γδ T cell migration into mouse pleural cavities during inflammatory responses triggered by LPS, Mycobacterium bovis BCG, or ovalbumin depends on leukotriene B4 and BLT1 receptor.
Keywords: inflammation, T cells, lipid mediators
Abstract
Herein, we investigated the involvement of the 5-LO-derived lipid mediator LTB4 in γδ T cell migration. When injected into the i.pl. space of C57BL/6 mice, LTB4 triggered γδ T lymphocyte mobilization in vivo, a phenomenon also observed in in vitro chemotaxis assays. The i.pl. injection of Escherichia coli endotoxin (LPS) triggered increased levels of LTB4 in pleural cavities. The in vivo inhibition of LTB4 biosynthesis by the 5-LO inhibitor zileuton or the FLAP inhibitor MK886 attenuated LPS-induced γδ T cell accumulation into pleural cavities. Accordingly, 5-LO KO mice failed to recruit γδ T cells into the inflammatory site after i.pl. LPS. Antagonists of the high-affinity LTB4 receptor BLT1, CP105,696, and LY292476 also attenuated LPS-induced γδ T cell accumulation in pleural cavities as well as in vitro chemotaxis toward pleural washes obtained from LPS-simulated mice. LTB4/BLT1 also accounted for γδ T cell migration induced by i.pl. administration of Mycobacterium bovis BCG or antigen in sensitized mice. BLT1 was expressed on naïve, resident as well as LPS-recruited γδ T cells. Isolated γδ T cells were found to undergo F-actin cytoskeleton reorganization when incubated with LTB4 in vitro, confirming that γδ T lymphocytes can respond directly to LTB4. In addition to its direct effect on γδ T cells, LTB4 triggered their accumulation indirectly, via modulation of CCL2 production in mouse pleural cavities. These data show that γδ T cell migration into the pleural cavity of mice during diverse inflammatory responses is dependent on LTB4/BLT1.
Introduction
γδ T lymphocytes are unconventional T cells that comprise a minor subset of T cells in lymphoid organs and are instead preferentially distributed in peripheral tissues, including lung and pleura [1,2,3]. These cells recognize a broad spectrum of nonpeptide antigens and play important roles in lung infections, exerting an early proinflammatory role followed by a subsequent regulatory role in an attempt to restrain the inflammatory response [4]. γδ T lymphocytes increase in number at inflammatory sites during infection and allergy [2, 5, 6], a phenomenon mediated via migration toward chemotactic factors [2, 7, 8] and/or local proliferation [9]. A mouse model of pleural inflammation induced by i.pl. administration of Escherichia coli endotoxin (LPS) is characterized by a massive influx of T lymphocytes that accompanies eosinophil accumulation [10, 11]. Among the T lymphocyte subsets present in the pleural space of LPS-challenged mice are γδ T lymphocytes, which are required for eosinophil tissue accumulation and maintenance of inflammation [11].
LTB4 is a lipid mediator derived from the metabolism of arachidonic acid by the enzyme 5-LO assisted by FLAP. It exerts its actions by ligating two G protein-coupled receptors, BLT1 and BLT2, with activation of downstream signaling events. Among its many biological functions, it is best known for its ability to stimulate leukocyte migration and activation [12], but it also enhances phagocytic and killing activities and expression of adhesion molecules in different leukocyte populations [13,14,15,16,17,18]. LTB4 levels are increased in the lungs during numerous inflammatory conditions, including LPS exposure, tuberculosis, and allergic responses [19,20,21,22]. Although LTB4 is a potent chemoattractant for myeloid cells [23] and αβ T lymphocytes via the BLT1 [24,25,26], its effects on γδ T lymphocytes are unknown.
In the present report, we show that LTB4 induces γδ T lymphocyte migration in vitro and in vivo via BLT1, revealing an important role for this lipid mediator in the recruitment of this lymphocyte subset into inflamed tissue in different murine models of pleural inflammation.
MATERIALS AND METHODS
Animals
C57BL/6 mice (18–20 g) were provided by Oswaldo Cruz Foundation Breeding Unit (Rio de Janeiro, Brazil) and bred at the Laboratory of Applied Pharmacology Experimental Animal Facility, Farmanguinhos (Fundação Oswaldo Cruz). Breeders of 5-LO KO (129-Alox5tm1Fun) and strain-matched WT sv129 mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and bred at BioRio Foundation (Laboratory of Transgenic Animals, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil). Mice were caged with free access to food and fresh water in a temperature-controlled room (22–24°C) with a 12-h light/dark cycle until used. All experimental procedures were performed according to The Committee on Ethical Use of Laboratory Animals of Fundação Oswaldo Cruz.
Antibodies and reagents
LPS (from E. coli serotype 0127:B8), chicken OVA grade V, PBS, RPMI 1640, EDTA, sodium azide, BSA, HEPES, HBSS, Histopaque 1077, goat anti-mouse IgG, and NP-40 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Aluminum hydroxide was purchased from EMS Sigma Pharma (São Paulo, Brazil). FITC-conjugated hamster IgG1 anti-murine CD3 (145-2C11), PerCP-conjugated hamster IgG1 anti-murine CD3 (145-2C11), PE-conjugated hamster IgG2 anti-murine γδ TCR (GL3), FITC-conjugated hamster IgG2 anti-murine γδ TCR (GL3) mAb, PerCP/PE/FITC-conjugated hamster IgG1 and IgG2, and goat IgG2a isotype controls were all purchased from BD PharMingen (San Diego, CA, USA). Mouse-Alexa Fluor 647-conjugated goat IgG2a anti-BLT1 (202/7B1) was obtained from AbD Serotec (Raleigh, NC, USA). MK886 (FLAP inhibitor) was obtained from Merck-Frosst (Montreal, QC, Canada). CP105,696 (selective BLT1 antagonist) was obtained from Pfizer Laboratories (Groton, CT, USA). LY292476 (selective BLT1 antagonist) was obtained from Eli Lilly (Indianapolis, IN, USA). Zileuton (5-LO inhibitor) was obtained from Abbott Laboratories (Chicago, IL, USA). Carboxymethylcellulose was purchased from Merck (Darmstadt, Germany). LTB4 and LTB4 EIA kits were purchased from Cayman Chemical (Ann Arbor, MI, USA). FBS was obtained from Hy-Clone (Logan, UT, USA). BCG was kindly provided by Fundação Ataulfo de Paiva (Rio de Janeiro, Brazil).
Pleurisy induction
Pleurisy was induced by an i.pl. injection of LTB4 (0.5 μg/cavity), LPS (250 ng/cavity), OVA (12.5 μg/cavity), or BCG (4×105 CFU/cavity), each diluted in sterile PBS to a final volume of 100 μl, via an adapted needle (13×0.45 mm) carefully inserted at a depth of 1 mm into the right side of the thoracic cavity of mice. Control groups received an i.pl. injection of 100 μl sterile PBS. LPS, LTB4, and BCG were injected in naive mice, whereas OVA challenge was induced in mice 14 days after prior sensitization by a s.c. injection of 200 μl of a mixture of OVA (50 μg) and aluminum hydroxide (5 mg). At specific time-points after stimulus injection, mice were killed in a carbon dioxide chamber. Pleural cells were recovered from thoracic cavities after washing with 500 μl PBS containing EDTA (10 mM, pH 7.4). For transmigration assays, pleural washes recovered from mice injected with SPW or LPW were pooled (n=10/group). SPW and LPW were centrifuged (420 g for 10 min), and cell-free supernatants were recovered, filtered (0.22 μm), and kept at –20°C until used.
Treatments
One hour before i.pl. injection of stimulus, CP105,696 (4 mg/kg) was injected i.p., and LY292476 (20 mg/kg) was injected s.c. after dilution in sterile saline to a final volume of 200 μl. Zileuton (3 mg/kg) was diluted in sterile saline containing 0.5% DMSO to a final volume of 200 μl and administered i.v. 1 h before stimulation. MK886 (1 mg/kg), diluted in 1% carboxymethylcellulose, was given orally (p.o.) to 12-h fasted animals 1 h before stimulation. The same volume of vehicle was administered in control groups.
Leukocyte counts
Total leukocyte counts were determined in a Neubauer chamber under an optical microscope after dilution in Turk fluid (2% acetic acid). Counts are reported as numbers of cells/cavity.
Flow cytometric analysis
Cells recovered from pleural cavities and spleen (106/100 μl) were incubated with the appropriate concentration of anti-TCR γδ mAb, anti-CD3 mAb, anti-BLT1 mAb, or IgG isotype controls for 30 min at 4°C, after incubation with rat serum to block nonspecific binding sites. Surface marker analysis was performed by using the Cell Quest program in a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA, USA). At least 104 lymphocytes were acquired/sample. All data were collected and displayed on a log scale of increasing fluorescence intensity and presented as histograms. Percentages of γδ lymphocytes were determined in a specific CD3+ T lymphocytes gate. Counts are reported as numbers of cells after multiplying the percentage of γδ T lymphocytes by the total number of leukocytes.
Transwell migration assay
Spleens from C57BL/6 mice were dissected, macerated in washing buffer (HBSS without Ca2+/Mg2+ containing 30 mM HEPES, 0.25% BSA, pH 7.4), and centrifuged (420 g for 10 min at 20°C). The cell pellet was resuspended in 3 ml HBSS without Ca2+/Mg2+ and subjected to centrifugation on a Histopaque-1083 gradient (400 g for 30 min) for mononuclear cell separation. Splenocytes (3×106 cells in 200 μl assay buffer) were added to the upper chamber of 3.0 μm pore diameter transwell tissue-culture inserts (Falcon, Berkeley, CA, USA), that were placed in individual wells of a 24-well cell-culture plate containing 300 μl assay buffer, stimulus (LTB4 100 nM, SPW or LPW), or stimulus plus CP105,696 (1 μM, 15 min prior stimulus). Plates were incubated for 2 h at 37°C and 5% CO2. Transmigrated cells were collected from the lower chamber, counted, stained with antibodies against CD3 and γδ TCR as described above, and analyzed by flow cytometry. Results are expressed as the chemotactic index, with accumulation in response to the vehicle having a chemotactic index of 1.
EIA for LTB4
Levels of LTB4 in cell-free pleural washes, recovered 1, 6, 9, and 24 h after i.pl. injection of LPS (250 ng/cavity), were determined by EIA using a commercial kit (Cayman Chemical), according to the manufacturer’s instructions. OD was determined at 412 nm. Results were expressed as pg LTB4/ml, based on a standard curve.
ELISA
Levels of CCL2 in cell-free pleural washes recovered 6 h after challenge were evaluated by sandwich ELISA using matched antibody pairs from R&D Systems (Minneapolis, MN, USA), according to the manufacturer’s instructions. Results are expressed as pg/cavity.
Filamentous actin staining
γδ T lymphocytes were magnetically isolated from total C57BL/6 mononuclear splenocytes by positive selection using the TCR γδ+ T cell isolation kit (Miltenyi Biotec, Germany), according to the protocols provided by the manufacturer. The enriched cell population contained 92% γδ T cells, as determined by cell-surface staining and flow cytometry analysis.
γδ T cells (5×104) were allowed to adhere for 1 h to coverslips treated previously with 0.1% nitric acid. Cells were stimulated with LTB4 (100 nM) for 15 min and fixed at room temperature with 4% paraformaldehyde (v/v) in PBS, pH 7.0. Thereafter, cells were permeabilized with 3% NP-40 for 40 min, followed by 15 min in –20°C acetone. γδ T cells were quenched using 50 mM ammonium chloride solution and 3% BSA in PBS for the next 20 min. Cells were covered with 0.4 unit rhodamine phalloidin (Invitrogen, Carlsbad, CA, USA) in methanol and incubated in a humidified chamber (1 h, 4°C). Cells were quenched in 3% BSA/PBS for 20 min and mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA). Cells were examined with a laser-scanning confocal microscope (Fluoview FV300, Olympus, Japan) under an oil immersion objective (100×). Images were obtained and processed using Fluoview 3.3 software (Olympus).
Statistical analysis
Data are reported as the mean ± sem and were analyzed statistically by means of ANOVA followed by Student-Newman-Keuls test or Student’s t-test. Values of P ≤ 0.05 were regarded as significant.
RESULTS
LTB4 induces γδ T lymphocyte migration
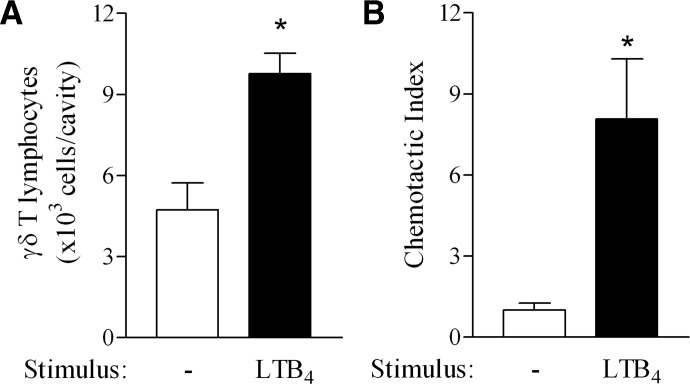
To examine whether LTB4 is capable of eliciting γδ T lymphocyte influx, we injected this lipid mediator directly into mouse pleural cavities. The i.pl. injection of LTB4 (0.5 μg/cavity) induced a twofold accumulation of γδ T lymphocytes in pleural cavities at 24 h (Fig. 1A), which was accompanied by CD3 T lymphocyte accumulation (saline: 59.4±7.5 vs. LTB4: 99.7±10.5 CD3 T lymphocytes×103/cavity; P=0.03). LTB4 (10−8 M) also triggered spleen γδ T lymphocytes chemotaxis in vitro in a transwell chamber within 2 h (Fig. 1B), demonstrating that LTB4 is able to attract γδ T cells directly. Interestingly, LTB4 also induced CD3 T lymphocyte chemotaxis (chemotactic index=3.8), however, to a lesser extent than the one observed for γδ T lymphocytes (chemotactic index=8.1).
Figure 1.
γδ T lymphocytes migrate toward LTB4. (A) In vivo γδ T lymphocyte accumulation in C57BL/6 mouse pleural cavities triggered by i.pl. injection of LTB4 (500 ng/cavity) 24 h after stimulation. Results are expressed as the mean ± sem from at least five animals/group in three different experiments. (B) In vitro chemotaxis of γδ T lymphocytes induced by LTB4 (100 nM). Spleen leukocytes (3×106/well) were placed in the upper chamber of 3 μm pore diameter transwell inserts and allowed to transmigrate toward LTB4 for 2 h. Cells that migrated into the bottom chamber were recovered, counted, and labeled for γδ TCR for FACS analysis as described in Materials and Methods. Results are expressed as the chemotactic index, and accumulation in response to vehicle had a chemotactic index of 1, as mean ± sem from triplicate values of a representative experiment out of three separate experiments. *, Statistically significant differences (P≤0.05).
LPS triggers LTB4 production in the pleural cavity
As an initial step in evaluating the participation of LTB4 in LPS-induced γδ T cell migration, we evaluated its production. As shown in Figure 2A, i.pl. LPS administration caused an increase of LTB4 in the pleural cavities of challenged mice 6 h after stimulation, returning to basal levels within 9 h. LPS stimulation also triggered γδ T cell influx into mouse pleural cavities from 6 h to 24 h after injection, with a peak response noted by 12 h (Fig. 2B).
Figure 2.
LPS triggers LTB4 production and γδ T lymphocyte accumulation. (A) LTB4 levels were determined by EIA in C57BL/6 mouse pleural fluid obtained at indicated time-points after i.pl. injection of saline or LPS (250 ng/cavity). Results are expressed as the mean ± sem from triplicate wells of one out of three separate experiments. (B) γδ T lymphocyte accumulation in the pleural cavity of C57BL/6 mice 6, 12, and 24 h after LPS i.pl. injection (250 ng/cavity). Results are expressed as the mean ± sem from at least six animals/group in three different experiments. *, Statistically significant differences (P≤0.05) compared with control group.
5-LO inhibition impairs LPS-induced γδ T lymphocyte migration in vivo
The enzyme 5-LO is responsible for the conversion of arachidonic acid to LTA4, which is hydrolyzed rapidly to form LTB4. The inhibition of 5-LO activity by zileuton (3 mg/kg, i.v.) diminished γδ T cell accumulation significantly 24 h after LPS injection (Fig. 3A). The helper protein FLAP facilitates 5-LO action, and the FLAP inhibitor MK886 (1 mg/kg, p.o.) had a similar effect (Fig. 3A). The importance of 5-LO products for γδ T cell influx, suggested by the pharmacological experiments described above, was confirmed in 5-LO−/− mice, in which LPS failed to trigger γδ T lymphocyte accumulation (Fig. 3B). The WT mice, used as control, were capable of mounting a marked response to LPS i.pl. injection, representing a substantial influx of γδ T cells to the inflamed pleura.
Figure 3.
5-LO metabolism is required for γδ T lymphocyte migration. (A) In vivo pretreatment of mice with zileuton (3 mg/kg, i.v.) or MK886 (1 mg/kg, p.o.) impaired γδ T cell accumulation in C57BL/6 mouse pleural cavities 24 h after LPS (250 ng/cavity) injection. Results are expressed as the mean ± sem from at least eight animals/group in three separate experiments. SAL, Saline. (B) γδ T lymphocyte numbers in pleural cavity 24 h after i.pl. injection of LPS (250 ng/cavity) in WT and 5-LO KO mice. Results are expressed as the mean ± sem from at least five animals/group in three different experiments. *, Statistically significant differences (P≤0.05) between nonstimulated and stimulated animals; +, significant differences between stimulated and treated groups or between WT- and KO-stimulated groups.
LTB4/BLT1 signaling is required for LPS-induced γδ T lymphocyte migration in vivo and in vitro
LTB4/BLT1 has been shown to play important roles in regulating the recruitment of αβ T cell subsets into inflammatory sites [24,25,26]. Therefore, the involvement of LTB4 and its high-affinity receptor in the recruitment of γδ T cells during LPS-induced pleurisy was investigated. The in vivo blockade of BLT1 by the antagonists CP105,696 (4 mg/kg, i.p.) or LY292476 (20 mg/kg, s.c.) caused a marked decrease in γδ T lymphocyte numbers in mouse pleural cavities 24 h after LPS i.pl. administration (Fig. 4A). We also analyzed the effect of in vitro blockade of BLT1 by CP105,696 (1 mM) on γδ T lymphocyte chemotaxis toward cell-free pleural washes recovered from mice 6 h after i.pl. LPS (LPW). Spleen γδ T lymphocytes migrated toward 6 h LPW to a higher extent than toward SPW (Fig. 4B). γδ T lymphocytes incubated previously with CP105,696 failed to migrate toward LPW, demonstrating that LTB4, present in LPW, accounts for γδ T lymphocyte chemotaxis through BLT1 activation.
Figure 4.
LPS-triggered γδ T lymphocyte accumulation requires BLT1. (A) In vivo pretreatment of mice with CP105,696 (4 mg/kg, i.p.) or with LY292476 (20 mg/kg, s.c.) impaired γδ T cell accumulation in C57BL/6 mice pleural cavities 24 h after LPS i.pl. (250 ng/cavity) stimulation. Results are expressed as the mean ± sem from at least eight animals/group in three separate experiments. (B) BLT1 blockade by CP105,696 (1 μM) inhibited in vitro γδ T lymphocyte migration induced by LPW. Spleen leukocytes (3×106/well) were placed in the upper chamber of 3 μm pore diameter transwell inserts and allowed to transmigrate toward LPW for 2 h. Migrated cells recovered from the bottom chamber were counted and labeled for FACS analysis. SPW were used as control. Results are expressed as the chemotactic index, and accumulation in response to vehicle had a chemotactic index of 1 as mean ± sem from triplicate wells from one out of three separate experiments. *, Statistically significant differences (P≤0.05) between LPW- and SPW-stimulated and stimulated groups; +, significant differences between stimulated and treated groups.
LTB4 accounts for γδ T cell migration in allergic and BCG-induced response in vivo
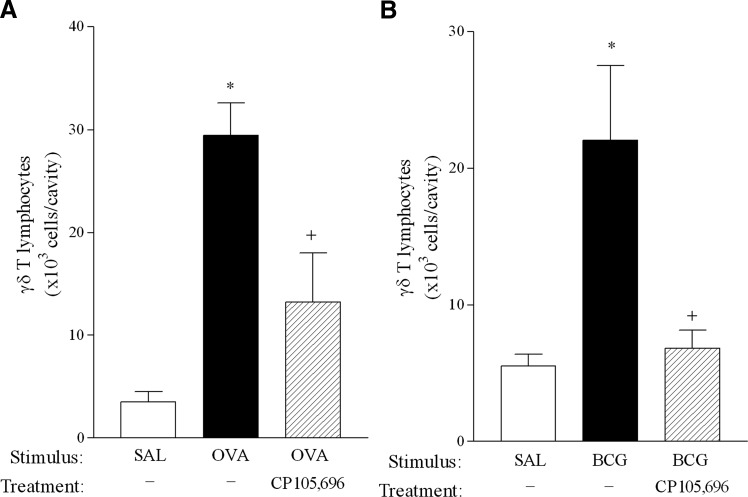
Previous reports by our group demonstrate that γδ T lymphocytes accumulate in inflamed pleura in inflammatory reactions induced by different stimuli [2, 11]. In a murine model of allergic pleurisy, γδ T cells were shown to migrate from secondary lymphoid organs to inflamed pleura via the peripheral circulation [3]. Here, we show that antigenic challenge with OVA (12.5 μg/cavity) into previously sensitized mice induced a significant increase in γδ T lymphocytes 24 h after stimulation (Fig. 5A). Blockade of BLT1 by CP105,696 treatment in vivo markedly diminished γδ T cell accumulation in the pleural cavity following OVA, demonstrating that LTB4 is required for attraction of these cells during allergy. Of note, OVA i.pl. administration into nonsensitized mice failed to induce accumulation of total leukocytes, CD3+ T cells, or γδ T lymphocytes (data not shown). Similar results were observed in BCG-injected mice, in which blockade of the LTB4 receptor impaired γδ T cell influx into inflamed pleura (Fig. 5B), demonstrating that LTB4 mediates γδ T cell migration in inflammatory reactions triggered by diverse stimuli.
Figure 5.
γδ T lymphocyte accumulation in mouse pleural cavity during allergic and BCG-induced response is inhibited by BLT1 blockade. In vivo pretreatment of mice with CP105,696 (4 mg/kg, i.p.) diminished γδ T cell accumulation induced by OVA (12.5 μg/cavity) challenge in previously sensitized C57BL/6 mice (A) or induced by BCG (4×105 CFU/cavity) in naïve C57BL/6 pleural cavities (B) 24 h after i.pl. stimulation. Results are expressed as the mean ± sem from five to eight animals/group in two separate experiments. *, Statistically significant differences (P≤0.05) between nonstimulated and stimulated groups; +, significant differences between stimulated and treated groups.
γδ T lymphocytes express BLT1
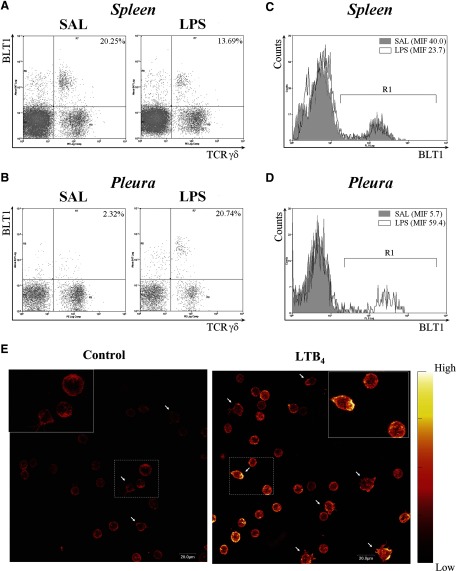
To verify that murine γδ T lymphocytes expressed the high-affinity receptor for LTB4, we assessed BLT1 expression by this T cell subset using two-color flow cytometry. As shown in representative dot plots in Figure 6, A and B, BTL1 is expressed on naïve γδ T lymphocytes recovered from spleen and pleural cavities from C57BL/6 mice. Interestingly the percentage of γδ T lymphocytes expressing BTL1 in spleen was diminished, whereas it was increased in the pleural cavity 12 h after LPS (250 ng/cavity, i.pl.) injection. In addition, BTL1 expression levels were increased 10 times on pleural γδ T lymphocytes after LPS stimulation (Fig. 6D), whereas no changes were observed on spleen γδ T cells (Fig. 6C). As during LPS-induced pleurisy, γδ T cells migrate from peripheral lymphoid organs to inflamed pleura [7], these data provide a basis for speculating that LTB4 is involved in this migratory route.
Figure 6.
BLT1 is expressed by γδ T lymphocytes and mediates F-actin polymerization. Representative dot plots of BLT1 expression by C57BL/6 murine γδ T lymphocytes recovered from spleen (A) and pleural cavities (B) 12 h after saline or LPS (250 ng/cavity) i.pl. injection. Histograms of gated γδ+ T lymphocytes from spleen (C) and pleura (D) are shown. R1 defines the BLT1-positive region, according to IgG isotype control. Numbers indicate mean intensity of fluorescence (MIF) of cells within R1. Leukocytes were stained with PE-labeled anti-γδ TCR plus Alexa 647-labeled anti-BLT1 for FACS analysis, as described in Materials and Methods. (E) Magnetically isolated spleen γδ T lymphocytes were stimulated for 15 min with LTB4 (100 nM) followed by F-actin cytoskeleton staining by rhodamine phalloidin. Cells were examined by confocal microscopy. Arrows indicate cells presenting a spread morphology. Color bar on the right of images represents the relative scale of fluorescence intensity.
LTB4 induces F-actin polymerization in γδ T lymphocytes
Chemotactic stimuli induce changes in cell morphology accompanied by cytoskeleton rearrangement [27,28,29]. To determine whether LTB4 elicits γδ T lymphocyte cytoskeleton changes, we analyzed the effect of LTB4 on F-actin polymerization in isolated γδ T lymphocytes. Purified, resting γδ T lymphocytes presented a spherical morphology and did not exhibit stress fibers, as shown by rhodamine phalloidin staining (Fig. 6E). After incubation with LTB4 for 15 min, the majority of γδ T cells exhibited intense staining of cortical F-actin fibers, indicating the rearrangement of cortical actin cytoskeleton. A few cells also showed a spread morphology with prominent cytoplasmic projections (filopodia extensions), as indicated by the arrows.
LTB4 modulates CCL2 production
We have demonstrated previously that CCL2 is an important mediator involved in γδ T lymphocytes recruitment into inflamed pleura during pleurisy induced by LPS, antigenic challenge, and BCG [2, 7]. We therefore analyzed whether LTB4, in addition to its direct effect on γδ T cells, could drive their migration into mouse pleural cavities indirectly via modulation of CCL2 production. As shown in Figure 7A, the i.pl. injection of LTB4 (0.5 μg/cavity) induced a significant increase in CCL2 levels in mouse pleural cavities within 6 h when compared with control mice. Interestingly, the in vivo blockade of BLT1 by CP105,696 (4 mg/kg, i.p.) impaired CCL2 production in mouse pleural cavities triggered by LPS (250 ng/cavity) stimulation (Fig. 7B), suggesting that LTB4 induces in vivo γδ T lymphocyte migration via this indirect, in addition to its direct, mechanism. Further supporting these data, LTB4 i.pl. injection into CCR2 KO mice was able to induce γδ T cell accumulation in the pleura, albeit to a lesser extent than in WT mice (Fig. 7C).
Figure 7.

LTB4 modulates CCL2 production. (A) Effect of LTB4 i.pl. injection (0.5 μg/cavity) on CCL2 production. (B) Effect of CP105,696 pretreatment (4 mg/kg, i.p.) on LPS (250 ng/cavity, i.pl.)-induced CCL2 synthesis. CCL2 protein levels were determined by ELISA in C57BL/6 mouse pleural fluids recovered 6 h after stimulation. Results are expressed as the mean ± sem from at least six animals/group from three different experiments. (C) Effect of LTB4 i.pl. injection (0.5 μg/cavity) on γδ T lymphocyte migration in WT or CCR2 KO mice within 24 h. *, Statistically significant differences (P≤0.05) between nonstimulated and stimulated animals; +, significant differences between stimulated and treated groups or between WT and KO animals. Data represent the mean ± sem from at least six animals.
DISCUSSION
The involvement of LTB4 in αβ T lymphocytes migration during inflammatory reactions has been increasingly appreciated; however, to our knowledge, the role of this lipid mediator in γδ T cell mobilization has not been explored previously [24, 26, 30, 31]. In the present study, we demonstrate that LTB4 is a chemoattractant for γδ T lymphocytes in vitro and in vivo and also mediates γδ T lymphocyte mobilization during inflammatory reactions in the pleura triggered by microbial components and antigen.
We have reported previously that LPS induced a marked increase in γδ T lymphocytes in pleural cavities of mice through an indirect mechanism that involves inflammatory mediators synthesized mainly by macrophages [7, 11]. Indeed, LPS is a potent inflammatory stimulus that triggers the production of a wide range of inflammatory chemoattractant mediators in vivo, including lipid mediators, in addition to cytokines and chemokines [32,33,34,35]. Furthermore, LTB4 production during inflammatory conditions has been reported previously to occur in the airways and other tissues, in which it displays the ability to attract different leukocyte populations to inflammatory sites [22, 36,37,38,39]. Previous reports demonstrated that the inhalation or the i.pl. in vivo administration of different stimuli, including LPS, up-regulates local production of LTB4 in mouse lung and pleura [20, 30, 40, 41]. Here, we show that LTB4 is produced during the early stage of LPS-induced pleurisy, in parallel to increased numbers of γδ T lymphocytes. LTs have been shown previously to play an essential role in mononuclear cell recruitment in in vivo models of inflammation, including αβ T lymphocyte recruitment to the airways [8, 24, 26, 30, 31]. αβ and γδ T lymphocytes have different mechanisms of migration into inflamed tissue. For example, Landgraf and coworkers [42] showed that cys-LTs do not mediate γδ T cell mobilization into the airways in a murine model of asthma, and they do mediate mobilization of αβ T lymphocytes.
We therefore investigated the ability of LTB4 to trigger γδ T cell migration and also to mediate γδ T cell influx in LPS-triggered inflammation. LTB4 induced γδ T lymphocyte migration in vivo and in vitro, suggesting that this lipid mediator is capable of direct effects on these cells. To assess the relevance of LTB4 in γδ T cell mobilization during inflammation, we blocked LT biosynthesis (with zileuton and MK886) and found significant inhibition of LPS-induced γδ T cell accumulation. Further data obtained with 5-LO−/− mice confirmed a striking degree of dependence of accumulation on endogenous LTs. Together, these findings establish that LTs play a crucial role in the recruitment of γδ T lymphocytes to inflamed tissue. Of note, the treatment of mice with MK571, a cys-LT receptor antagonist, failed to influence γδ T lymphocyte migration induced by LPS (data not shown), suggesting that LTB4 is the major 5-LO product mediating this phenomenon.
The confirmation of LTB4 as an important mediator for LPS-induced γδ T cell mobilization was established further by treatment with two different LTB4 BLT1 antagonists (CP105,696 and LY292476). Moreover, isolated, naïve splenocytes (γδ T cell source) incubated with CP105,696 and exposed to LPW, in which LTB4 presence was demonstrated, failed to migrate, suggesting that this T lymphocyte subset can be stimulated directly by LTB4 produced in response to LPS.
The relevance of LTB4, for γδ T cell migration in the inflammatory response driven by other stimuli, was also investigated. First, using the mouse model of allergic pleurisy, we found that LTB4 is also crucial to γδ T cell migration during allergy, in accordance with data obtained by Tager and coworkers [24] regarding αβ T subsets CD4+ and CD8+ cells. γδ T lymphocytes play important roles as the first line of defense against microorganisms, and their reactivity to mycobacteria reflects their involvement in the pathophysiology of mycobacterial infections. Indeed, γδ T lymphocytes are activated by Mycobacterium tuberculosis and BCG in vitro [43, 44] and also accumulate in lymphoid and nonlymphoid tissues after in vivo stimulation [43, 45]. In agreement with these data, we have demonstrated previously that the i.pl. administration of BCG induced the accumulation of γδ T lymphocytes in the pleural cavity of C57BL/6 mice [7]. The involvement of 5-LO products in host immune response to mycobacteria has not been investigated extensively; however, 5-LO inhibition has been shown recently to suppress the murine immune response to M. tuberculosis via down-regulation of Th1 responses [46]. However, in this model of repeated i.t. infection with M. tuberculosis, 5-LO inhibition exerted no effect on mononuclear cell accumulation in infected mice airways [46]. Here, we show that LTB4 mediated Mycobacterium bovis BCG-induced γδ T lymphocyte migration, suggesting that this lipid mediator is required for γδ T cell migration during inflammatory reactions.
The fact that LTB4 was capable of attracting γδ T lymphocytes in vitro suggested that it might act directly to do so in vivo. However, LTB4 has also been shown to modulate the production of other inflammatory mediators, including cytokines and chemokines [47], making it possible that LTB4 could also be acting indirectly. LTB4 and the CC chemokine CCL2 have been demonstrated to influence the production of each other in different experimental models in vivo and in vitro [22, 32, 48, 49], implicating CCL2 as an intermediate for LTB4 actions (or vice versa). We have shown previously that the CCL2/CCR2 pathway is important for γδ T lymphocyte migration in LPS- and OVA-induced pleurisy [2, 7]. Here, we show that the i.pl. administration of LTB4 induced significant increases in CCL2 levels in mouse pleural cavities and that CP105,696 treatment diminished LPS-induced CCL2 production (via BLT1 expressed by CCL2-producing cells, for example, macrophages). These data suggest a cross-talk between LTB4 and CCL2 in the evolution of LPS pleurisy, and induction of CCL2 production likely represents an additional mechanism by which LTB4 promotes γδ T lymphocytes recruitment to the inflamed pleura. We found that only a small proportion of the γδ T lymphocytes found in spleen and pleura was BLT1+. It is possible that only those γδ T cells expressing BLT1 respond directly to LTB4, whereas other subtype(s) depend on CCL2. Indeed, preliminary studies found that Vγ4 lymphocytes, which comprise approximately only one-third of total γδ T lymphocytes found in the pleura after LTB4 i.pl. stimulation, failed to accumulate in CCR2 KO mice, indicating that the migration of this subset depends mainly on CCL2 rather than on the direct action of LTB4 (data not shown). Further work will be necessary to better elucidate the involvement of different γδ T lymphocyte subsets in this response.
Alternatively, it is possible that the small number of γδ T cells exhibiting surface expression of BLT1 might result from ligand-dependent internalization during the inflammatory response. Indeed, BLT1, as most G protein-coupled receptors, has been shown to be readily internalized following activation in diverse cell types [50,51,52]. Despite the modest number of BLT1-positive cells, the direct BLT1-dependent chemotactic activity of LTB4 on γδ T lymphocytes was established by the studies in CCR2 KO mice.
BLT1 expression has been described on CD4+ and CD8+ T lymphocytes and human γδ T cells [24, 53]. However, the expression of BLT1 on murine γδ T cells has not been shown previously. Our results demonstrate that resident pleural γδ T cells in naive animals express BLT1, and after LPS stimulation, the percentage of BLT1+γδ T cells as well as the expression levels of BLT1 were enhanced. Indeed, overexpression of BLT1 has been demonstrated in inflamed lung tissue [24] and also, specifically in T lymphocytes recovered from the airways of patients with broncholitis [31]. In addition, we found that a population of spleen γδ T lymphocytes obtained from nonstimulated mice was BLT1-positive and that this population decreased after LPS i.pl. stimulation. Whether BLT1+γδ T cells found in inflamed pleura originate from secondary lymphoid tissues, such as spleen, needs further investigation. It is noteworthy that γδ T cells comprised the majority of the cells expressing BLT1 in the spleen, and these migrated toward LTB4 to a greater extent than did total CD3 T lymphocytes. These data support the functional importance of BLT1 for γδ T cell migration during the inflammatory response.
Cell migration is a dynamic process that involves F-actin polymerization leading to cytoskeleton reorganization accompanied by formation of filopodia and lammellipodia [54]. BLT1, like other G protein-coupled receptors, is known to activate small GTPases and induce the reorganization of actin cytoskeleton [55]. The ability of LTB4 to accomplish this in γδ T cells lends plausibility to its capacity to activate γδ T lymphocytes directly via BLT1, providing a possible basis for LTB4 participation in the recruitment of γδ T cells to the inflamed pleura.
In conclusion, we provide evidence for the first time that LPS recruits γδ T lymphocytes to inflamed sites via a mechanism dependent on the synthesis of LTB4 and signaling via BLT1. LTB4 also up-regulates CCL2 production, which contributes to γδ T lymphocyte accumulation in inflamed pleura via the CCR2 receptor. The fact that LTB4 also mediates γδ T lymphocyte mobilization induced by other stimuli, such as M. bovis BCG and antigenic challenge, suggests that this mediator is broadly required for the migration of γδ T cells to inflammatory sites and reinforces a role for LTB4 in linking innate and acquired immune responses. Further experiments will be required to determine if this important role for LTB4 in γδ T cell recruitment applies to tissues other than the pleural cavity.
AUTHORSHIP
R. S-M. and M. F. S. C. performed the experiments and analyzed data. B. P. and B. L. D. performed F-actin assay and analysis. C. F. B. contributed with study design of CCR2 KO mice experiments. M. C. S. contributed with BCG study design, performance, and analysis. R. S-M., M. F. S. C., and M. C. S. helped draft the manuscript. M. G. H. and M. P-G. contributed to the study design and edited the manuscript. C. P. and C. C. designed research, supervised the work, analyzed data, and wrote the manuscript.
ACKNOWLEDGMENTS
This work was supported by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho de Desenvolvimento Científico e Tecnológico (CNPq), and Fundação Oswaldo Cruz (Fiocruz). M. F. S. C. and R. S-M. are students of the Post-Graduation Program in Cellular and Molecular Biology of Fiocruz. The authors thank André L. P. Candéa (Fiocruz) for confocal image acquisition and Victor Ugarte Bornstein (Fiocruz) and Fausto K. Ferraris (Fiocruz) for technical assistance.
Footnotes
Abbreviations: 5-LO=5-lipoxygenase, BCG=bacille Calmette-Guérin, BLT1=LTB4 receptor 1, cys-LT=cysteinyl-LT, EIA=enzyme immunoassay, FLAP=5-LO activating protein, i.pl.=intrapleural, KO=knockout, LPW=LPS pleural wash, LTB4=leukotriene B4, NP-40=Nonidet P-40, p.o.=per os, SPW=saline pleural wash, WT=wild-type
References
- Dodd J., Riffault S., Kodituwakku J. S., Hayday A. C., Openshaw P. J. Pulmonary V γ 4+ γ δ T cells have proinflammatory and antiviral effects in viral lung disease. J Immunol. 2009;182:1174–1181. doi: 10.4049/jimmunol.182.2.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penido C., Costa M. F. S., Souza M. C., Costa K. A., Candéa A. L., Benjamim C. F., Henriques M. G. Involvement of CC chemokines in γδ T lymphocyte trafficking during allergic inflammation: the role of CCL2/CCR2 pathway. Int Immunol. 2008;20:129–139. doi: 10.1093/intimm/dxm128. [DOI] [PubMed] [Google Scholar]
- Costa M. F. S., Nihei J., Mengel J., Henriques M. G., Penido C. Requirement of L-selection for γδ T lymphocyte activation and migration during allergic pleurisy: co-relation with eosinophil accumulation. Int Immunopharmacol. 2009;9:303–312. doi: 10.1016/j.intimp.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Carding S. R., Egan P. J. γδ T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- Nakasone C., Yamamoto N., Nakamatsu M., Kinjo T., Miyagi K., Uezu K., Nakamura K., Higa F., Ishikawa H., O'Brien R. L., Ikuta K., Kaku M., Fujita J., Kawakami K. Accumulation of γ/δ T cells in the lungs and their roles in neutrophil-mediated host defense against pneumococcal infection. Microbes Infect. 2007;9:251–258. doi: 10.1016/j.micinf.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Souza M. C., Penido C., Costa M. F. S., Henriques M. G. Mechanisms of T-lymphocyte accumulation during experimental pleural infection induced by Mycobacterium bovis BCG. Infect Immun. 2008;76:5686–5693. doi: 10.1128/IAI.00133-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penido C., Vieira-de-Abreu A., Bozza M. T., Castro-Faria-Neto H. C., Bozza P. T. Role of monocyte chemotactic protein 1/CC chemokine ligand 2 on γ δ T lymphocyte trafficking during inflammation induced by lipopolysaccharide or Mycobacterium bovis bacille Calmette-Guérin. J Immunol. 2003;171:6788–6794. doi: 10.4049/jimmunol.171.12.6788. [DOI] [PubMed] [Google Scholar]
- Prinz I., Gregoire C., Mollenkopf H., Aguado E., Wang Y., Malissen M., Kaufmann S. H., Malissen B. The type 1 cysteinyl leukotriene receptor triggers calcium influx and chamotaxis in mouse α β- and γ δ effector T cells. J Immunol. 2005;175:713–719. doi: 10.4049/jimmunol.175.2.713. [DOI] [PubMed] [Google Scholar]
- Shen Y., Zhou D., Qiu L., Lai X., Simon M., Shen L., Kou Z. S., Wang Q., Jiang L., Estep J., Hunt R., Clagett M., Sehgal P. K., Li Y., Zeng X., Morita C. T., Brenner M. B., Lentvin N. L., Chen Z. W. Adaptive immune response of Vγ2Vδ2+ T cells during mycobacteriral infections. Science. 2002;295:2255–2258. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza P. T., Castro-Faria-Neto H. C., Penido C., Laranjeira A. P., Henriques M. G., Silva P. M., Martins M. A., dos Santos R. R., Cordeiro R. S. Requirement for lymphocytes and resident macrophages in LPS-induced pleural eosinophil accumulation. J Leukoc Biol. 1994;56:151–158. doi: 10.1002/jlb.56.2.151. [DOI] [PubMed] [Google Scholar]
- Penido C., Castro-Faria-Neto H. C., Laranjeira A. P., Rosas E. C., Ribeiro-dos-Santos R., Bozza P. T., Henriques M. G. The role of γδ T lymphocytes in lipopolysaccharide-induced eosinophil accumulation into the mouse pleural cavity. J Immunol. 1997;159:853–860. [PubMed] [Google Scholar]
- Peters-Golden M. Expanding roles for leukotrienes in airway inflammation. Curr Allergy Asthma Rep. 2008;8:367–373. doi: 10.1007/s11882-008-0057-z. [DOI] [PubMed] [Google Scholar]
- Migliorisi G., Folkes E., Pawlowski N. In vitro studies of human monocyte migration across endothelium in response to leukotriene B4 and fMet-Leu-Phe. Am J Pathol. 1987;127:157–167. [PMC free article] [PubMed] [Google Scholar]
- Bailie M. B., Standiford T. J., Laichalk L. L., Coffey M. J., Strieter R., Peters-Golden M. Leukotriene-deficient mice manifest enhanced lethality from Klebsiella pneumonia in association with decreased alveolar macrophage phagocytic and bactericidal activities. J Immunol. 1996;157:5221–5224. [PubMed] [Google Scholar]
- Mancuso P., Standiford T. J., Marshall T., Peters-Golden M. 5-Lipoxygenase reaction products modulate alveolar macrophage phagocytosis of Klebsiella pneumoniae. Infect Immun. 1998;66:5140–5146. doi: 10.1128/iai.66.11.5140-5146.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canetti C. A., Leung B. P., Culshaw S., McInnes I. B., Cunha F. Q., Liew F. Y. IL-18 enhances collagen-induced arthritis by recruiting neutrophils via TNF-α and leukotriene B4. J Immunol. 2003;171:1009–1015. doi: 10.4049/jimmunol.171.2.1009. [DOI] [PubMed] [Google Scholar]
- Serezani C. H., Aronoff D. M., Jancar S., Mancuso P., Peters-Golden M. Leukotrienes enhance the bactericidal activity of alveolar macrophages against Klebsiella pneumoniae through the activation of NADPH oxidase. Blood. 2005;106:1067–1075. doi: 10.1182/blood-2004-08-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamand L., Tremblay M. J., Borgeat P. Leukotriene B4 triggers the in vitro and in vivo release of potent antimicrobial agents. J Immunol. 2007;178:8036–8045. doi: 10.4049/jimmunol.178.12.8036. [DOI] [PubMed] [Google Scholar]
- Pace E., Profita M., Melis M., Bonanno A., Paterno A., Mody C. H., Spatafora M., Ferraro M., Siena L., Vignola A. M., Bonsignore G., Gjomarkaj M. LTB4 is present in exudative pleural effusions and contributes actively to neutrophil recruitment in the inflamed pleural space. Clin Exp Immunol. 2004;135:519–527. doi: 10.1111/j.1365-2249.2003.02387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittirsch D., Flierl M. A., Day D. E., Nadeau B. A., MacGuire S. R., Hoesel L. M., Ipaktchi K., Zetoune F. S., Sarma J. V., Leng L., Huber-Lang M. S., Neff T. A., Bucala R., Ward P. A. Acute lung injury induced by lipopolysaccharide is independent of complement activation. J Immunol. 2008;180:7664–7672. doi: 10.4049/jimmunol.180.11.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsopoulos P., Omri A., Alipour M., Vermeulen N., Smith M. G., Suntres Z. E. Effectiveness of liposomal-N-acetylcysteine against LPS-induced injuries in rodents. Int J Pharm. 2008;363:106–111. doi: 10.1016/j.ijpharm.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Cheraim A. B., Xavier-Elsas P., de Oliveira S. H., Batistella T., Russo M., Gaspar-Elsas M. I., Cunha F. Q. Leukotriene B4 is essential for selective eosinophil recruitment following allergen challenge of CD4+ cells in a model of chronic eosinophilic inflammation. Life Sci. 2008;83:214–222. doi: 10.1016/j.lfs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Davies D., Larbi K., Allen A., Sanz M., Weg V. B., Haskard D. O., Lobb R. R., Nourshargh S. VCAM-1 contributes to rapid eosinophil accumulation induced by the chemoattractants PAF and LTB4: evidence for basal expression of functional VCAM-1 in rat skin. Immunology. 1999;97:150–158. doi: 10.1046/j.1365-2567.1999.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager A. M., Bromley S. K., Medoff B. D., Islam S. A., Bercury S. D., Friedrich E. B., Carafone A. D., Gerszten R. E., Luster A. D. Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nat Immunol. 2003;4:982–990. doi: 10.1038/ni970. [DOI] [PubMed] [Google Scholar]
- Goodarzi K., Goodarzi M., Tager A. M., Luster A. D., von Andrian U. H. Leukotriene B4 and BLT1 control cytotoxic effector T cell recruitment to inflamed tissues. Nat Immunol. 2003;4:965–973. doi: 10.1038/ni972. [DOI] [PubMed] [Google Scholar]
- Miyahara N., Takeda K., Miyahara S., Taube C., Joetham A., Koya T., Matsubara S., Dakhama A., Tager A. M., Luster A. D., Gelfand E. W. Leukotriene B4 receptor-1 is essencial for allergen-mediated recreitment of CD8+ T cells and airway hyperresponsiveness. J Immunol. 2005;174:4979–4984. doi: 10.4049/jimmunol.174.8.4979. [DOI] [PubMed] [Google Scholar]
- Sakata D., Taniguchi H., Yasuda S., Adachi-Morishima A., Hamazaki Y., Nakayama R., Miki T., Minato N., Narumiya S. Impaired T lymphocyte trafficking in mice deficient in an actin-nucleating protein, mDia1. J Exp Med. 2007;204:2031–2038. doi: 10.1084/jem.20062647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern D., Lloyd C. M., Robinson D. S. Chemokine responsiveness of CD4+ CD25+ regulatory and CD4+ CD25– T cells from atopic and nonatopic donors. Allergy. 2009;64:1121–1129. doi: 10.1111/j.1398-9995.2008.01962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. S., Mitchell J. S., DeNucci C. C., Martin A. L., Shimizu Y. The p110γ isoform of phosphatidylinositol 3-kinase regulates migration of effector CD4 T lymphocytes into peripheral inflammatory sites. J Leukoc Biol. 2008;84:814–823. doi: 10.1189/jlb.0807561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube C., Miyahara N., Ott V., Swanson B., Takeda K., Loader J., Shultz L. D., Tager A. M., Luster A. D., Dakhama A., Gelfand E. W. The leukotriene B4 receptor (BLT1) is required for effector CD8+ T cell-mediated, mast cell-dependent airway hyperresponsiveness. J Immunol. 2006;176:3157–3164. doi: 10.4049/jimmunol.176.5.3157. [DOI] [PubMed] [Google Scholar]
- Medoff B. D., Seung E., Wain J. C., Means T. K., Campanella G. S., Islam S. A., Thomas S. Y., Ginns L. C., Grabie N., Lichtman A. H., Tager A. M., Luster A. D. BLT1-mediated T cell trafficking is critical for rejection and obliterative bronchiolitis after lung transplantation. J Exp Med. 2005;202:97–110. doi: 10.1084/jem.20042481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco P., Vieira-de-Abreu A., Gomes R. N., Barbosa-Lima G., Wermelinger L. B., Maya-Monteiro C. M., Silva A. R., Bozza M. T., Castro-Faria-Neto H. C., Bandeira-Melo C., Bozza P. T. Monocyte chamoattractant protein-1/CC chemokine ligand 2 controls microtube-driven biogenesis and leukotriene B4-synthesizing function of macro-phage lipid bodies elicited by innate immune response. J Immunol. 2007;179:8500–8508. doi: 10.4049/jimmunol.179.12.8500. [DOI] [PubMed] [Google Scholar]
- Zeldin D. C., Wohlford-Lenane C., Chulada P., Bradbury J. A., Scarborough P. E., Roggli V., Langenbach R., Schawatz D. A. Airway inflammation and responsiveness in prostaglandin H synthase-deficient mice exposed to bacterial lipopolysaccharide. Am J Respir Cell Mol Biol. 2001;25:457–465. doi: 10.1165/ajrcmb.25.4.4505. [DOI] [PubMed] [Google Scholar]
- Penido C., Castro-Faria-Neto H. C., Vieira-de-Abreu A., Figueiredo R. T., Pelled A., Martins M. A., Jose P. J., Williams T. J., Bozza P. T. LPS induces eosinophil migration via CCR3 signaling through a mechanism independent of RANTES and eotaxin. Am J Respir Cell Mol Biol. 2001;25:707–716. doi: 10.1165/ajrcmb.25.6.4401. [DOI] [PubMed] [Google Scholar]
- Johnston C. J., Finkelstein J. N., Gelein R., Oberdörster G. Pulmonary cytokine and chemokine mRNA levels after inhalation of lipopolysaccharide in C57BL/6 mice. Toxicol Sci. 1998;46:300–307. doi: 10.1006/toxs.1998.2557. [DOI] [PubMed] [Google Scholar]
- Henderson W. R., Jr, Lewis D. B., Albert R. K., Zhang Y., Lamm W. J., Chiang G. K., Jones F., Eriksen P., Tien Y. T., Jonas M., Chi E. Y. The importance of leukotrienes in airway inflammation in a mouse model of asthma. J Exp Med. 1996;184:1483–1494. doi: 10.1084/jem.184.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley L., Kim J., Bolgos G. L., Siddiqui J., Remick D. G. Allergens induce enhanced bronchoconstriction and leukotriene production in C5 deficient mice. Respir Res. 2006;7:129. doi: 10.1186/1465-9921-7-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte Fde P., Barja-Fidalgo C., Verri W. A., Jr, Cunha F. Q., Rae G. A., Penido C., Henriques M. G. Endothelins modulate inflammatory reaction in zymosan-induced arthritis: participation of LTB4, TNF-α, and CXCL-1. J Leukoc Biol. 2008;84:652–660. doi: 10.1189/jlb.1207827. [DOI] [PubMed] [Google Scholar]
- Guerrero A. T., Verri W. A., Jr, Cunha T. M., Silva T. A., Schivo I. R., Dal-Secco D., Canetti C., Rocha F. A., Parada C. A., Cunha F. Q., Ferreira S. H. Involvement of LTB4 in zymosan-induced joint nociception in mice: participation of neutrophils and PGE2. J Leukoc Biol. 2008;83:122–130. doi: 10.1189/jlb.0207123. [DOI] [PubMed] [Google Scholar]
- Klein A., Talvani A., Silva P. M., Martins M. A., Wells T. N., Proudfoot A., Luckacs N. W., Teixeira M. M. Stem cell factor-induced leukotriene B4 production cooperates with eotaxin to mediate the recruitment of eosinophils during allergic pleurisy in mice. J Immunol. 2001;167:524–531. doi: 10.4049/jimmunol.167.1.524. [DOI] [PubMed] [Google Scholar]
- Van Heeckeren A. M., Schluchter M. D., Drumm M. L., Davis P. B. Role of Cftr genotype in the response to chronic Pseudomonas aeruginosa lung infection in mice. Am J Physiol Lung Cell Mol Physiol. 2004;287:L944–L952. doi: 10.1152/ajplung.00387.2003. [DOI] [PubMed] [Google Scholar]
- Landgraf R. G., Nossi D. F., Sirois P., Jancar S. Prostaglandins, leukotrienes and PAF selectively modulate lymphocyte subset and eosinophil infiltration into the airways in a murine model of asthma. Prostaglandins Leukot Essent Fatty Acids. 2007;77:163–172. doi: 10.1016/j.plefa.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Inoue T., Yoshikai Y., Matsuzaki G., Nomoto K. Early appearing γ/δ-bearing T cells during infection with Calmette Guérin bacillus. J Immunol. 1991;146:2754–2762. [PubMed] [Google Scholar]
- Cipriani B., Borsellino G., Poccia F., Placido R., Tramonti D., Bach S., Battistini L., Brosnan C. F. Activation of C-C β-chemokines in human peripheral blood γδ T cells by isopentenyl pyrophosphate and regulation by cytokines. Blood. 2000;95:39–47. [PubMed] [Google Scholar]
- Griffin J. P., Harshan K. V., Born W. K., Orme I. M. Kinetics of accumulation of γ δ receptor-bearing T lymphocytes in mice infected with live mycobacteria. Infect Immun. 1991;59:4263–4265. doi: 10.1128/iai.59.11.4263-4265.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peres C. M., de Paula L., Medeiros A. I., Sorgi C. A., Soares E. G., Carlos D., Peters-Golden M., Silva C. L., Faccioli L. H. Inhibition of leukotriene biosynthesis abrogates the host control of Mycobacterium tuberculosis. Microbes Infect. 2007;9:483–489. doi: 10.1016/j.micinf.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters-Golden M., Canetti C., Mancuso P., Coffey M. J. Leukotrienes: underappreciated mediators of innate immune responses. J Immunol. 2005;174:589–594. doi: 10.4049/jimmunol.174.2.589. [DOI] [PubMed] [Google Scholar]
- Huang L., Zhao A., Wong F., Ayala J. M., Struthers M., Ujjainwalla F., Wright S. D., Springer M. S., Evans J., Cui J. Leukotriene B4 strongly increases monocyte chamoattractant protein-1 in human monocytes. Arterioscler Thromb Vasc Biol. 2004;24:1783–1788. doi: 10.1161/01.ATV.0000140063.06341.09. [DOI] [PubMed] [Google Scholar]
- Matsukawa A., Hogaboam C. M., Lukacs N. W., Lincoln P. M., Strieter R. M., Kunkel S. L. Endogenous monocyte chemoattractant protein-1 (MCP-1) protects mice in a model of acute septic peritonitis: cross-talk between MCP-1 and leukotriene B4. J Immunol. 1999;163:6148–6154. [PubMed] [Google Scholar]
- Jala V. R., Shao W. H., Haribabu B. Phosphorylation independent β-arrestin translocation and internalization of leukotriene B4 receptors. J Biol Chem. 2004;280:4880–4887. doi: 10.1074/jbc.M409821200. [DOI] [PubMed] [Google Scholar]
- Gaudreault E., Thompson C., Stankova J., Rola-Pleszczynski M. Involvement of BLT1 endocytosis and Yes kinase activation in leukotriene B4-induced neutrophil degranulation. J Immunol. 2005;174:3617–3625. doi: 10.4049/jimmunol.174.6.3617. [DOI] [PubMed] [Google Scholar]
- Chen Z., Gaudreau R., Le Gouill C., Rola-Pleszczynski M., Stankova J. Agonist-induced internalization of leukotriene B4 receptor 1 requires G-protein-coupled receptor kinase 2 but not arrestins. Mol Pharmacol. 2004;66:377–384. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- Islam S. A., Thomas S. Y., Hess C., Medoff B. D., Means T. K., Brander C., Lilly C. M., Tager A. M., Luster A. D. The leukotriene B4 lipid chemoattractant receptor BLT1 defines antigen-primed T cells in humans. Blood. 2006;107:444–453. doi: 10.1182/blood-2005-06-2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Madrid F., Pozo M. A. Leukocyte polarization in cell migration and immune interactions. EMBO J. 1999;18:501–511. doi: 10.1093/emboj/18.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavelaars A., Vroon A., Raatgever R. P., Fong A. M., Premont R. T., Patel D. D., Lefkowitz R. J., Heijnen C. J. Increased acute inflammation, leukotriene B4-induced chemotaxis, and signaling in mice deficient for G protein-coupled receptor kinase 6. J Immunol. 2003;171:6128–6134. doi: 10.4049/jimmunol.171.11.6128. [DOI] [PubMed] [Google Scholar]