Review on alcohol abuse increasing the risk for acute respiratory distress syndrome through alterations in pulmonary function, and implications to potential therapeutic targets.
Keywords: lung injury, oxidative stress
Abstract
ARDS is a severe form of lung injury characterized by increased permeability of the alveolar capillary membrane, diffuse alveolar damage, the accumulation of proteinaceous interstitial and intra-alveolar edema, and the presence of hyaline membranes. These pathological changes are accompanied by physiological alterations, including severe hypoxemia, an increase in pulmonary dead space, and decreased pulmonary compliance. Approximately 200,000 individuals develop ARDS in the United States each year [1], and nearly 50% of these patients have a history of alcohol abuse. We have identified alcohol abuse as an independent risk factor for the development of ARDS [2,3,4,5], and more recent studies have validated these findings in patients following lung resection and blood transfusion [2, 3]. In ARDS survivors, alcohol abuse is also associated with an increased duration of mechanical ventilation and prolonged ICU length of stay [5]. Despite studies aimed at improving outcomes in patients with ARDS, the mortality remains high at >40% [6]. For those who abuse alcohol, the mortality is even higher, at 65% [4]. In this review, we will discuss the relationship between alcohol abuse and ARDS, the effects of alcohol abuse on pulmonary function, and future directions and potential therapeutic targets for patients at risk for ARDS as a result of alcohol abuse, which impairs immune function, decreases pulmonary antioxidant capacity, decreases alveolar epithelial cell function, alters activation of the renin angiotensin system, and impairs GM-CSF signaling. These pathways represent potential therapeutic targets for patients at risk for ARDS as a result of alcohol abuse.
Introduction
Alcohol is the most frequently abused drug in the world [7], and 15–20 million individuals in the United States meet the diagnosis of alcohol-dependence [8]. The lifetime prevalence of alcohol use in the United States is 66%, and nearly 50% of adult Americans have used alcohol at some time during the last 12 months [9]. In some countries, such as the Czech Republic, alcohol consumption is virtually ubiquitous, as 98% of adult men and women drank alcohol during the previous 12 months [10]. Drinking more than two glasses of alcohol per day has deleterious health effects and is associated with increased mortality [11, 12]. The regular use of alcohol also imposes a tremendous economic burden. In the Unites States alone, alcohol use costs $166 billion annually, of which nearly 20% is for direct medical expenses [13, 14].
Alcohol abuse is common among critically ill patients [15, 16] and is responsible for up to 40% of admissions to the ICU in some U.S. hospitals. It also has direct adverse affects on patient outcomes, including higher likelihood of ICU admission, longer hospital length of stay, increased sepsis, and an increased requirement for mechanical ventilation [1, 5, 17, 18]. Jensen and colleagues [19] examined the effects of alcohol abuse in a cohort of ICU patients with a similar preadmission health status, general indication for ICU admission, and age [19]. A history of alcohol abuse was associated with a doubling of the mortality rate in this cohort of all patients that require ICU care. As these critically ill patients cannot always communicate as a result of the need for mechanical ventilation or sedative agents, a history of alcohol abuse is often not obtained. Subsequently, important prognostic information may go unnoticed, and specific alcohol-related interventions may not be initiated routinely.
Although alcohol is a systemic drug that affects almost every organ system in the body, the effects of alcohol on the lung have been relatively unexplored. The most significant pulmonary effects of alcohol abuse involve an increased risk of bacterial infection and acute lung injury. In this article, we will review the effects of alcohol abuse on the epidemiology of pneumonia and ARDS. The effects of alcohol abuse on various aspects of pulmonary function will also be addressed, including alcohol-related effects on pulmonary antioxidant capacity. Finally, we will discuss future directions for investigation of alcohol abusers as well as potential therapies for critically ill patients at risk for ARDS.
ALCOHOL ABUSE INCREASES THE RISK OF PNEUMONIA AND ARDS
The relationship between alcohol abuse and pulmonary disease has been known for centuries. Dr. Benjamin Rush [20], in his 1785 An Inquiry Into the Effects of Ardent Spirits Upon the Human Body and Mind, described the relationship between alcohol and disease, stating that ardent spirits “dispose to every form of acute disease.” Included in his discussion was the observation that alcohol abuse often results in the development of “hoarseness, and a husky cough, which often terminate in consumption, and sometimes in an acute and fatal disease of the lungs”. Since that time, multiple studies have linked alcohol abuse with worsened pulmonary disease and increased mortality.
Alcohol abuse and pneumonia
The incidence of CAP is greater in individuals with a history of alcohol abuse. Patients with pneumonia have significantly higher daily alcohol intake prior to hospitalization and are more likely to have used alcohol chronically when compared with patients without pneumonia, even after adjusting for the presence of cirrhosis and cigarette smoking [15]. Alcohol abuse is also associated with increased morbidity in patients with CAP, resulting in a greater incidence of bacteremia, delayed time to recovery, and a higher frequency of persistent pulmonary infiltrates on chest radiograph [15, 21, 22]. In patients with CAP as a result of Streptococcus pneumoniae, alcohol abuse is associated with a higher rate of empyema [23]. Most importantly, alcohol abuse is associated with increased mortality in patients with CAP [24]. In a pre-antibiotic study published in 1923 [24], a dose-response effect of alcohol abuse on mortality from bacterial pneumonia was observed that remained after age adjustment. The deleterious effect of alcohol abuse on mortality has persisted despite the use of antibiotics. In a meta-analysis of CAP, mortality was significantly higher in patients with a history of alcohol abuse [25]. Economic implications associated with CAP in patients with a history of alcohol abuse are also apparent, as reflected by increased total hospital charges and more frequent ICU use [16].
HAP is the second most common nosocomial infection and represents nearly 50% of all acquired infections in the ICU. When HAP occurs in the subset of patients receiving mechanical ventilation, it is termed VAP. In one study of 223 mechanically ventilated patients, where patients were simply classified as current, former, or never drinkers, alcohol abuse was not a risk factor for the development of VAP [26]. However, these negative results may have been related to misclassification of alcohol abuse as a result of the use of an imprecise definition. In a case-controlled study of patients with VAP and matched ICU controls, a multivariable analysis revealed that chronic alcohol abuse was the strongest risk factor for ICU mortality (odds ratio 2.6) [27]. More recent evidence demonstrates that at-risk drinkers, as defined by the NIAAA criteria, were at higher risk for developing VAP than not-at-risk drinkers, even after adjusting for age, gender, Simplified Acute Physiology Score II, length of hospital stay before ICU admission, prior antibiotic administration within 24 h before ICU admission, type of admission, immunosuppression, duration of mechanical ventilation, and central venous and urinary catheter exposure (hazard ratio 1.76) [28].
Alcohol abuse and ARDS
ARDS is a form of diffuse lung injury that is characterized pathologically by disruption of the pulmonary capillary-alveolar membrane. Although a healthy lung effectively regulates alveolar fluid movement, disruption of the pulmonary capillary-alveolar membrane in ARDS results in the accumulation of excess interstitial and alveolar fluid. The consequences of these changes include impaired gas exchange, decreased compliance, and increased pulmonary arterial pressure. Clinically, ARDS presents as the sudden onset of severe hypoxemia in conjunction with bilateral infiltrates on chest radiographs that are noncardiogenic in origin [29]. Criteria for the diagnosis of ARDS include [1] the presence of bilateral pulmonary infiltrates [2], a ratio of the arterial partial pressure of oxygen/fraction of inspired oxygen of <200 mmHg, and [3] the absence of clinical evidence for left heart failure (or a pulmonary capillary wedge pressure ≤18 mmHg, if measured). Approximately 200,000 individuals develop ARDS in the United States each year [1], and the mortality from this disease remains unacceptably high at >40% [6]. There are many risk factors for the development of ARDS, including sepsis, pneumonia, surgery, trauma, burn, blood transfusion, pancreatitis, aspiration, and others. Recent evidence has also shown that a history of alcohol abuse is an independent risk factor for the development of ARDS.
Several studies have demonstrated that a prior history of alcohol abuse is associated with an increased risk of developing ARDS. In one study of 351 critically ill patients with one of seven at-risk diagnoses for the development of ARDS, 43% of the patients who abused alcohol developed ARDS, as opposed to only 22% of those without a history of alcohol abuse [4]. In patients with sepsis, this difference was even more impressive, with 52% of the patients with a prior history of alcohol abuse developing ARDS, compared with only 20% in patients without a history of alcohol abuse. In those patients who developed ARDS, the in-hospital mortality rate was 65% in patients with a prior history of alcohol abuse compared with only 36% in patients without a history of alcohol abuse. Importantly, the effect of chronic alcohol abuse on the development of ARDS remained significant after adjusting for severity of illness and co-morbidities.
In a more recent study of 220 patients with septic shock, 30% of patients had a history of alcohol abuse. In these individuals, the incidence of ARDS in patients with a history of alcohol abuse was 70%, compared with 31% in individuals without a history of chronic alcohol abuse [5]. In addition, patients with a history of chronic alcohol abuse had more severe nonpulmonary organ dysfunction. Importantly, these effects again remained significant after adjusting for differences in the source of infection, sex, age, chronic hepatic dysfunction, diabetes, severity of illness, nutritional status, and smoking status.
As ALI may complicate thoracic surgery and is a major contributor to postoperative mortality, risk factors for ALI were examined in 879 patients who underwent pulmonary resections for non-small cell lung carcinoma. In this cohort, the total incidence of ALI was 4.2%, and the majority of cases occurred in the absence of a predisposing clinical adverse event. In those patients who developed ALI in the absence of an inciting event, a preoperative history of alcohol abuse was identified as an independent risk factors for ALI (odds ratio 1.9) [30].
TRALI is a form of ALI that typically develops within 6 h following transfusion and is the leading cause of transfusion-related mortality. To determine risk factors for the development of TRALI, 901 consecutive transfused critically ill patients were observed closely for the development of ALI. Risk factors were then compared between patients who developed ALI after transfusion and transfused control patients and matched by age, sex, and admission diagnosis. Seventy-four transfused patients developed ALI within 6 h of transfusion (8%), and compared with transfused control subjects, patients with ALI were more likely to have a history of chronic alcohol abuse (37% in those who developed ALI compared with 18% in those who did not develop ALI) [2].
PATHOGENESIS OF ALCOHOL-RELATED PULMONARY DYSFUNCTION
Acute alcohol intoxication and pulmonary immune function
Acute alcohol exposure has been shown to alter neutrophil function in a variety of ways, including decreased adhesion molecule expression, impaired margination and adhesion, impaired release of granule contents, decreased chemotaxis, and decreased phagocytosis and bacterial killing [31,32,33,34,35,36,37,38]. For example, acute alcohol exposure inhibits up-regulation of CD18, an adhesion molecule that helps bind target receptors on endothelial cells [39,40,41,42]. The production of important neutrophil chemotactic cytokines, including MIP-2 and IL-8, is also decreased by acute ethanol intoxication [43, 44].
The importance of these alcohol-induced defects in neutrophil function is highlighted by studies aimed at improving neutrophil function in the intoxicated host. G-CSF, a hematopoietic growth factor that regulates neutrophil production and function [45], improves neutrophil function in the setting of acute alcohol intoxication. When rats are pretreated with G-CSF prior to acute alcohol intoxication, neutrophil recruitment and bacterial clearance are enhanced following intrapulmonary challenge with Klebsiella pneumoniae [46]. Additional studies have demonstrated the effects of G-CSF pretreatment on neutrophil adhesion molecule expression in control and acutely intoxicated rats following i.v. or intratracheal challenge with LPS [38, 47]. Following LPS challenge, alcohol impairs the up-regulation of neutrophil CD18 and CD11b/c expression following LPS challenge; however, G-CSF pretreatment attenuates this alcohol-induced defect. Overall, neutrophil function is impaired by alcohol, resulting in impaired host defense against infection and increased morbidity and mortality. Therapy involving improvement of neutrophil function in the intoxicated host may prove useful in the treatment of patients with pulmonary infection, many of whom abuse alcohol.
In addition to its effects on neutrophil function, acute alcohol exposure suppresses the production of proinflammatory cytokines, including TNF-α and IL-1β, by alveolar macrophages and blood monocytes [48]. Critical functions of TNF-α include enhancing the release of additional proinflammatory cytokines, increasing adhesion molecule expression on vascular endothelial cells and neutrophils, augmenting the function of neutrophils at an inflammatory site, and increasing bacterial clearance and survival following bacterial challenge. The release of other proinflammatory cytokines and chemokines has also been shown to be impaired by acute alcohol intoxication, including GM-CSF, G-CSF, IL-6, IL-8, and MIP-2 [31, 42, 49,50,51]. Conversely, alcohol exposure has the opposite affect on the production of IL-10 and TGF-β, increasing their spontaneous release and augmenting their production in response to inflammatory stimuli [51]. As IL-10 and TGF-β are anti-inflammatory cytokines, alcohol-induced enhancement of their release results in further suppression of the immune response.
Alcohol alters cytokine production in macrophages in part by inhibiting the activation of the intracellular messenger NF-κβ, which is required for the transcription of genes that encode for TNF-α and other chemokines [52]. Other studies have demonstrated that alcohol alters expression of TLRs, which are responsible for recognition of bacterial antigens and transmembrane signaling leading to cytokine production [53,54,55,56]. These changes appear to be a result, in part, of alterations of components of the TLR complex within lipid rafts, with subsequent changes in actin cytoskeleton rearrangement, receptor clustering, and cell signaling [57].
Chronic alcohol abuse and pulmonary immune function
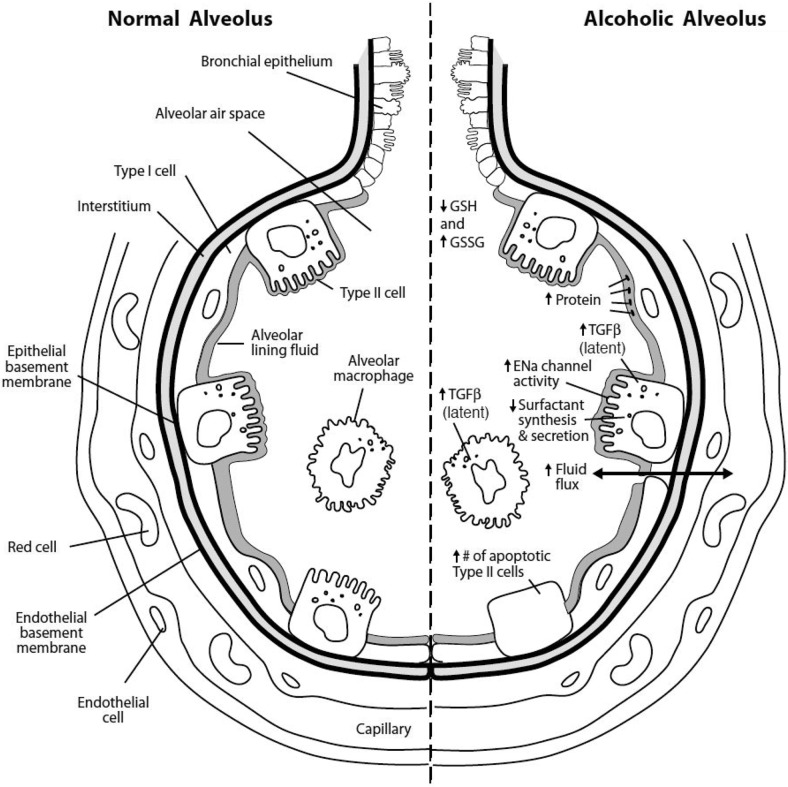
Chronic alcohol exposure modulates immune function at different sites than acute alcohol exposure, and Figure 1 outlines many of the pulmonary changes associated with chronic alcohol abuse [58]. Although acute alcohol intoxication has been reported to impair proinflammatory cytokine production, chronic alcohol abuse, particularly in association with alcoholic liver disease, has been reported to increase levels of circulating TNF, IL-1, and IL-6 [59]. Production of the neutrophil chemotactic cytokine MIP-2 from alveolar macrophages remains inhibited during chronic alcohol exposure [60,61]. In a cohort of trauma patients, chronic alcohol abuse has been reported to increase plasma levels of the cytokines IL-6 and IL-10 and the endothelial cell adhesion molecule E-selectin [61]. In long-term alcohol abuse, the production of white blood cells in the bone marrow is decreased, and superoxide production in neutrophils is reduced [62, 63].
Figure 1.
Basal changes in the alveolus as a result of chronic alcohol abuse. A variety of differences in the basal functioning of alveoli from patients with alcohol abuse compared with abstainers has been reported, which contributes to the propensity for adverse respiratory conditions described in these patients, such as ARDS. A normal alveolus is shown on the left, and the effects of alcohol abuse on an alveolus are on the right. Alcohol-related changes include lower concentrations of alveolar GSH and an increase in the proportion of GSSG; increased production of alveolar macrophage and type II cell TGF-β; increased protein in the alveolar space with enhanced fluid flux across the alveolar-epithelial membrane; and abnormalities of the alveolar type II cells including increased numbers of epithelial sodium (ENa) channels, decreased surfactant synthesis, and increased cellular apoptosis. (Figure reprinted with permission [58].)
Chronic alcohol abuse and alveolar epithelial dysfunction
Given the important role of alveolar epithelial function in the pathophysiology of ARDS, many studies of the relationship between alcohol abuse and ARDS have focused on the effects of alcohol on the permeability of this barrier. Chronic alcohol ingestion in animal models has been shown to impair surfactant production and increase oxidant-mediated necrosis and apoptosis in alveolar epithelial cells [64,65,66,67]. These alterations are associated with impaired alveolar epithelial barrier function, as demonstrated by increased protein leak and decreased alveolar liquid clearance [68], and result in increased susceptibility to endotoxin-mediated acute lung injury [67]. After i.p. exposure to endotoxin, the lungs from alcohol-fed rats have increased activity of matrix metalloproteinases that degrade the extracellular matrix of the lung [62, 63, 69]. There is also an increased release of biologically active TGF-β into the alveolar space, resulting in disruption of epithelial barrier function [67]. Similar to patients with ARDS, these changes result in increases in alveolar capillary permeability, enhanced pulmonary edema formation, and increased protein concentrations in the alveolar lining fluid [70].
Chronic alcohol abuse and pulmonary antioxidant capacity
One of the central alterations related to chronic ethanol ingestion is a decreased concentration of the antioxidant GSH throughout the alveolar lining fluid of the lung and within alveolar type II cells [63, 64, 67, 68]. The alveolar lining fluid from the lungs of chronic alcoholics without any other medical diagnoses is GSH-deficient [71], and many of the deleterious effects of alcohol, including abnormal surfactant synthesis and secretion, increases in type II cell apoptosis, increases in basal expression of TGF-β, and alterations in alveolar-capillary barrier function and permeability, are associated with deficiencies in pulmonary GSH [72, 73]. Additionally, protein concentrations in the alveolar lining fluid of these individuals are increased, consistent with changes in alveolar-capillary permeability. Although these basal changes in the lung induced by chronic alcohol exposure do not cause ARDS independently, they do render the lung more vulnerable to the increased systemic oxidative stress that occurs during acute critical illnesses. In patients with ARDS, a prior history of alcohol abuse is associated with an increased concentration of E-selectin, an endothelial-specific adhesion molecule, in the alveolar lining fluid of the lung and the increased accumulation of extravascular lung water [74]. These findings are consistent with enhanced endothelial cell activation and increased permeability defects during ARDS related to chronic alcohol abuse.
Alcohol abuse and angiotensin II
Angiotensin II is formed by the sequential conversion of angiotensinogen to angiotensin I and then to angiotensin II. The conversion of angiotensin I to angiotensin II is carried out within the lung by ACE. Interestingly, angiotensin II levels are elevated in patients with ARDS and in rats following chronic alcohol ingestion [75, 76]. Interestingly, mice deficient in ACE have been shown to have less lung injury following acid aspiration or sepsis [77]. Clinical data also suggest that individuals who express the ACE allele (which is associated with increased ACE activity) are at higher risk for ARDS [78]. In contrast, ACE2 is a more recently identified negative modulator of the renin angiotensin system that inactivates angiotensin II, and treatment with rACE2 also protects mice from lung injury [77]. The biological effects of angiotensin II depend on its interaction with specific angiotensin II receptors, of which AT1 and AT2 have been best characterized. Activation of the AT1 receptor by angiotensin II has been shown to mediate alcohol-induced oxidative stress in the lung [79], and angiotensin II appears to activate NADPH oxidase expression and subsequent production of superoxide within the lung [80]. On the other hand, chronic alcohol ingestion has been shown to markedly increase the relative expression of the AT2 receptor within alveolar epithelial cells and predispose these cells to apoptosis when exposed to oxidative stress or proinflammatory cytokines [81]. In contrast, inhibition of the AT2 receptor in alcohol-fed rats inhibits angiotensin II- and TNF-α-induced apoptosis of alveolar epithelial cells [81].
Alcohol abuse and pulmonary GM-CSF signaling
GM-CSF is a 23-kDa glycosylated monomeric peptide secreted by multiple cell types, including the alveolar epithelial type II cell. GM-CSF has been shown to stimulate the growth of a variety of blood cells, including granulocytes, macrophages, eosinophils, erythrocytes, megakaryocytes, and dendritic cells. Interestingly, targeted deletion of the GM-CSF gene in mice produced an unexpected lung-specific phenotype that was essentially identical to pulmonary alveolar proteinosis [82]. The role of chronic alcohol ingestion on this pathway was demonstrated when rGM-CSF delivered via the upper airway restored alveolar epithelial barrier function and fluid transport in alcohol-fed rats, even during endotoxemia [83]. Subsequent studies have identified that chronic alcohol ingestion decreases the expression of GM-CSF receptors in airway epithelial cells and macrophages and in turn, dampens intracellular signaling to the GM-CSF master transcription factor PU.1. Importantly, rGM-CSF treatment restores GM-CSF receptor expression and signaling and normalizes alveolar epithelial barrier function [84] and alveolar macrophage immune function [85].
POSSIBLE THERAPIES FOR ALCOHOL-ASSOCIATED ARDS
Data from the NIAAA’s 1991–1992 National Longitudinal Alcohol Epidemiologic Survey and the 2001–2002 National Epidemiologic Survey on Alcohol and Related Conditions reported that the prevalence of AUDs increased significantly across most demographics for men and women [86]. Even when people attempt to stop harmful alcohol consumption, the rate of recidivism with AUDs is notoriously high. As a result, the prevalence of critical illnesses such as ARDS stands to increase in this population still further in the future. NAC and GM-CSF have been investigated for use in critically ill patients, including studies in patients with ARDS. Findings have been largely encouraging, and the safety profile for these agents has been good. Unfortunately, none of these drugs has been used in trials specifically focused on individuals with AUDs, who are at greater risk for ARDS. The plausibility of these drugs, as well as other agents that alter the effects of alcohol, to effectively remediate abnormal alveolar-capillary permeability and other alcohol-induced abnormalities is detailed below. Investigating these drugs in individuals with AUDs has the potential to translate into new treatment strategies to decrease the risk for ARDS.
NAC is a tripeptide that can function as a direct antioxidant or an indirect antioxidant through its ability to donate cysteine in the synthesis of GSH [87, 88]. In situations of increased GSH use, NAC can be given to increase cysteine levels, thereby increasing GSH levels. NAC is a commonly used medication for acetaminophen toxicity, where it has a long safety history, orally and i.v. [89]. In an animal model of sepsis, NAC prevented activation of NF-κB and IL-8 and decreased neutrophilic alveolitis [90, 91]. Very high levels of GSH normally exist in the lung [92]; however, in the lungs of patients with ARDS, GSH is decreased significantly [93, 94]. Based on these observations, trials of NAC in patients with ARDS have been performed. Two small studies, each with <30 subjects, found that i.v. NAC compared with placebo was efficacious in increasing the number of organ failure-free days (days a patient was alive and without organ failure) [95, 96].
Notably, neither of these clinical trials nor any others in the literature focused specifically on repletion of GSH in subjects with AUDs, although such individuals might benefit most from GSH replacement. This is illustrated in animal models, where oral NAC administered to rodents in an AUD model increased the amount of cytosolic GSH significantly [65], as well as normalized type II cell mitochondrial GSH levels and surfactant synthesis [66]. NAC administration in animals also correlates with important pulmonary outcomes, including reductions in lung edema after endotoxin administration [97] and restoration of the functional surfactant pool [98]. Of importance, decreased pulmonary GSH levels and increased levels of the GSSG have been observed in human subjects with AUDs [70, 99], and these correlate with alveolar protein concentrations [100]. Therefore, restoring GSH homeostasis within the lung via NAC represents a therapeutic intervention, whereby the predisposition for ARDS could be decreased in patients who abuse alcohol.
As mentioned above, GM-CSF is a protein known to affect progenitor cell proliferation and monocyte-to-macrophage differentiation. It also influences adhesion molecule expression, phagocytosis of bacteria [101], and phagocytic clearance of apoptotic inflammatory cells [102]. Additionally, it has effects on nonhematopoietic cells including alveolar epithelial cells [103]. In humans with ARDS, higher levels of GM-CSF in epithelial lining fluid are associated with improved survival [104]. Based on these observations, a small, randomized controlled trial of GM-CSF in 28 patients with severe sepsis and respiratory dysfunction was performed. It demonstrated that low-dose GM-CSF administration in these patients led to improved oxygenation and a decreased incidence of frank lung injury, without significant adverse effects [105]. Since publication of this trial, a multicenter investigation of GM-CSF in acute lung injury has been underway. Notably, safety concerns about the use of this drug have been minimal.
Based on recent animal data, therapy with GM-CSF can normalize alcohol-induced lung pathology, which in turn may decrease the predisposition to develop ARDS. In animal models of AUD, ethanol feeding affected the signaling capacity of GM-CSF by decreasing expression of its receptors (α and β) on alveolar macrophages and the alveolar epithelium [84, 85]. Treating alcohol-fed animals with intranasal GM-CSF reversed these abnormalities. More importantly, GM-CSF improved endotoxin-induced secretion of TNF-α and bacterial phagocytosis by alveolar macrophages in animal models [85]. It also attenuated lung edema in animals subjected to intratracheal saline challenge (with and without concomitant endotoxemia) and decreased lung lavage protein concentrations. Finally, alveolar epithelial monolayers consisting of cells from these ethanol-fed animals cultured in the presence of GM-CSF were significantly less permeable [83]. Collectively, these animal data provide evidence that GM-CSF administration may be efficacious in maintaining normal alveolar-capillary permeability and alveolar macrophage function in subjects with AUDs, thereby decreasing risk for ARDS.
Finally, angiotensin II inhibition is an intriguing therapeutic target and if effective, may limit alcohol-induced oxidative stress. As ACE inhibitors and angiotensin receptor blockers (which act to block the AT1 receptor) are being used currently on a widespread basis, a systematic trial of these agents in patients who abuse alcohol may be possible. Unfortunately, the use of these agents in the critically ill patient may be impossible as a result of concern about hypotension and renal dysfunction. As a result, although these agents may ultimately have a role in chronic treatment of individuals who abuse alcohol, their role in critically ill patients with ARDS would be limited significantly. In the future, agents that block the AT2 receptor specifically may offer benefit and may be more tolerable in the critically ill patient.
FUTURE DIRECTIONS
As we gain more insight into the mechanisms by which alcohol predisposes patients to the development of ARDS, additional novel therapies will be identified that will improve the incidence and severity of ARDS in this important patient population. As discussed above, the pulmonary effects of alcohol abuse are broad and include such defects as pulmonary GSH depletion, abnormalities in angiotensin II production and receptor expression, and abnormalities in GM-CSF signaling. These effects lead to physiological changes consistent with ARDS and therefore represent future targets for therapy in patients who abuse alcohol.
Given the widespread use of alcohol in our critically ill patients, the clear role of alcohol abuse in increasing the risk for ARDS, and the current lack of specific treatments for individuals with alcohol abuse and ARDS, further investigations into the mechanisms by which alcohol predisposes to ARDS and the effects of specific therapies are necessary. Until such therapies are available, primary prevention is imperative to decrease the excessive morbidity seen in critically ill patients with a prior history of alcohol abuse.
Footnotes
Abbreviations: ACE=angiotensin-converting enzyme, ALI=acute lung injury, ARDS=acute respiratory distress syndrome, AT1=angiotensin type 1 receptor, AUD=alcohol use disorder, CAP=community acquired bacterial pneumonia, GSH=glutathione, GSSG=oxidized form of GSH, HAP=hospital acquired pneumonia, ICU=Intensive Care Unit, NAC=N-acetylcysteine, NIAAA=National Institute on Alcohol Abuse and Alcoholism, TRALI=transfusion-related lung injury, VAP=ventilator-associated pneumonia
References
- Goss C. H., Brower R. G., Hudson L. D., Rubenfeld G. D. Incidence of acute lung injury in the United States. Crit Care Med. 2003;31:1607–1611. doi: 10.1097/01.CCM.0000063475.65751.1D. [DOI] [PubMed] [Google Scholar]
- Gajic O., Rana R., Winters J. L., Yilmaz M., Mendez J. L., Rickman O. B., O'Byrne M. M., Evenson L. K., Malinchoc M., DeGoey S. R., Afessa B., Hubmayr R. D., Moore S. B. Transfusion-related acute lung injury in the critically ill: prospective nested case-control study. Am J Respir Crit Care Med. 2007;176:886–891. doi: 10.1164/rccm.200702-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licker M., de Perrot M., Spiliopoulos A., Robert J., Diaper J., Chevalley C., Tschopp J. M. Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg. 2003;97:1558–1565. doi: 10.1213/01.ANE.0000087799.85495.8A. [DOI] [PubMed] [Google Scholar]
- Moss M., Bucher B., Moore F. A., Moore E. E., Parsons P. E. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA. 1996;275:50–54. [PubMed] [Google Scholar]
- Moss M., Parsons P. E., Steinberg K. P., Hudson L. D., Guidot D. M., Burnham E. L., Eaton S., Cotsonis G. A. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med. 2003;31:869–877. doi: 10.1097/01.CCM.0000055389.64497.11. [DOI] [PubMed] [Google Scholar]
- Phua J., Badia J. R., Adhikari N. K., Friedrich J. O., Fowler R. A., Singh J. M., Scales D. C., Stather D. R., Li A., Jones A., Gattas D. J., Hallett D., Tomlinson G., Stewart T. E., Ferguson N. D. Has mortality from acute respiratory distress syndrome decreased over time? a systematic review. Am J Respir Crit Care Med. 2009;179:220–227. doi: 10.1164/rccm.200805-722OC. [DOI] [PubMed] [Google Scholar]
- Angell M., Kassirer J. P. Alcohol and other drugs—toward a more rational and consistent policy. N Engl J Med. 1994;331:537–539. doi: 10.1056/NEJM199408253310810. [DOI] [PubMed] [Google Scholar]
- Halpern N. A., Bettes L., Greenstein R. Federal and nationwide intensive care units and healthcare costs: 1986–1992. Crit Care Med. 1994;22:2001–2007. [PubMed] [Google Scholar]
- Grant B. F. Prevalence and correlates of alcohol use and DSM-IV alcohol dependence in the United States: results of the National Longitudinal Alcohol Epidemiologic Survey. J Stud Alcohol. 1997;58:464–473. doi: 10.15288/jsa.1997.58.464. [DOI] [PubMed] [Google Scholar]
- Wilsnack R. W., Vogeltanz N. D., Wilsnack S. C., Harris T. R., Ahlstrom S., Bondy S., Csémy L., Ferrence R., Ferris J., Fleming J., Graham K., Greenfield T., Guyon L., Haavio-Mannila E., Kellner F., Knibbe R., Kubicka L., Loukomskaia M., Mustonen H., Nadeau L., Narusk A., Neve R., Rahav G., Spak F., Teichman M., Trocki K., Webster I., Weiss S. Gender differences in alcohol consumption and adverse drinking consequences: cross-cultural patterns. Addiction. 2000;95:251–265. doi: 10.1046/j.1360-0443.2000.95225112.x. [DOI] [PubMed] [Google Scholar]
- Boffetta P., Garfinkel L. Alcohol drinking and mortality among men enrolled in an American Cancer Society prospective study. Epidemiology. 1990;1:342–348. doi: 10.1097/00001648-199009000-00003. [DOI] [PubMed] [Google Scholar]
- Camargo C. A., Jr, Hennekens C. H., Gaziano J. M., Glynn R. J., Manson J. E., Stampfer M. J. Prospective study of moderate alcohol consumption and mortality in US male physicians. Arch Intern Med. 1997;157:79–85. [PubMed] [Google Scholar]
- Garbutt J. C., West S. L., Carey T. S., Lohr K. N., Crews F. T. Pharmacological treatment of alcohol dependence: a review of the evidence. JAMA. 1999;281:1318–1325. doi: 10.1001/jama.281.14.1318. [DOI] [PubMed] [Google Scholar]
- Saitz R. Clinical practice Unhealthy alcohol use. N Engl J Med. 2005;352:596–607. doi: 10.1056/NEJMcp042262. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sola J., Junque A., Estruch R., Monforte R., Torres A., Urbano-Marquez A. High alcohol intake as a risk and prognostic factor for community-acquired pneumonia. Arch Intern Med. 1995;155:1649–1654. doi: 10.1001/archinte.1995.00430150137014. [DOI] [PubMed] [Google Scholar]
- Saitz R., Ghali W. A., Moskowitz M. A. The impact of alcohol-related diagnoses on pneumonia outcomes. Arch Intern Med. 1997;157:1446–1452. [PubMed] [Google Scholar]
- De Wit M., Best A. M., Gennings C., Burnham E. L., Moss M. Alcohol use disorders increase the risk for mechanical ventilation in medical patients. Alcohol Clin Exp Res. 2007;31:1224–1230. doi: 10.1111/j.1530-0277.2007.00421.x. [DOI] [PubMed] [Google Scholar]
- O'Brien J. M., Jr, Lu B., Ali N. A., Martin G. S., Aberegg S. K., Marsh C. B., Lemeshow S., Douglas I. S. Alcohol dependence is independently associated with sepsis, septic shock, and hospital mortality among adult intensive care unit patients. Crit Care Med. 2007;35:345–350. doi: 10.1097/01.CCM.0000254340.91644.B2. [DOI] [PubMed] [Google Scholar]
- Jensen N. H., Dragsted L., Christensen J. K., Jorgensen J. C., Qvist J. Severity of illness and outcome of treatment in alcoholic patients in the intensive care unit. Intensive Care Med. 1988;15:19–22. doi: 10.1007/BF00255630. [DOI] [PubMed] [Google Scholar]
- Rush B. An inquiry into the effects of ardent spirits upon the human body and mind. Q J Stud Alcohol. 1943;4:321–341. [Google Scholar]
- Carpenter J. L., Huang D. Y. Community-acquired pulmonary infections in a public municipal hospital in the 1980s. South Med J. 1991;84:299–306. doi: 10.1097/00007611-199103000-00004. [DOI] [PubMed] [Google Scholar]
- Ortqvist A., Hedlund J., Grillner L., Jalonen E., Kallings I., Leinonen M., Kalin M. Aetiology, outcome and prognostic factors in community-acquired pneumonia requiring hospitalization. Eur Respir J. 1990;3:1105–1113. [PubMed] [Google Scholar]
- Van Metre T. E. Pneumococcal pneumonia treated with antibiotics; the prognostic significance of certain clinical findings. N Engl J Med. 1954;251:1048–1052. doi: 10.1056/NEJM195412232512604. [DOI] [PubMed] [Google Scholar]
- Capps J. A., Coleman G. H. Influence of alcohol on prognosis of pneumonia in Cook County Hospital: a statistical report. JAMA. 1923;80:750–752. [Google Scholar]
- Fine M. J., Smith M. A., Carson C. A., Mutha S. S., Sankey S. S., Weissfeld L. A., Kapoor W. N. Prognosis and outcomes of patients with community-acquired pneumonia A meta-analysis. JAMA. 1996;275:134–141. [PubMed] [Google Scholar]
- George D. L., Falk P. S., Wunderink R. G., Leeper K. V., Jr, Meduri G. U., Steere E. L., Corbett C. E., Mayhall C. G. Epidemiology of ventilator-acquired pneumonia based on protected bronchoscopic sampling. Am J Respir Crit Care Med. 1998;158:1839–1847. doi: 10.1164/ajrccm.158.6.9610069. [DOI] [PubMed] [Google Scholar]
- Bercault N., Boulain T. Mortality rate attributable to ventilator-associated nosocomial pneumonia in an adult intensive care unit: a prospective case-control study. Crit Care Med. 2001;29:2303–2309. doi: 10.1097/00003246-200112000-00012. [DOI] [PubMed] [Google Scholar]
- Gacouin A., Legay F., Camus C., Volatron A. C., Barbarot N., Donnio P. Y., Thomas R., Le Tulzo Y. At-risk drinkers are at higher risk to acquire a bacterial infection during an intensive care unit stay than abstinent or moderate drinkers. Crit Care Med. 2008;36:1735–1741. doi: 10.1097/CCM.0b013e318174dd75. [DOI] [PubMed] [Google Scholar]
- Ware L. B., Matthay M. A. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- Licker M., de Perrot M., Spiliopoulos A., Robert J., Diaper J., Chevalley C., Tschopp J. M. Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg. 2003;97:1558–1565. doi: 10.1213/01.ANE.0000087799.85495.8A. [DOI] [PubMed] [Google Scholar]
- Boe D. M., Nelson S., Zhang P., Bagby G. J. Acute ethanol intoxication suppresses lung chemokine production following infection with Streptococcus pneumoniae. J Infect Dis. 2001;184:1134–1142. doi: 10.1086/323661. [DOI] [PubMed] [Google Scholar]
- Gluckman S. J., MacGregor R. R. Effect of acute alcohol intoxication on granulocyte mobilization and kinetics. Blood. 1978;52:551–559. [PubMed] [Google Scholar]
- MacGregor R. R., Spagnuolo P. J., Lentnek A. L. Inhibition of granulocyte adherence by ethanol, prednisone, and aspirin, measured with an assay system. N Engl J Med. 1974;291:642–646. doi: 10.1056/NEJM197409262911302. [DOI] [PubMed] [Google Scholar]
- MacGregor R. R., Louria D. B. Alcohol and infection. Curr Clin Top Infect Dis. 1997;17:291–315. [PubMed] [Google Scholar]
- Nilsson E., Lindstrom P., Patarroyo M., Ringertz B., Lerner R., Rincon J., Palmblad J. Ethanol impairs certain aspects of neutrophil adhesion in vitro: comparisons with inhibition of expression of the CD18 antigen. J Infect Dis. 1991;163:591–597. doi: 10.1093/infdis/163.3.591. [DOI] [PubMed] [Google Scholar]
- Szabo G., Chavan S., Mandrekar P., Catalano D. Acute alcohol consumption attenuates interleukin-8 (IL-8) and monocyte chemoattractant peptide-1 (MCP-1) induction in response to ex vivo stimulation. J Clin Immunol. 1999;19:67–76. doi: 10.1023/a:1020518703050. [DOI] [PubMed] [Google Scholar]
- Zhang P., Nelson S., Summer W. R., Spitzer J. A. Acute ethanol intoxication suppresses the pulmonary inflammatory response in rats challenged with intrapulmonary endotoxin. Alcohol Clin Exp Res. 1997;21:773–778. [PubMed] [Google Scholar]
- Zhang P., Bagby G. J., Xie M., Stoltz D. A., Summer W. R., Nelson S. Acute ethanol intoxication inhibits neutrophil β2-integrin expression in rats during endotoxemia. Alcohol Clin Exp Res. 1998;22:135–141. [PubMed] [Google Scholar]
- Kolls J. K., Xie J., Lei D., Greenberg S., Summer W. R., Nelson S. Differential effects of in vivo ethanol on LPS-induced TNF and nitric oxide production in the lung. Am J Physiol. 1995;268:L991–L998. doi: 10.1152/ajplung.1995.268.6.L991. [DOI] [PubMed] [Google Scholar]
- Nelson S., Bagby G. J., Bainton B. G., Summer W. R. The effects of acute and chronic alcoholism on tumor necrosis factor and the inflammatory response. J Infect Dis. 1989;160:422–429. doi: 10.1093/infdis/160.3.422. [DOI] [PubMed] [Google Scholar]
- Stoltz D. A., Nelson S., Kolls J. K., Zhang P., Bohm R. P., Jr, Murphey-Corb M., Bagby G. Y. In vitro ethanol suppresses alveolar macrophage TNF-α during simian immunodeficiency virus infection. Am J Respir Crit Care Med. 2000;161:135–140. doi: 10.1164/ajrccm.161.1.9905016. [DOI] [PubMed] [Google Scholar]
- Szabo G., Mandrekar P., Catalano D. Inhibition of superantigen-induced T cell proliferation and monocyte IL-1 β, TNF-α, and IL-6 production by acute ethanol treatment. J Leukoc Biol. 1995;58:342–350. doi: 10.1002/jlb.58.3.342. [DOI] [PubMed] [Google Scholar]
- Mandrekar P., Catalano D., Szabo G. Inhibition of lipopolysaccharide-mediated NFκB activation by ethanol in human monocytes. Int Immunol. 1999;11:1781–1790. doi: 10.1093/intimm/11.11.1781. [DOI] [PubMed] [Google Scholar]
- Nelson S., Kolls J. K. Alcohol, host defence and society. Nat Rev Immunol. 2002;2:205–209. doi: 10.1038/nri744. [DOI] [PubMed] [Google Scholar]
- Nelson S. Role of granulocyte colony-stimulating factor in the immune response to acute bacterial infection in the nonneutropenic host: an overview. Clin Infect Dis. 1994;18(Suppl. 2):S197–S204. doi: 10.1093/clinids/18.supplement_2.s197. [DOI] [PubMed] [Google Scholar]
- Nelson S., Summer W., Bagby G., Nakamura C., Stewart L., Lipscomb G., Andresen J. Granulocyte colony-stimulating factor enhances pulmonary host defenses in normal and ethanol-treated rats. J Infect Dis. 1991;164:901–906. doi: 10.1093/infdis/164.5.901. [DOI] [PubMed] [Google Scholar]
- Zhang P., Bagby G. J., Stoltz D. A., Spitzer J. A., Summer W. R., Nelson S. Modulation of the lung host response by granulocyte colony-stimulating factor in rats challenged with intrapulmonary endotoxin. Shock. 1997;7:193–199. doi: 10.1097/00024382-199703000-00007. [DOI] [PubMed] [Google Scholar]
- Standiford T. J., Danforth J. M. Ethanol feeding inhibits proinflammatory cytokine expression from murine alveolar macrophages ex vivo. Alcohol Clin Exp Res. 1997;21:1212–1217. [PubMed] [Google Scholar]
- Bagby G. J., Zhang P., Stoltz D. A., Nelson S. Suppression of the granulocyte colony-stimulating factor response to Escherichia coli challenge by alcohol intoxication. Alcohol Clin Exp Res. 1998;22:1740–1745. [PubMed] [Google Scholar]
- Bermudez L. E., Wu M., Martinelli J., Young L. S. Ethanol affects release of TNF and GM-CSF and membrane expression of TNF receptors by human macrophages. Lymphokine Cytokine Res. 1991;10:413–419. [PubMed] [Google Scholar]
- Szabo G., Mandrekar P., Girouard L., Catalano D. Regulation of human monocyte functions by acute ethanol treatment: decreased tumor necrosis factor-α, interleukin-1 β and elevated interleukin-10, and transforming growth factor-β production. Alcohol Clin Exp Res. 1996;20:900–907. doi: 10.1111/j.1530-0277.1996.tb05269.x. [DOI] [PubMed] [Google Scholar]
- Von Heymann C., Langenkamp J., Dubisz N., von Dossow V., Schaffartzik W., Kern H., Kox W. J., Spies C. Posttraumatic immune modulation in chronic alcoholics is associated with multiple organ dysfunction syndrome. J Trauma. 2002;52:95–103. doi: 10.1097/00005373-200201000-00017. [DOI] [PubMed] [Google Scholar]
- Dai Q., Pruett S. B. Different effects of acute and chronic ethanol on LPS-induced cytokine production and TLR4 receptor behavior in mouse peritoneal macrophages. J Immunotoxicol. 2006;3:217–225. doi: 10.1080/15476910601080156. [DOI] [PubMed] [Google Scholar]
- Mandrekar P., Catalano D., Girouard L., Szabo G. Human monocyte IL-10 production is increased by acute ethanol treatment. Cytokine. 1996;8:567–577. doi: 10.1006/cyto.1996.0076. [DOI] [PubMed] [Google Scholar]
- Pruett S. B., Schwab C., Zheng Q., Fan R. Suppression of innate immunity by acute ethanol administration: a global perspective and a new mechanism beginning with inhibition of signaling through TLR3. J Immunol. 2004;173:2715–2724. doi: 10.4049/jimmunol.173.4.2715. [DOI] [PubMed] [Google Scholar]
- Yamashina S., Wheeler M. D., Rusyn I., Ikejima K., Sato N., Thurman R. G. Tolerance and sensitization to endotoxin in Kupffer cells caused by acute ethanol involve interleukin-1 receptor-associated kinase. Biochem Biophys Res Commun. 2000;277:686–690. doi: 10.1006/bbrc.2000.3738. [DOI] [PubMed] [Google Scholar]
- Szabo G., Dolganiuc A., Dai Q., Pruett S. B. TLR4, ethanol, and lipid rafts: a new mechanism of ethanol action with implications for other receptor-mediated effects. J Immunol. 2007;178:1243–1249. doi: 10.4049/jimmunol.178.3.1243. [DOI] [PubMed] [Google Scholar]
- Moss M., Burnham E. L. Alcohol abuse in the critically ill patient. Lancet. 2006;368:2231–2242. doi: 10.1016/S0140-6736(06)69490-7. [DOI] [PubMed] [Google Scholar]
- Deviere J., Content J., Denys C., Vandenbussche P., Schandene L., Wybran J., Dupont E. High interleukin-6 serum levels and increased production by leucocytes in alcoholic liver cirrhosis Correlation with IgA serum levels and lymphokines production. Clin Exp Immunol. 1989;77:221–225. [PMC free article] [PubMed] [Google Scholar]
- Liu Y. K. Leukopenia in alcoholics. Am J Med. 1973;54:605–610. doi: 10.1016/0002-9343(73)90118-6. [DOI] [PubMed] [Google Scholar]
- Sachs C. W., Christensen R. H., Pratt P. C., Lynn W. S. Neutrophil elastase activity and superoxide production are diminished in neutrophils of alcoholics. Am Rev Respir Dis. 1990;141:1249–1255. doi: 10.1164/ajrccm/141.5_Pt_1.1249. [DOI] [PubMed] [Google Scholar]
- Lois M., Brown L. A., Moss I. M., Roman J., Guidot D. M. Ethanol ingestion increases activation of matrix metalloproteinases in rat lungs during acute endotoxemia. Am J Respir Crit Care Med. 1999;160:1354–1360. doi: 10.1164/ajrccm.160.4.9811060. [DOI] [PubMed] [Google Scholar]
- Bechara R. I., Brown L. A., Roman J., Joshi P. C., Guidot D. M. Transforming growth factor β1 expression and activation is increased in the alcoholic rat lung. Am J Respir Crit Care Med. 2004;170:188–194. doi: 10.1164/rccm.200304-478OC. [DOI] [PubMed] [Google Scholar]
- Brown L. A., Harris F. L., Bechara R., Guidot D. M. Effect of chronic ethanol ingestion on alveolar type II cell: glutathione and inflammatory mediator-induced apoptosis. Alcohol Clin Exp Res. 2001;25:1078–1085. [PubMed] [Google Scholar]
- Brown L. A., Harris F. L., Guidot D. M. Chronic ethanol ingestion potentiates TNF-α-mediated oxidative stress and apoptosis in rat type II cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L377–L386. doi: 10.1152/ajplung.2001.281.2.L377. [DOI] [PubMed] [Google Scholar]
- Guidot D. M., Brown L. A. Mitochondrial glutathione replacement restores surfactant synthesis and secretion in alveolar epithelial cells of ethanol-fed rats. Alcohol Clin Exp Res. 2000;24:1070–1076. [PubMed] [Google Scholar]
- Holguin F., Moss I., Brown L. A., Guidot D. M. Chronic ethanol ingestion impairs alveolar type II cell glutathione homeostasis and function and predisposes to endotoxin-mediated acute edematous lung injury in rats. J Clin Invest. 1998;101:761–768. doi: 10.1172/JCI1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidot D. M., Modelska K., Lois M., Jain L., Moss I. M., Pittet J. F., Brown L. A. Ethanol ingestion via glutathione depletion impairs alveolar epithelial barrier function in rats. Am J Physiol Lung Cell Mol Physiol. 2000;279:L127–L135. doi: 10.1152/ajplung.2000.279.1.L127. [DOI] [PubMed] [Google Scholar]
- Ware L. B., Matthay M. A. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- Moss M., Guidot D. M., Wong-Lambertina M., Ten H. T., Perez R. L., Brown L. A. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. Am J Respir Crit Care Med. 2000;161:414–419. doi: 10.1164/ajrccm.161.2.9905002. [DOI] [PubMed] [Google Scholar]
- Burnham E. L., Brown L. A., Halls L., Moss M. Effects of chronic alcohol abuse on alveolar epithelial barrier function and glutathione homeostasis. Alcohol Clin Exp Res. 2003;27:1167–1172. doi: 10.1097/01.ALC.0000075821.34270.98. [DOI] [PubMed] [Google Scholar]
- Burnham E. L., Moss M., Harris F., Brown L. A. Elevated plasma and lung endothelial selectin levels in patients with acute respiratory distress syndrome and a history of chronic alcohol abuse. Crit Care Med. 2004;32:675–679. doi: 10.1097/01.ccm.0000114824.65158.4e. [DOI] [PubMed] [Google Scholar]
- Martin G. S., Eaton S., Mealer M., Moss M. Extravascular lung water in patients with severe sepsis: a prospective cohort study. Crit Care. 2005;9:R74–R82. doi: 10.1186/cc3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham E. L., Moss M., Harris F., Brown L. A. Elevated plasma and lung endothelial selectin levels in patients with acute respiratory distress syndrome and a history of chronic alcohol abuse. Crit Care Med. 2004;32:675–679. doi: 10.1097/01.ccm.0000114824.65158.4e. [DOI] [PubMed] [Google Scholar]
- Wenz M., Steinau R., Gerlach H., Lange M., Kaczmarczyk G. Inhaled nitric oxide does not change transpulmonary angiotensin II formation in patients with acute respiratory distress syndrome. Chest. 1997;112:478–483. doi: 10.1378/chest.112.2.478. [DOI] [PubMed] [Google Scholar]
- Wright J. W., Morseth S. L., Abhold R. H., Harding J. W. Elevations in plasma angiotensin II with prolonged ethanol treatment in rats. Pharmacol Biochem Behav. 1986;24:813–818. doi: 10.1016/0091-3057(86)90416-8. [DOI] [PubMed] [Google Scholar]
- Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M. A., Fukamizu A., Hui C. C., Hein L., Uhlig S., Slutsky A. S., Jiang C., Penninger J. M. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R. P., Webb S., Bellingan G. J., Montgomery H. E., Chaudhari B., McAnulty R. J., Humphries S. E., Hill M. R., Laurent G. J. Angiotensin converting enzyme insertion/deletion polymorphism is associated with susceptibility and outcome in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002;166:646–650. doi: 10.1164/rccm.2108086. [DOI] [PubMed] [Google Scholar]
- Bechara R. I., Pelaez A., Palacio A., Joshi P. C., Hart C. M., Brown L. A., Raynor R., Guidot D. M. Angiotensin II mediates glutathione depletion, transforming growth factor-β1 expression, and epithelial barrier dysfunction in the alcoholic rat lung. Am J Physiol Lung Cell Mol Physiol. 2005;289:L363–L370. doi: 10.1152/ajplung.00141.2005. [DOI] [PubMed] [Google Scholar]
- Polikandriotis J. A., Rupnow H. L., Elms S. C., Clempus R. E., Campbell D. J., Sutliff R. L., Brown L. A., Guidot D. M., Hart C. M. Chronic ethanol ingestion increases superoxide production and NADPH oxidase expression in the lung. Am J Respir Cell Mol Biol. 2006;34:314–319. doi: 10.1165/rcmb.2005-0320OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara R. I., Brown L. A., Eaton D. C., Roman J., Guidot D. M. Chronic ethanol ingestion increases expression of the angiotensin II type 2 (AT2) receptor and enhances tumor necrosis factor-α- and angiotensin II-induced cytotoxicity via AT2 signaling in rat alveolar epithelial cells. Alcohol Clin Exp Res. 2003;27:1006–1014. doi: 10.1097/01.ALC.0000071932.56932.53. [DOI] [PubMed] [Google Scholar]
- Huffman J. A., Hull W. M., Dranoff G., Mulligan R. C., Whitsett J. A. Pulmonary epithelial cell expression of GM-CSF corrects the alveolar proteinosis in GM-CSF-deficient mice. J Clin Invest. 1996;97:649–655. doi: 10.1172/JCI118461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaez A., Bechara R. I., Joshi P. C., Brown L. A., Guidot D. M. Granulocyte/macrophage colony-stimulating factor treatment improves alveolar epithelial barrier function in alcoholic rat lung. Am J Physiol Lung Cell Mol Physiol. 2004;286:L106–L111. doi: 10.1152/ajplung.00148.2003. [DOI] [PubMed] [Google Scholar]
- Joshi P. C., Applewhite L., Mitchell P. O., Fernainy K., Roman J., Eaton D. C., Guidot D. M. GM-CSF receptor expression and signaling is decreased in lungs of ethanol-fed rats. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1150–L1158. doi: 10.1152/ajplung.00150.2006. [DOI] [PubMed] [Google Scholar]
- Joshi P. C., Applewhite L., Ritzenthaler J. D., Roman J., Fernandez A. L., Eaton D. C., Brown L. A., Guidot D. M. Chronic ethanol ingestion in rats decreases granulocyte-macrophage colony-stimulating factor receptor expression and downstream signaling in the alveolar macrophage. J Immunol. 2005;175:6837–6845. doi: 10.4049/jimmunol.175.10.6837. [DOI] [PubMed] [Google Scholar]
- Grant B. F., Dawson D. A., Stinson F. S., Chou S. P., Dufour M. C., Pickering R. P. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Aruoma O. I., Halliwell B., Hoey B. M., Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989;6:593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- Moldéus P., Cotgreave I. A., Berggren M. Lung protection by a thiol-containing antioxidant: N-acetylcysteine. Respiration. 1986;50(Suppl. 1):31–42. doi: 10.1159/000195086. [DOI] [PubMed] [Google Scholar]
- Smilkstein M. J., Knapp G. L., Kulig K. W., Rumack B. H. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose Analysis of the national multicenter study (1976 to 1985) N Engl J Med. 1988;319:1557–1562. doi: 10.1056/NEJM198812153192401. [DOI] [PubMed] [Google Scholar]
- Blackwell T. S., Blackwell T. R., Holden E. P., Christman B. W., Christman J. W. In vivo antioxidant treatment suppresses nuclear factor-κ B activation and neutrophilic lung inflammation. J Immunol. 1996;157:1630–1637. [PubMed] [Google Scholar]
- Eklund A., Eriksson O., Hakansson L., Larsson K., Ohlsson K., Venge P., Bergstrand H., Björnson A., Brattsand R., Glennow C., et al. Oral N-acetylcysteine reduces selected humoral markers of inflammatory cell activity in BAL fluid from healthy smokers: correlation to effects on cellular variables. Eur Respir J. 1988;1:832–838. [PubMed] [Google Scholar]
- Cantin A. M., North S. L., Hubbard R. C., Crystal R. G. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol. 1987;63:152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- Pacht E. R., DeMichele S. J., Nelson J. L., Hart J., Wennberg A. K., Gadek J. E. Enteral nutrition with eicosapentaenoic acid, γ-linolenic acid, and antioxidants reduces alveolar inflammatory mediators and protein influx in patients with acute respiratory distress syndrome. Crit Care Med. 2003;31:491–500. doi: 10.1097/01.CCM.0000049952.96496.3E. [DOI] [PubMed] [Google Scholar]
- Bunnell E., Pacht E. R. Oxidized glutathione is increased in the alveolar fluid of patients with the adult respiratory distress syndrome. Am Rev Respir Dis. 1993;148:1174–1178. doi: 10.1164/ajrccm/148.5.1174. [DOI] [PubMed] [Google Scholar]
- Bernard G. R., Wheeler A. P., Arons M. M., Morris P. E., Paz H. L., Russell J. A., Wright P. E. A trial of antioxidants N-acetylcysteine and procysteine in ARDS The Antioxidant in ARDS Study Group. Chest. 1997;112:164–172. doi: 10.1378/chest.112.1.164. [DOI] [PubMed] [Google Scholar]
- Soltan-Sharifi M. S., Mojtahedzadeh M., Najafi A., Reza Khajavi M., Reza Rouini M., Moradi M., Mohammadirad A., Abdollahi M. Improvement by N-acetylcysteine of acute respiratory distress syndrome through increasing intracellular glutathione, and extracellular thiol molecules and anti-oxidant power: evidence for underlying toxicological mechanisms. Hum Exp Toxicol. 2007;26:697–703. doi: 10.1177/0960327107083452. [DOI] [PubMed] [Google Scholar]
- Holguin F., Moss I., Brown L. A., Guidot D. M. Chronic ethanol ingestion impairs alveolar type II cell glutathione homeostasis and function and predisposes to endotoxin-mediated acute edematous lung injury in rats. J Clin Invest. 1998;101:761–768. doi: 10.1172/JCI1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez A., Bechara R. I., Lewis J. F., Malloy J., McCaig L., Brown L. A., Guidot D. M. Glutathione replacement preserves the functional surfactant phospholipid pool size and decreases sepsis-mediated lung dysfunction in ethanol-fed rats. Alcohol Clin Exp Res. 2002;26:1245–1251. doi: 10.1097/01.ALC.0000024269.05402.97. [DOI] [PubMed] [Google Scholar]
- Yeh M. Y., Burnham E. L., Moss M., Brown L. A. Chronic alcoholism alters systemic and pulmonary glutathione redox status. Am J Respir Crit Care Med. 2007;176:270–276. doi: 10.1164/rccm.200611-1722OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham E. L., Brown L. A., Halls L., Moss M. Effects of chronic alcohol abuse on alveolar epithelial barrier function and glutathione homeostasis. Alcohol Clin Exp Res. 2003;27:1167–1172. doi: 10.1097/01.ALC.0000075821.34270.98. [DOI] [PubMed] [Google Scholar]
- Lieschke G. J., Burgess A. W. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (1) N Engl J Med. 1992;327:28–35. doi: 10.1056/NEJM199207023270106. [DOI] [PubMed] [Google Scholar]
- Galati G., Rovere P., Citterio G., Bondanza A., Scagliette U., Bucci E., Heltai S., Fascio U., Rugarli C., Manfredi A. A. In vivo administration of GM-CSF promotes the clearance of apoptotic cells: effects on monocytes and polymorphonuclear leukocytes. J Leukoc Biol. 2000;67:174–182. doi: 10.1002/jlb.67.2.174. [DOI] [PubMed] [Google Scholar]
- Trapnell B. C., Whitsett J. A. GM-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu Rev Physiol. 2002;64:775–802. doi: 10.1146/annurev.physiol.64.090601.113847. [DOI] [PubMed] [Google Scholar]
- Matute-Bello G., Liles W. C., Radella F., II, Steinberg K. P., Ruzinski J. T., Hudson L. D., Martin T. R. Modulation of neutrophil apoptosis by granulocyte colony-stimulating factor and granulocyte/macrophage colony-stimulating factor during the course of acute respiratory distress syndrome. Crit Care Med. 2000;28:1–7. doi: 10.1097/00003246-200001000-00001. [DOI] [PubMed] [Google Scholar]
- Presneill J. J., Harris T., Stewart A. G., Cade J. F., Wilson J. W. A randomized phase II trial of granulocyte-macrophage colony-stimulating factor therapy in severe sepsis with respiratory dysfunction. Am J Respir Crit Care Med. 2002;166:138–143. doi: 10.1164/rccm.2009005. [DOI] [PubMed] [Google Scholar]