attack
In terms of CV risk factors, age ≥75 years, dyslipidaemia, and hypertension were more frequent in women compared with men, whereas men more frequently reported habitual smoking. More women had a previous history of angina pectoris, chronic heart failure, TIA or non-haemorrhagic stroke, and more men had previously had manifestations of PAD, MI, PCI, or CABG. Median serum glucose and glycosylated haemoglobin (HbA1c) levels were higher in women than in men. The median estimated glomerular filtration rate was higher in men. Following study entry, women more frequently had a final diagnosis of unstable angina than men, but had fewer final diagnoses of ST-elevation myocardial infarction (STEMI). Planned and received procedures, including CABG, were more frequent in men.
Association between sex and outcomes, independent of randomized treatment
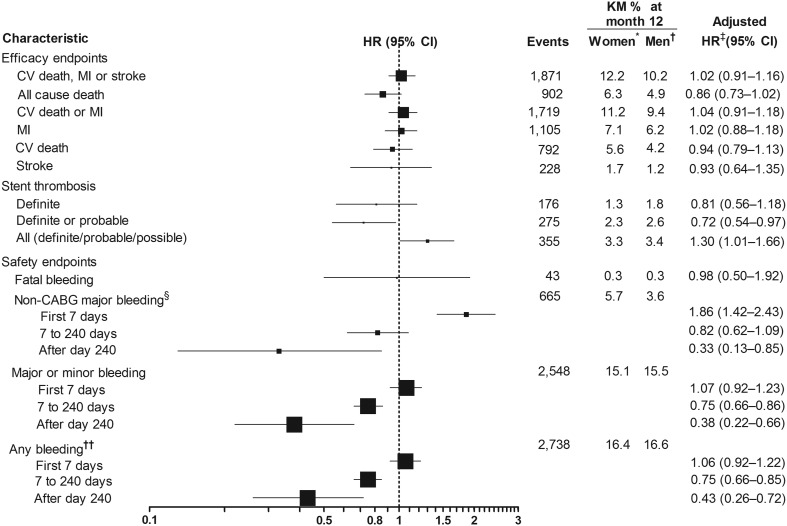
In the overall population, female sex was not significantly associated with the risk of the primary composite endpoint [adjusted HR: 1.02 (0.91−1.16); Figure 1]. Female sex was also not significantly associated with all-cause death [adjusted HR: 0.86 (0.73−1.02)], incidence of stent thrombosis [adjusted HR: 0.81 (0.56−1.18)], or other efficacy endpoints. Unadjusted data for efficacy endpoints in females and males are described in Supplementary material online, Table S2A.
Figure 1.
Association between sex and clinical outcome. *n = 5288. †n = 13 336. ‡Adjustment variables selected from the following: age, weight, height, body mass index, waist circumference, race, smoking status, diabetes, hypertension, heart rate, systolic blood pressure, changes in electrocardiogram at entry, electrocardiogram depression, killip class at entry, age, haemoglobin, white blood cells, dyslipidaemia, creatinine, angina pectoris, prior myocardial infarction, congestive heart failure, prior GI bleeding, prior percutaneous coronary intervention, prior coronary artery bypass grafting, prior transient ischaemic attack, prior non-haemorrhagic stroke, peripheral arterial disease, renal disease, chronic obstructive pulmonary disease, final diagnosis, onset of symptoms to randomized treatment, glycoprotein IIb/IIIa inhibitors at randomization, randomized treatment, treatment approach, and region. §PLATO-defined and adjudicated19. ††Any bleeding includes major, minor, and minimal bleedings. CABG, coronary artery bypass grafting; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CV death, death from cardiovascular causes; ECG, electrocardiogram; GI, gastrointestinal bleeding; HR, hazard ratio; KM, Kaplan–Meier analysis; MI, myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; TIA, transient ischaemic stroke.
Regarding safety endpoints, rates of non-CABG-related major bleeding did not differ significantly between sexes [adjusted HR: 1.16 (0.96−1.40)], but women experienced a lower rate of CABG-related major bleeding events [adjusted HR: 0.57 (0.49−0.67)]. PLATO-defined and adjudicated overall major bleeding rate was lower in women than in men [adjusted HR: 0.81 (0.71−0.92)], but this difference may be explained by the lower CABG-related bleeding rate and less use of CABG in women. When major and minor bleeding were combined, there was still significantly less bleeding in women compared with men (Figure 1). The same was true for any bleeding including major, minor, and minimal bleeding (Figure 1). Age and body mass index were univariately associated with major bleeding but the association of body mass index disappeared when adjusted by age. Women were more likely to have low body mass index than men (data not shown). Unadjusted data describing the association of gender with endpoints are provided in Supplementary material online, Table S2A.
Association between sex and efficacy of randomized treatment
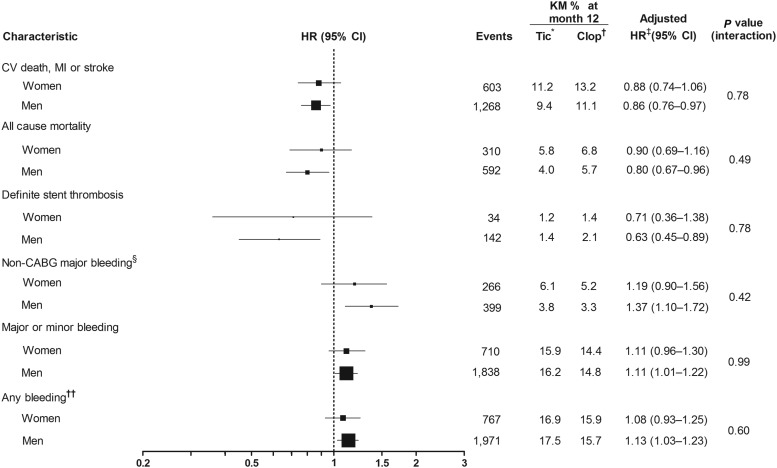
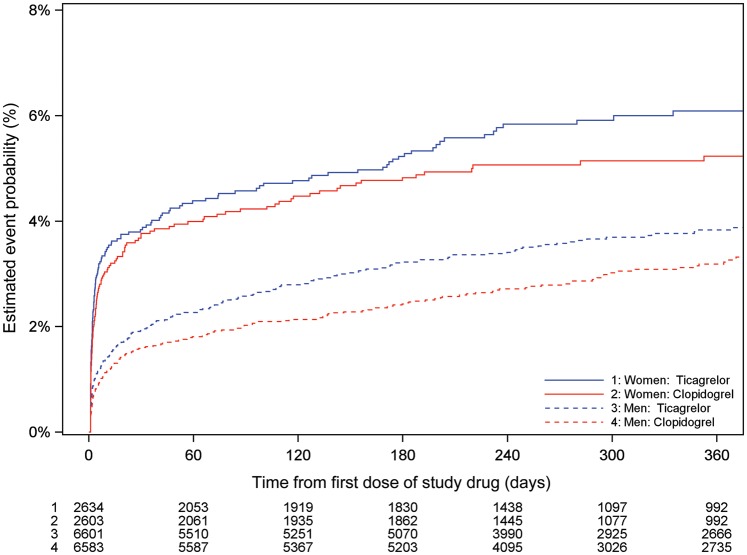
When the association of sex with outcomes was assessed by randomized treatment, ticagrelor was associated with similar reductions among women and men in CV death, MI, or stroke [women: adjusted HR: 0.88 (0.74–1.06); men: adjusted HR: 0.86 (0.76–0.97); Figures 2 and 3]; all-cause death [women: adjusted HR: 0.90 (0.69–1.16); men: adjusted HR: 0.80 (0.67–0.96) Figures 2 and 4]; and definite stent thrombosis [women: adjusted HR: 0.71 (0.36–1.38); men: adjusted HR: 0.63 (0.45–0.89)] compared with clopidogrel. For these outcomes, the interaction between randomized treatment and sex remained non-significant (interaction range P = 0.49−0.78). Supplementary material online, Table S3A presents unadjusted data.
Figure 2.
Association between sex and treatment, and clinical outcome. *Tic = ticagrelor (n = 9333). †Clop = clopidogrel (n = 9291). ‡Adjustment variables selected from the following: age, weight, waist circumference, smoking status, diabetes, hypertension, heart rate, systolic blood pressure, changes in electrocardiogram at entry, killip class at entry, haemoglobin, white blood cells, dyslipidaemia, angina pectoris, prior myocardial infarction, congestive heart failure, prior GI bleeding, prior percutaneous coronary intervention, prior coronary artery bypass grafting, prior transient ischaemic attack, non-haemorrhagic stroke, peripheral arterial disease, chronic renal disease, chronic obstructive pulmonary disease, final diagnosis, onset of symptoms to randomized treatment, GP Iib/IIIa inhibitors at randomization, randomized treatment, treatment approach, and region. §PLATO-defined and adjudicated19. ††Any bleeding includes major, minor, and minimal bleedings. CABG, coronary artery bypass grafting; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CV death, death from cardiovascular causes; ECG, electrocardiogram; GI, gastrointestinal bleeding; HR, hazard ratio; KM, Kaplan–Meier analysis; MI, myocardial infarction; PCI, percutaneous coronary intervention; TIA, transient ischaemic stroke.
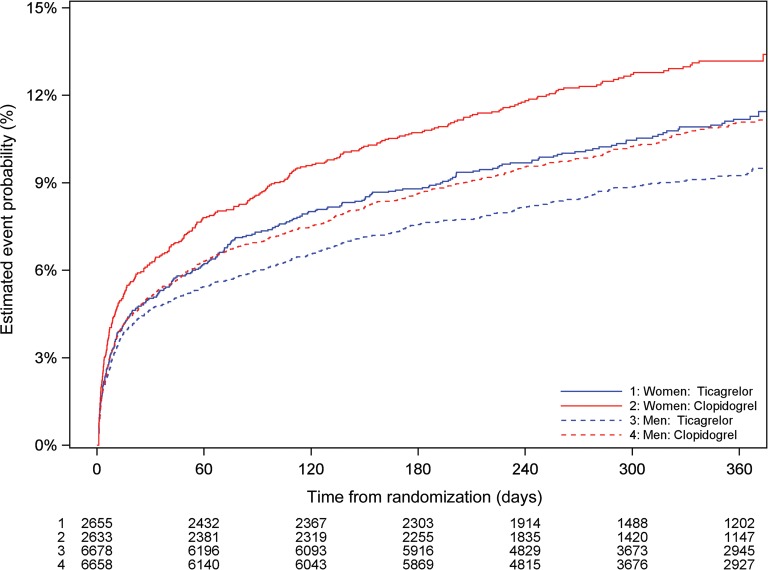
Figure 3.
Cumulative Kaplan–Meier estimates of the incidence of the primary composite outcome—cardiovascular death/myocardial infarction/stroke, by sex and treatment (ticagrelor vs. clopidogrel). Estimated event rate at 12 months, ticagrelor vs. clopidogrel, interaction P = 0.88. CV death, death from cardiovascular causes; MI, myocardial infarction.
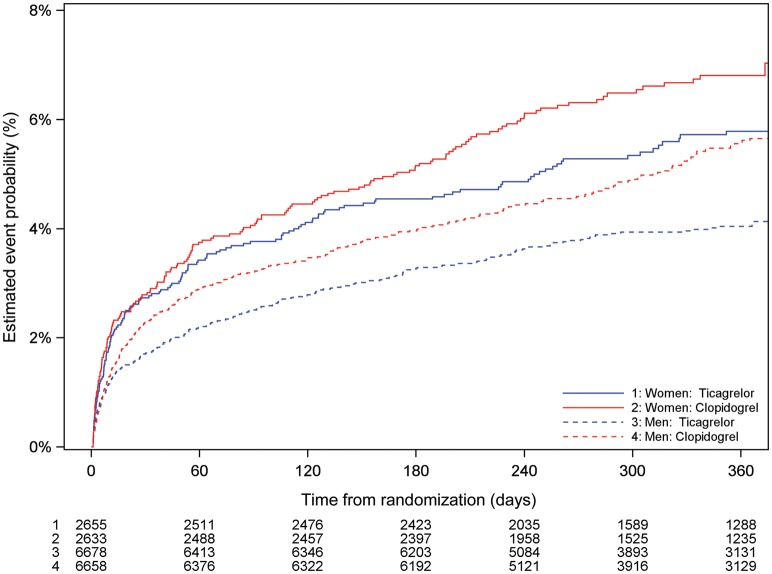
Figure 4.
Cumulative Kaplan–Meier estimates of incidence of all-cause death, by sex and treatment (ticagrelor vs. clopidogrel). Estimated event rate at 12 months ticagrelor vs. clopidogrel, interaction P = 0.50.
Further analyses of the associations between sex, randomized treatment, and either final diagnosis (STEMI vs. NSTEMI, unstable angina or ‘other’) or planned treatment (invasive vs. medically managed) revealed no significant interactions for studied endpoints (interaction P-value range 0.20−0.83).
Association between sex and safety of randomized treatment
There were no differences between ticagrelor and clopidogrel for PLATO-defined overall major bleeding episodes either in women [adjusted HR: 1.01 (0.83−1.23)] or men [adjusted HR: 1.10 (0.98−1.24); Figures 2 and 5]. There was no evidence of randomized treatment by sex interaction for this outcome (P = 0.43) Unadjusted data are presented in Supplementary material online, Table S3B. No differences in major or minor bleeding, as well as any bleeding (a composite of major, minor, and minimal bleeding), were observed among women and men treated with either ticagrelor or clopidogrel (Figure 2).
Figure 5.
Cumulative Kaplan–Meier estimates of incidence of overall major bleeding complications (PLATO-defined), by sex and treatment (ticagrelor vs. clopidogrel). Estimated event rate at 12 months ticagrelor vs. clopidogrel, interaction P = 0.43. CABG, coronary artery bypass.
Consistent with the main study data, ticagrelor was associated with an increase in non-CABG-related major bleeding when compared with clopidogrel in both women [adjusted HR: 1.19 (0.90−1.56)] and men [adjusted HR: 1.37 (1.10−1.72); Figure 2]. There was no significant interaction between treatment and sex (P = 0.42; Figure 2).
Additional analyses of the associations between sex, randomized treatment, and either final diagnosis or planned treatment also revealed no significant interactions for the bleeding endpoints (interaction P-value range: 0.17−0.67).
Higher rates of dyspnoea were reported during ticagrelor treatment than with clopidogrel treatment in both women [16.3 vs. 9.0%; HR: 1.86 (1.58−2.20)] and men [KM estimate at 1 year: 14.2 vs. 8.3%; HR: 1.81 (1.62−2.01); Table 2]. Ventricular pauses were more frequent in patients receiving ticagrelor compared with those receiving clopidogrel; however, a significant difference in rates was only observed for pauses of ≥3 s during the first week in men [6.0 vs. 3.7%; HR: 1.64 (1.09−2.47)]. There was no evidence of interaction between randomized treatment and sex for dyspnoea and ventricular pauses (interaction P-value range: 0.34−0.89).
Table 2.
The incidence of dyspnoea and ventricular pauses by sex and treatment
| Ticagrelor | Clopidogrel | Interaction P-value |
||
|---|---|---|---|---|
| Dyspnoea | KM % (n) | KM % (n) | HR (95% CI) | |
| Women | 16.3 (389) | 9.0 (214) | 1.86 (1.58–2.20) | 0.7610 |
| Men | 14.2 (870) | 8.3 (500) | 1.81 (1.62–2.01) | |
| Ventricular pauses | % (n/N) | % (n/N) | OR (95% CI) | |
| First week | ||||
| ≥3 s | ||||
| Women | 5.2 (20/386) | 3.4 (13/381) | 1.55 (0.76–3.16) | 0.8860 |
| Men | 6.0 (64/1075) | 3.7 (39/1051) | 1.64 (1.09–2.47) | |
| ≥5 s | ||||
| Women | 1.3 (5/386) | 1.3 (5/381) | 0.99 (0.28–3.44) | 0.3408 |
| Men | 2.2 (24/1075) | 1.1 (12/1051) | 1.98 (0.98–3.97) | |
| At 30 days | ||||
| ≥3 s | ||||
| Women | 1.2 (3/249) | 1.1 (3/265) | 1.07 (0.21–5.33) | 0.8241 |
| Men | 2.4 (18/743) | 1.9 (14/747) | 1.30 (0.64–2.63) | |
| ≥5 s | ||||
| Women | 0.4 (1/249) | 0.4 (1/265) | 1.07 (0.07–17.1) | 0.8541 |
| Men | 0.9 (7/743) | 0.7 (5/747) | 1.41 (0.45–4.47) | |
CI, confidence interval; HR, hazard ratio; KM, Kaplan–Meier analysis; OR, odds ratio.
Discussion
The results of the present adjusted analysis largely correspond with those reported for the large registry studies, in that men and women with ACS encounter similar mortality.1,2,6,9
In the present study, unadjusted data showed a significantly higher incidence of the efficacy endpoints of CV death, myocardial infarction, or stroke (individually and in combination) in women; however, this difference disappeared after adjustment for baseline characteristics including age.
Women with ACS have been reported to have a higher risk of bleeding complications than men.21 The suggested important risk factors for bleeds have included a smaller body and vessel size, older age, reduced creatinine clearance, higher prevalence of comorbidities, higher rate of overdosed antithrombotic medication, and differences in response to antithrombotics between women and men.21 In the present study, unadjusted data showed no differences in bleeding risk between men and women. However, after adjustment for various baseline characteristics, including age, women had significantly fewer major, major or minor, and any bleeding events.
In the present analysis, the rate of overall major bleeding events appeared lower in women than men contrary to previous evidence.21–23 However, it is likely that this observation mainly reflects the significantly lesser use of CABG surgery and the lower rate of CABG-related major bleeding in women in the PLATO trial. Thus the non-CABG-related bleeding rates in the present study were not different between women when compared with men. This difference from the previously reported figures may be due to the careful registration of and adjustment for differences in baseline characteristics.
This analysis of the effect of female sex on outcomes related to treatment with ticagrelor and clopidogrel in ACS patients reveals results that are consistent with those reported for the primary study outcomes.18 This is true for both unadjusted and adjusted data. Both in women and men with ACS, ticagrelor reduced the rates of adverse CV events and mortality compared with clopidogrel, without an increase in the rate of PLATO-defined overall bleeding. In both sexes, however, and in agreement with the main study results, there was a similar increase in the rate of non-CABG-related major bleeding complications in the ticagrelor-treated patients. This finding was consistent for both unadjusted and adjusted data.
With regard to other phase III studies, the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction (TRITON-TIMI 38) study reported similar efficacy for prasugrel in men (risk reduction in CV death, non-fatal MI, or non-fatal stroke = 21%) and the overall study population (risk reduction = 19%).24 In the TRITON-TIMI 38 study, 26% of the patients were women and compared with men they were older, lighter in body weight, more often had diabetes mellitus, hypertension, and renal impairment, but less often had prior MI and tobacco use.25 Rate of CV death was higher for women than men (2.9 vs. 2.0%; P = 0.01), but the difference disappeared after adjustment for baseline differences that included age [HR: 1.08 (0.80–1.46)]. Non-CABG TIMI major bleeding events occurred more frequently in women than in men, but after adjustment the difference disappeared [HR: 1.20 (0.90–1.61)]. However, the incidence of non-CABG TIMI minor bleeding was significantly higher in women before and after adjustment [HR: 2.15 (1.66–2.80)]. Sex and management with prasugrel and clopidogrel did not interact for CV death and major bleeding complications.
A numerically lower absolute reduction in efficacy endpoints was reported for women receiving clopidogrel in the Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) study, but the difference was not significant.26 A meta-analysis of blinded randomized trials comparing clopidogrel and placebo (including CURE, CREDO, CLARITY-TIMI 28, COMMIT, and CHARISMA) reported no significant difference in treatment effect between men and women.27
A meta-analysis on early invasive therapy vs. conservative treatment strategies in women and men with non-ST-elevation ACS (NSTE-ACS) demonstrated a comparable benefit of invasive therapy in men and high-risk women, with positive cardiac biomarkers without benefits in the low-risk women.28 Similarly, the benefit of GPI in women with NSTE-ACS in contrast to men was restricted to the patients with a positive troponin concentration.29
In a separate pre-planned analysis of PLATO data, the effect and treatment-related complications of ticagrelor vs. clopidogrel in patients aged ≥75 years were compared with those aged <75 years.30 The clinical benefit of ticagrelor over clopidogrel (with regard to the primary composite endpoint, definite stent thrombosis, or all-cause death), or PLATO-defined overall major bleeds, did not differ significantly between the age groups after adjustment of data for important differences in baseline characteristics including sex.
Study limitations
In the present study, comparisons were not corrected for multiplicity of analyses. The analyses were therefore susceptible to an increased type I error and subsequent increase in the likelihood of false-positive statistical differences. The study was not powered to show gender differences in efficacy and safety, but with a study population of 18 624 patients, including 5288 (28.4%) women, the total number of adjudicated events in both men and women were high, with a suitable power to show a possible sex-related heterogeneity in observed event rates.
Conclusion
In aspirin-treated patients with either STEMI managed with primary PCI, or NSTE-ACS, there were no differences between women and men for adverse clinical outcomes or concerning efficacy or safety outcomes of treatment with ticagrelor compared with clopidogrel.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work and the PLATelet inhibition and patient Outcomes (PLATO) study were supported by AstraZeneca. Support for the analysis and interpretation of results and preparation of the manuscript was provided through funds to the Uppsala Clinical Research Center and Duke Clinical Research Institute as part of the Clinical Study Agreement. Additional editorial support during manuscript preparation was funded by AstraZeneca. PLATO Clinical Trial Registration: www.clinicaltrials.gov; NCT00391872. Funding to pay the Open Access publication charges for this article was provided by AstraZeneca.
Conflicts of interest: S.H.: advisory board member for AstraZeneca, Bristol-Myers Squibb, Pfizer, and Bayer; research support from GlaxoSmithKline, Pfizer, and Sanofi-Aventis. S.K.J.: research grant from AstraZeneca, Eli Lilly, Bristol-Myers Squibb, Terumo, Inc., Medtronic, and Vascular Solutions; honoraria from The Medicines Company, AstraZeneca, Eli Lilly, Bristol-Myers Squibb, and IROKO; consultant/advisory board from AstraZeneca, Eli Lilly, Merck, Medtronic, and Sanofi. R.G.B.: research grants from AstraZeneca, Eli Lilly, Bristol-Myers Squibb and Merck/Schering-Plough; consultant (clinical event committee activity only) for Roche and Pfizer. R.C.B.: no honoraria. Scientific advisory for Merck, Portola, Boehringer Ingelheim, Bayer, Daiichi-Sankyo. Research grant from AstraZeneca. A.B.: consulting fees from Sanofi-Aventis, Eli Lilly, Boehringer Ingelheim, AstraZeneca, GlaxoSmithKline, Novartis and Bristol-Myers Squibb/Pfizer. M.H.: board membership in AstraZeneca; consultancy fees from AstraZeneca, Menarini, Novartis; institutional grants from GlaxoSmithKline; honoraria from Lilly, AstraZeneca, Menarini, Boehringer Ingelheim, Rovi, Bayer; travel support from AstraZeneca, Almirall. A. Himmelmann: reports being an employee of AstraZeneca. J.H.: reports being an employee of AstraZeneca and having equity ownership in AstraZeneca. H.A.K.: honoraria from AstraZeneca, Eli Lilly, GlaxoSmithKline, Roche, and Bayer; holds a patent jointly with Roche and receives royalties for this patent. R.L.: advisory board member for AstraZeneca, Bayer, Boehringer Ingelheim, Pfizer, Novo Nordisk and Leo Pharma. J.M.: research grant from Servier; consultant and speaker fees from Bayer Healthcare, Merck Sharp & Dohme, Boehringer Ingelheim, Jaba Recordati, Pfizer/ Bristol-Myers Squibb; speaker fees from AstraZeneca. J.C.N.: research grant support and consulting fees/honorarium from Schering-Plough/Merck; board membership with AstraZeneca; research grant support and payment for lectures, including service on speakers' bureaus from Astra Zeneca and Eli Lilly; travel/accommodations/meeting expenses from Astra Zeneca. P.G.S.: research grant (to INSERM U698): NYU School of Medicine, Sanofi, Servier; speaking or consulting: Amarin, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, GlaxoSmithKline, Lilly, Medtronic, Otsuka, Pfizer, Roche, Sanofi, Servier, The Medicines Company, Vivus; stockholding in Aterovax. R.F.S.: research grants from AstraZeneca, Eli Lilly/Daiichi-Sankyo, and Merck; research support from Accumetrics; honoraria from AstraZeneca, Eli Lilly/Daiichi-Sankyo, Merck, Iroko, Accumetrics, and Medscape; consultancy fees from AstraZeneca, Merck, Novartis, Accumetrics, Sanofi-Aventis/Regeneron, Bristol-Myers Squibb, Eisai, Roche and Daiichi-Sankyo. D.M.W.: reports no conflicts of interest. L.W.: research grants from AstraZeneca, Merck & Co., Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, GlaxoSmithKline; consultant for Merck & Co., Regado Biosciences, Evolva, Portola, C.S.L. Behring, Athera Biotechnologies, Boehringer Ingelheim, AstraZeneca, GlaxoSmithKline, and Bristol-Myers Squibb/Pfizer; lecture fees from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, GlaxoSmithKline, and Merck & Co.; honoraria from Boehringer Ingelheim, AstraZeneca, Bristol-Myers Squibb/Pfizer, GlaxoSmithKline, and Merck & Co.; travel support from AstraZeneca and Bristol-Myers Squibb/Pfizer.
Supplementary Material
Acknowledgements
David Evans, Josh Collis, and Neil Fisher are acknowledged for writing support, and Ulla Nässander Schikan, PhD, at Uppsala Clinical Research Center, Uppsala, Sweden, for providing editorial assistance.
References
- 1.Maynard C, Every NR, Martin JS, Kudenchuk PJ, Weaver WD. Association of gender and survival in patients with acute myocardial infarction. Arch Intern Med. 1997;157:1379–1384. [PubMed] [Google Scholar]
- 2.Moen EK, Asher CR, Miller DP, Weaver WD, White HD, Califf RM, Topol EJ. Long-term follow-up of gender-specific outcomes after thrombolytic therapy for acute myocardial infarction from the GUSTO-I trial. Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries. J Womens Health. 1997;6:285–293. doi: 10.1089/jwh.1997.6.285. [DOI] [PubMed] [Google Scholar]
- 3.Chandra NC, Ziegelstein RC, Rogers WJ, Tiefenbrunn AJ, Gore JM, French WJ, Rubison M. Observations of the treatment of women in the United States with myocardial infarction: a report from the National Registry of Myocardial Infarction-I. Arch Intern Med. 1998;158:981–988. doi: 10.1001/archinte.158.9.981. [DOI] [PubMed] [Google Scholar]
- 4.Hochman JS, Tamis JE, Thompson TD, Weaver WD, White HD, Van de Werf F, Aylward P, Topol EJ, Califf RM. Sex, clinical presentation, and outcome in patients with acute coronary syndromes. Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes IIb Investigators. N Engl J Med. 1999;341:226–232. doi: 10.1056/NEJM199907223410402. [DOI] [PubMed] [Google Scholar]
- 5.Gan SC, Beaver SK, Houck PM, MacLehose RF, Lawson HW, Chan L. Treatment of acute myocardial infarction and 30-day mortality among women and men. N Engl J Med. 2000;343:8–15. doi: 10.1056/NEJM200007063430102. [DOI] [PubMed] [Google Scholar]
- 6.Kim C, Schaaf CH, Maynard C, Every NR. Unstable angina in the myocardial infarction triage and intervention registry (MITI): short- and long-term outcomes in men and women. Am Heart J. 2001;141:73–77. doi: 10.1067/mhj.2001.111546. [DOI] [PubMed] [Google Scholar]
- 7.Anand SS, Xie CC, Mehta S, Franzosi MG, Joyner C, Chrolavicius S, Fox KA, Yusuf S CURE Investigators. Differences in the management and prognosis of women and men who suffer from acute coronary syndromes. J Am Coll Cardiol. 2005;46:1845–1851. doi: 10.1016/j.jacc.2005.05.091. [DOI] [PubMed] [Google Scholar]
- 8.Blomkalns AL, Chen AY, Hochman JS, Peterson ED, Trynosky K, Diercks DB, Brogan GX, Jr, Boden WE, Roe MT, Ohman EM, Gibler WB, Newby LK CRUSADE Investigators. Gender disparities in the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes: large scale observations from the CRUSADE (Can rapid risk stratification of unstable angina patients suppress adverse outcomes with early implementation of the American College of Cardiology/American Heart Association guidelines) National Quality Improvement Initiative. J Am Coll Cardiol. 2005;45:832–837. doi: 10.1016/j.jacc.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 9.Heer T, Gitt AK, Juenger C, Schiele R, Wienbergen H, Towae F, Gottwitz M, Zahn R, Zeymer U, Senges J ACOS Investigators. Gender differences in acute non-ST-segment elevation myocardial infarction. Am J Cardiol. 2006;98:160–166. doi: 10.1016/j.amjcard.2006.01.072. [DOI] [PubMed] [Google Scholar]
- 10.Alfredsson J, Stenestrand U, Wallentin L, Swahn E. Gender differences in management and outcome in non-ST-elevation acute coronary syndrome. Heart. 2007;93:1357–1362. doi: 10.1136/hrt.2006.102012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radovanovic D, Erne P, Urban P, Bertel O, Rickli H, Gaspoz JM AMIS Plus Investigators. Gender differences in management and outcomes in patients with acute coronary syndromes: results on 20,290 patients from the AMIS Plus Registry. Heart. 2007;93:1369–1375. doi: 10.1136/hrt.2006.106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poon S, Goodman SG, Yan RT, Bugiardini R, Bierman AS, Eagle KA, Johnston N, Huynh T, Grondin FR, Schenck-Gustafsson K, Yan AT. Bridging the gender gap: insights from a contemporary analysis of sex-related differences in the treatment and outcomes of patients with acute coronary syndromes. Am Heart J. 2012;163:66–73. doi: 10.1016/j.ahj.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 13.Tavris D, Shoaibi A, Chen AY, Uchida T, Roe MT, Chen J. Gender differences in the treatment of non-ST-segment elevation myocardial infarction. Clin Cardiol. 2010;33:99–103. doi: 10.1002/clc.20691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tizón-Marcos H, Bertrand OF, Rodés-Cabau J, Larose E, Gaudreault V, Bagur R, Gleeton O, Courtis J, Roy L, Poirier P, Costerousse O, De Larochellière R. Impact of female gender and transradial coronary stenting with maximal antiplatelet therapy on bleeding and ischemic outcomes. Am Heart J. 2009;157:740–745. doi: 10.1016/j.ahj.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Husted S, van Giezen JJJ. Ticagrelor: the first reversibly binding oral P2Y12 receptor antagonist. Cardiovasc Ther. 2009;27:259–274. doi: 10.1111/j.1755-5922.2009.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.AstraZeneca UK Limited. Brilinta full prescribing information. 2011a. http://www1.astrazeneca-us.com/pi/brilinta.pdf. (24 May 2013)
- 17.AstraZeneca UK Limited. Brilique 90 mg film coated tablets. Summary of product characteristics. 2011b http://www.medicines.org.uk/emc/medicine/23935/PC/brilique%2090%20mg%20film%20coated%20tablets/ (24 May 2013) [Google Scholar]
- 18.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA. Freij A, Thorsén M, editors. PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 19.James S, Akerblom A, Cannon CP, Emanuelsson H, Husted S, Katus H, Skene A, Steg PG, Storey RF, Harrington R, Becker R, Wallentin L. Comparison of ticagrelor, the first reversible oral P2Y(12) receptor antagonist, with clopidogrel in patients with acute coronary syndromes: rationale, design, and baseline characteristics of the PLATelet Inhibition and Patient Outcomes (PLATO) trial. Am Heart J. 2009;157:599–605. doi: 10.1016/j.ahj.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 20.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80:557–572. [Google Scholar]
- 21.Steg PG, Huber K, Andreotti F, Arnesen H, Atar D, Badimon L, Bassand JP, De Caterina R, Eikelboom JA, Gulba D, Hamon M, Helft G, Fox KA, Kristensen SD, Rao SV, Verheugt FW, Widimsky P, Zeymer U, Collet JP. Bleeding in acute coronary syndromes and percutaneous coronary interventions: position paper by the Working Group on Thrombosis of the European Society of Cardiology. Eur Heart J. 2011;32:1854–1864. doi: 10.1093/eurheartj/ehr204. [DOI] [PubMed] [Google Scholar]
- 22.Moscucci M, Fox KA, Cannon CP, Klein W, López-Sendón J, Montalescot G, White K, Goldberg RJ. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE) Eur Heart J. 2003;24:1815–1823. doi: 10.1016/s0195-668x(03)00485-8. [DOI] [PubMed] [Google Scholar]
- 23.Subherwal S, Bach RG, Chen AY, Gage BF, Rao SV, Newby LK, Wang TY, Gibler WB, Ohman EM, Roe MT, Pollack CV, Jr, Peterson ED, Alexander KP. Baseline risk of major bleeding in non-ST-segment elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress Adverse outcomes with Early implementation of the ACC/AHA guidelines) bleeding score. Circulation. 2009;119:1873–1882. doi: 10.1161/CIRCULATIONAHA.108.828541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 25.Mega JL, Wiviott SD, Mohanavelu S, Nicolau JC, McCabe CH, Antman EM, Braunwald E. Cardiovascular outcomes of women and men with acute coronary syndromes and angiographically confirmed coronary artery disease in TRITON-TIMI 38. Circulation. 2008;118(Suppl. 2):S967. [Google Scholar]
- 26.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK Clopidogrel in Unstable angina to prevent Recurrent Events trial (CURE) Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 27.Berger JS, Bhatt DL, Cannon CP, Chen Z, Jiang L, Jones JB, Mehta SR, Sabatine MS, Steinhubl SR, Topol EJ, Berger PB. The relative efficacy and safety of clopidogrel in women and men: a sex-specific collaborative meta-analysis. Am Coll Cardiol. 2009;54:1935–1945. doi: 10.1016/j.jacc.2009.05.074. [DOI] [PubMed] [Google Scholar]
- 28.O'Donoghue M, Boden WE, Braunwald E, Cannon CP, Clayton TC, de Winter RJ, Fox KA, Lagerqvist B, McCullough PA, Murphy SA, Spacek R, Swahn E, Wallentin L, Windhausen F, Sabatine MS. Early invasive vs. conservative treatment strategies in women and men with unstable angina and non-ST-segment elevation myocardial infarction. A meta-analysis. JAMA. 2008;300:71–80. doi: 10.1001/jama.300.1.71. [DOI] [PubMed] [Google Scholar]
- 29.Boersma E, Harrington RA, Moliterno DJ, White H, Simoons ML. Platelet glycoprotein IIb/IIIa inhibitors in acute coronary syndromes. Lancet. 2002;360:342–343. doi: 10.1016/s0140-6736(02)09532-6. [DOI] [PubMed] [Google Scholar]
- 30.Husted S, James S, Becker RC, Horrow J, Katus H, Storey RF, Cannon CP, Heras M, Lopes RD, Morais J, Mahaffey KW, Bach RG, Wojdyla D, Wallentin L PLATO study group. Ticagrelor versus clopidogrel in elderly patients with acute coronary syndromes: a substudy from the Prospective Randomized PLATelet Inhibition and Patient Outcomes (PLATO) trial. Circ Cardiovasc Qual and Outcomes. 2012;5:680–688. doi: 10.1161/CIRCOUTCOMES.111.964395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.