Abstract
4-Aminoantipyrine was utilized as key intermediate for the synthesis of pyrazolone derivatives bearing biologically active moieties. The newly synthesized compounds were characterized by IR, 1H- and 13C-NMR spectral and microanalytical studies. The compounds were screened as anticancer agents against a human tumor breast cancer cell line MCF7, and the results showed that (Z)-4-((3-amino-5-imino-1-phenyl-1H-pyrazol-4(5H)-ylidene)methylamino)-1,5-dimethyl-2-phenyl-1,2-dihydropyrazol-3-one 5, 3-(4-bromophenyl) -1-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile 13, 1-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1-Hpyrazol- 4-yl)-3-(4-iodophenyl)-4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile 14, 3,3′-(4,4′-sulfonylbis(4,1-phenylene))bis(1-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol- 4-yl)-4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile) 16, (Z)-1-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-2-hydrazono-4-oxo-3-phenyl-1,2,3,4-tetrahydropyrimidine-5-carbonitrile 17, (Z)-1-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-4-oxo-3-phenyl-2-(2-phenylhydrazono)-1,2,3,4-tetrahydro pyrimidine-5-carbonitrile 18, and (Z)-4-(3-amino-6-hydrazono-7-phenyl-6,7-dihydro pyrazolo[3,4-d]pyrimidin-5-yl)-1,5-dimethyl-2-phenyl-1,2-dihydropyrazol-3-one 19 were the most active compounds with IC50 values ranging from 30.68 to 60.72 μM compared with Doxorubicin as positive control with the IC50 value 71.8 μM.
Keywords: pyrazoles, sulfonamides, anti-breast cancer
1. Introduction
Pyrazolone derivatives such as antipyrine, aminopyrine, and dipyrone are well known compounds used mainly as analgesic and antipyretic drugs and their pharmacological molecular mechanism has been widely surveyed [1,2]. One of the best known antipyrine derivatives is 4-aminoantipyrine which is used for the protection against oxidative stress as well as prophylactic of some diseases including cancer, and these are important directions in medical applications [3]. Several derivatives of antipyrine were also biologically evaluated, and analgesic [4], anti-inflammatory [5], antimicrobial [6], and anticancer activity [7–9] have been reported. Antipyrine derivatives are strong inhibitors of cycloxygenase isoenzymes, platelet tromboxane synthesis, and prostanoids synthesis [10], which catalyze the rate-limiting step of prostaglandin synthesis. Pyrazolones are also a well-known elicitor of hypersensitivity [11]. Recently, Al-Haiza et al. [12] synthesized a new compound with pyrazolone moiety with antimicrobial and antifungal activities. In the last decade, several pyrazole derivatives proved to have potent anticancer action by the inhibition of the cyclindependent kinases (CDKs) which are members of the large family of protein kinases and are responsible for the eukaryotic cell cycle regulation; they are intensively studied for their cancer implication [13]. Based on the above information and due to our interest in pyrazole as a biologically active pharmacophore [14–18], we synthesized a new series of heterocycles incorporating antipyrine moiety starting from 4-aminoantipyrine to be evaluated for their anticancer activity against human tumor breast cell line (MCF7).
2. Results and Discussion
2.1. Chemistry
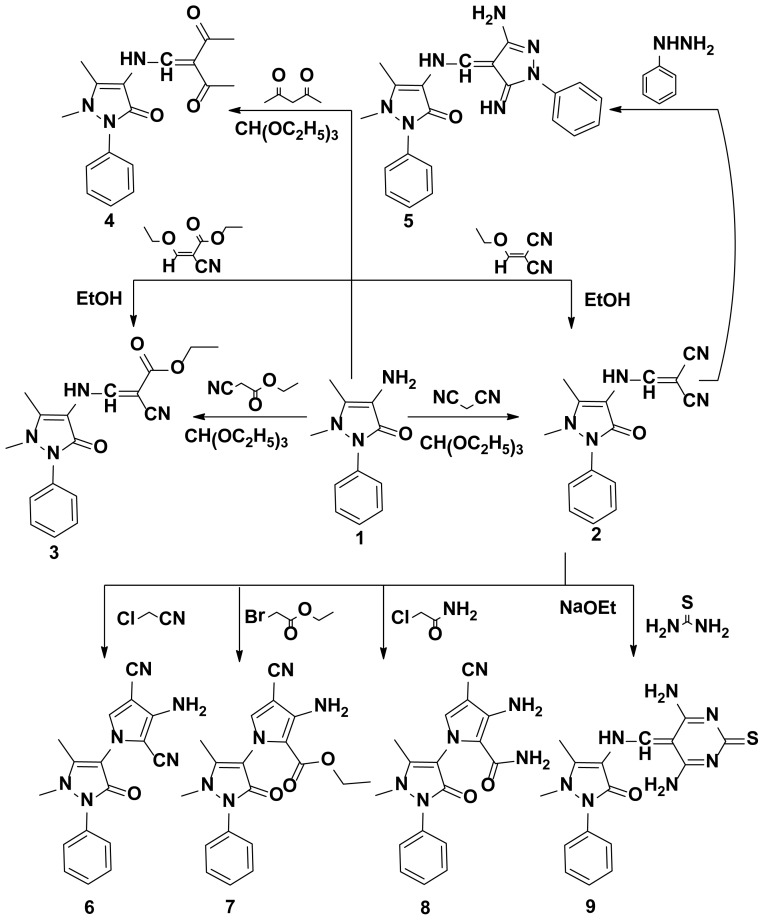
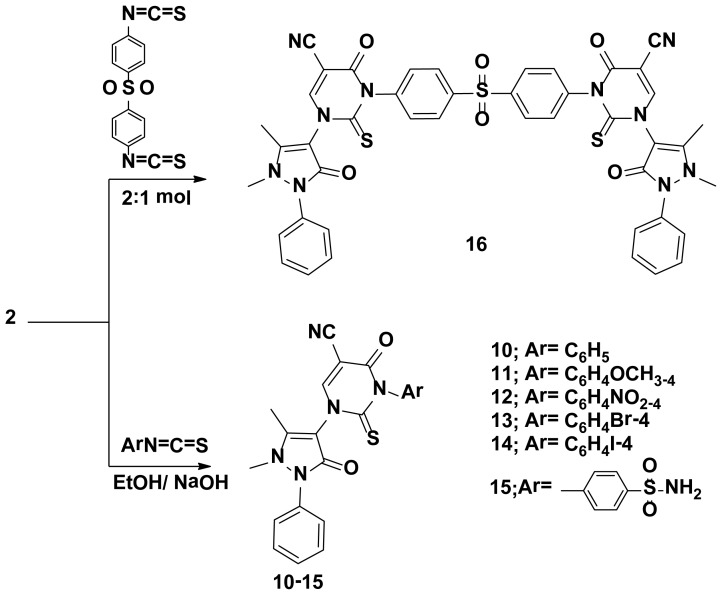
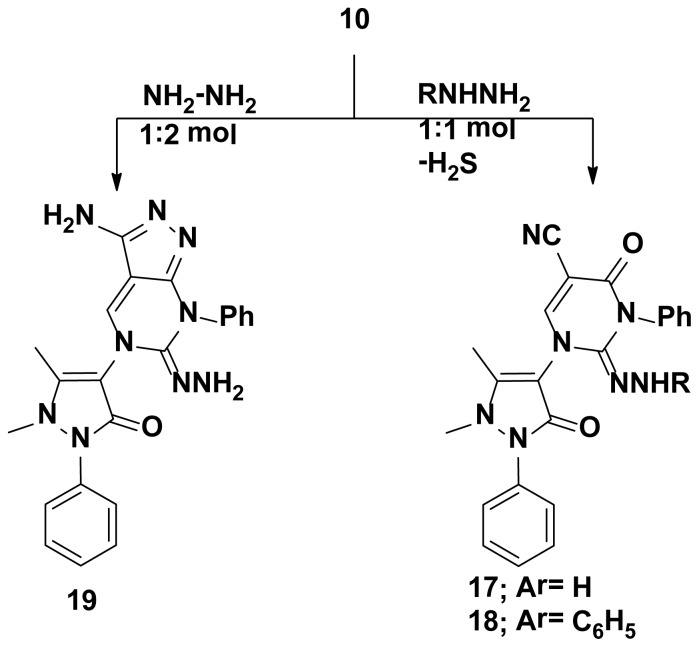
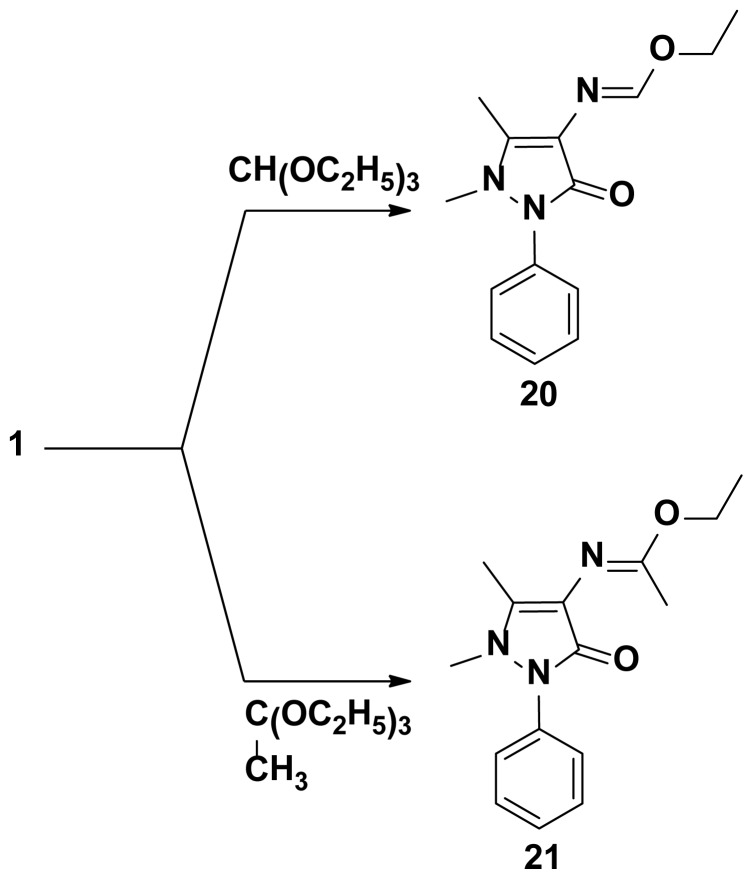
The starting key reagent 4-aminoantipyrine was purchased from Sigma-Aldrich chemical company (St. Louis, MO, USA). In this work, the reactivity of 4-aminoantipyrine with active methylene containing compounds (malononitrile, 2-(ethoxymethylene)malononitrile, ethylcyanoacetate, (ethoxymethylene)ethylcyanoacetate and acetylacetone) was studied and the reaction proceeded in the presence of triethylorthoformate in methanol containing catalytic amounts of acetic acid following the reported reaction condition [19]. The obtained pyrazolone derivatives 2–4, respectively, were identified by elemental and spectral data. Due to the biological importance of pyrazole, pyrrole and pyrimidine rings as anticancer agents [20–22], the pyrazolone derivative 2 was reacted with different nucleophiles in order to obtain biologically active pyrazole 5, pyrrole 6–8 and pyrimidine 9–16 derivatives bearing pyrazolone moieties. Thus, interaction of compound 2 with phenyl hydrazine yielded the corresponding pyrazole derivative 5. On the other hand, interaction of compound 2 with 2-chloroacetonitrile, ethylbromoacetate or 2-chloroacetamide in dioxane containing a catalytic amount of triethylamine yielded the corresponding pyrrole derivatives 6–8, respectively. Reaction of compound 2 with thiourea in refluxing ethanol containing sodium ethoxide gave the corresponding pyrimidine derivative 9 (Scheme 1). The reactivity of compound 2 towards different aryl isothiocyanates/NaOH was studied and the reaction proceeded via an addition reaction onto the isothiocyanato group followed by intramolecular cyclization to produce the pyrimidine ring as in compounds 10–15. Similarly, the bis-compound 16 was obtained expectedly via the reaction of one mole of 1-(4-isothio cyanatophenylsulfonyl)-4-isothiocyanatobenzene with two moles of compound 2 in ethanol containing sodium ethoxide (Scheme 2). The hydrazono pyrimidine derivatives 17, 18 were synthesized from compound 10 through reaction with either hydrazine hydrate or phenyl hydrazine, respectively, and the reaction proceeded via elimination of H2S which was detected by a lead acetate paper. On the other hand, double cyclization took place when one mole of compound 10 was reacted with two moles of hydrazine hydrate to yield the corresponding pyrazolo[3,4-d]pyrimidine 19 (Scheme 3). Finally, treatment of 4-aminoantipyrine 1 with either triethylorthoformate or acetate in acetic anhydride resulted in the formation of compounds 20, 21, respectively (Scheme 4). The structures of compounds were proved by microanalytical and spectral data and were consistent with the proposed structures.
Scheme 1.
Synthetic pathways for compounds 2–9.
Scheme 2.
Synthetic pathways for compounds 10–16.
Scheme 3.
Synthetic pathways for compounds 17–19.
Scheme 4.
Synthetic pathways for compounds 20, 21.
2.2. In-Vitro Anticancer Screening
Doxorubicin, the positive control used in this study, is an anticancer drug used to treat several cancer diseases including breast cancer. The relationship between surviving fraction and drug concentration was plotted; the response parameters calculated was IC50 value, which corresponds to the compound concentration causing 50% mortality in net cells (Table 1). The present work describes the synthesis of novel derivatives starting from 4-aminoantipyrine 1 by incorporating biologically active moieties, pyrazole, pyrrole, pyrimidine and pyrazolopyrimidine. From Table 1, we can observe that some of the tested compounds were found to be equipotent or even more potent than Doxorubicin on MCF7 cell line with IC50 values ranging from 30.68 to 70.65 μM compared to the reference drug (71.8 μM). The most potent compounds in this study were found to be those belonging to the pyrimidine derivatives of antipyrine especially the halogenated ones, where, the iodophenyl 14 (IC50 = 30.68 μM) and the bromophenyl 13 (IC50 = 43.41 μM) derivatives showed significant activities, also, the biscompound 16 (IC50 = 37.22 μM). In addition, pyrazole 5, pyrimidines 17, 18, pyrazolopyrimidine 19 with IC50 values 60.72, 54.23, 44.99, and 44.49 μM exhibited a higher activity when compared with the Doxorubicin as positive control. The phenyl and 4-nitro phenyl derivatives 10 and 12 (IC50 = 72.04 and 70.65 μM) are nearly as active as Doxorubicin. On the other hand, reaction of one mole of hydrazine hydrate or phenyl hydrazine with compound 10 yielded the pyrimidine derivatives 17 and 18 with significant activities (IC50 = 54.23 and 44.99 μM), while, addition of two moles of hydrazine hydrate yielded the pyrazolopyrimidine 19 (IC50 = 44.49 μM) which is nearly as potent as compounds 17 and 18. Moreover, the reaction of phenylhydrazine with compound 2 was also successful as it yielded the pyrazole derivative 5 which showed high activity (IC50 = 60.72 μM). All these compounds are more active than the reference drug. Considering the pyrrole derivatives 6, 7 and 8, they were found to exhibit lower activity compared to the reference drug (IC50 = 128.61, 104.11 and 85.12 μM, respectively). Compounds 2–4, 9, 11, 15, 20 and 21 are the least potent in this study with IC50 ranging from 109.58 to 173.83 μM.
Table 1.
In-vitro anticancer screening of compounds 2–21 against human breast cell line (MCF7).
| Compound No. | IC50 (μg/mL) | IC50 (μM) |
|---|---|---|
| 2 | 48.2 | 173.83 |
| 3 | 38.0 | 116.56 |
| 4 | 34.9 | 111.50 |
| 5 | 23.5 | 60.72 |
| 6 | 40.9 | 128.61 |
| 7 | 38.0 | 104.11 |
| 8 | 28.6 | 85.12 |
| 9 | 38.9 | 109.58 |
| 10 | 29.9 | 72.04 |
| 11 | NA | NA |
| 12 | 32.5 | 70.65 |
| 13 | 21.4 | 43.41 |
| 14 | 16.6 | 30.68 |
| 15 | NA | NA |
| 16 | 33.2 | 37.22 |
| 17 | 22.4 | 54.23 |
| 18 | 22.0 | 44.99 |
| 19 | 19.0 | 44.49 |
| 20 | 35.8 | 138.22 |
| 21 | NA | NA |
|
| ||
| Doxorubicin | 39.0 | 71.8 |
NA: Compound having IC50 value > 100 μg/mL.
Generally, incorporation of pyrimidine ring yielded the most potent compounds and these results point to the possible use of pyrimidine derivatives of antipyrine for treatment of breast tumors.
3. Experimental Section
3.1. General
Reagents were obtained from commercial suppliers and were used without purification. Melting points were determined in open capillary tubes using Thermo system FP800 Mettler FP80 central processor supplied with FP81 MBC cell apparatus (Stuart Scientific, Redhill, UK), and were uncorrected. Elemental analyses (C, H, N) were performed on a Perkin-Elmer 2400 Instrument (Perkin-Elmer, Norwalk, CT, USA). All compounds were within ±0.4% of the theoretical values. Infrared (IR) spectra (KBr disc) were recorded on FT-IR spectrophotometer (Perkin Elmer, Norwalk, CT, USA) at the Research Center, College of Pharmacy, King Saud University, Saudi Arabia.1H and 13C NMR spectra were recorded on a Ultra Shield Plus 500 MHz (Bruker, Munich, Germany) spectrometer operating at 500 MHz for proton and 125 MHz for carbon, respectively. The chemical shift values are reported in δ (ppm) relative to the residual solvent peak, the coupling constants (J) are reported in Hertz (Hz).
3.2. Chemistry
3.2.1. 2-((1,5-Dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-ylamino)methylene)-malononitrile 2
A mixture of 4-aminoantipyrine (0.01 mol), malononitrile (0.01 mol), triethylorthoformate (0.01 mol), and acetic acid (1 mL) in methanol (30 mL) was refluxed for 5 h, the reaction mixture was cooled, filtered, the filtered solid was crystallized from ethanol to give 2. Yield%: 90, m.p. = 254.7 °C, IR, cm−1: 3210 (NH), 3055 (CH arom.), 2941, 2818 (CH aliph.), 2200 (CN), 1658 (C=N). 1H (DMSO-d6, ppm): 2.2 [s, 3H, CH3], 3.2 [s, 3H, N–CH3], 7.3–7.9 [m, 5H, Ar–H], 8.1 [s, 1H, CH], 10.5 [s, 1H, NH, D2O-exchangeable]. 13C (DMSO-d6, ppm): 10.3 (CH3), 29.1 (N–CH3), 50.3, 104.6, 114.1(2), 116.4(2), 117.2, 127.2(2), 129.1, 134.5, 160.1 (C=O), 165.5. Anal. Calcd. for C15H13N5O (279): C, 64.51; H, 4.69; N, 25.07. Found: C, 64.91; H, 4.80; N, 25.34.
3.2.2. (Z)-Ethyl-2-cyano-3-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-ylamino)acrylate 3
A mixture of 4-aminoantipyrine (0.01 mol), ethylcyanoacetate (0.01 mol), triethylorthoformate (0.01 mol), and acetic acid (1 mL) in methanol (30 mL) was refluxed for 5 h, the reaction mixture was filtered, the filtered solid was crystallized from ethanol to give 3. Yield%: 83, m.p. = 154.8 °C, IR, cm−1: 3192 (NH), 3065 (CH arom.), 2965, 2841 (CH aliph.), 2214 (CN), 1710, 1658 (2C=O). 1H (DMSO-d6, ppm): 1.2 [t, 3H, CH3, J = 7.9 Hz], 2.3 [s, 3H, CH3], 3.2 [s, 3H, N–CH3], 4.2 [q, 2H, CH2, J = 8.1 Hz], 7.3–8.0 [m, 5H, Ar–H], 8.2 [d, 1H, CH, J = 7.12 Hz], 10.0 [d, 1H, NH, D2O-exchangeable, J = 7.3 Hz]. 13C (DMSO-d6, ppm): 10.3, 14.3, 39.0, 60.2, 72.6, 109.7, 115.7(2), 116.0, 118,1, 129.0(2), 129.1, 134.3, 156.8, 161.0, 166.3. Anal. Calcd. for C17H18N4O3 (326): C, 62.57; H, 5.56; N, 17.17. Found: C, 62.90; H, 5.89; N, 17.25.
3.2.3. 3-((1,5-Dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-ylamino)methylene)-pentane- 2,4-dione 4
A mixture of 4-aminoantipyrine (0.01 mol), acetylacetone (0.01 mol), triethylorthoformate (0.01 mol), and acetic acid (1 mL) in methanol (30 mL) was refluxed for 5 h, the reaction mixture was filtered, the filtered solid was crystallized from ethanol to give 4. Yield%: 90, m.p. = 201.8 °C, IR, cm−1: 3209 (NH), 3100 (CH arom.), 2971, 2848 (CH aliph.), 1716, 1692, 1661 (3C=O), 1612 (C=N). 1H (DMSO-d6, ppm): 1.9 [s, 3H, CH3], 2.1 [s, 6H, 2COCH3], 3.0 [s, 3H, N–CH3], 7.3–7.5 [m, 6H, Ar–H + CH], 9.0 [s, 1H, NH, D2O-exchangeable]. 13C (DMSO-d6, ppm): 11.1, 22.4(2), 39.1, 104.4, 107.8(2), 108.4, 121.8, 128.8(2), 129.0, 135.0, 151.0, 161.8, 195.1(2). Anal. Calcd. for C17H19N3O3 (313): C, 65.16; H, 6.11; N, 13.41. Found: C, 65.87; H, 6.37; N, 13.21.
3.2.4. (Z)-4-((3-Amino-5-imino-1-phenyl-1H-pyrazol-4(5H)-ylidene)methylamino)-1,5-dimethyl-2- phenyl-1,2-dihydropyrazol-3-one 5
Compound 2 (0.01 mol) was mixed with phenyl hydrazine (0.01 mol) in dioxane (20 mL) and refluxed for 5 h, the reaction mixture was cooled, poured onto ice water. The precipitated solid products were filtered and crystallized from methanol to give compound 5. Yield%: 76, m.p. = 172.7 °C, IR, cm−1: 3386, 3348, 3276 (NH, NH2), 3026 (CH arom.), 2981, 2872 (CH aliph.), 1660 (C=O), 1595 (C=N). 1H (DMSO-d6, ppm): 2.2 [s, 3H, CH3], 3.2 [s, 3H, N–CH3], 7.2–7.9 [m, 10H, Ar–H], 8.3 [s, 1H, CH], 8.7 [s, 1H, NH, D2O-exchangeable], 13.2 [s, 1H, NH-imino, D2O-exchangeable]. 13C (DMSO-d6, ppm): 11.5, 39.5, 106.2, 108.0, 112.4(2), 116.1(2), 117.6, 124.1, 127.7(2), 128.9, 129.5(2), 137.9, 141.2, 148.4, 156.5, 163.1, 166.2. Anal. Calcd. for C21H21N7 (387): C, 65.10; H, 5.46; N, 25.31. Found: C, 65.32; H, 5.09; N, 25.55.
3.2.5. 3-Amino-1-(1.5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-1H-pyrrole-2,4-dicarbonitrile 6
Compound 2 (0.01 mol) was mixed with 2-chloroacetonitrile (0.01 mol) in dioxane (20 mL) containing 3 drops of triethylamine and refluxed for 5 h, the reaction mixture was cooled, poured onto ice water. The precipitated solid products were filtered and crystallized from methanol to give compound 6. Yield%: 70, m.p. > 350 °C, IR, cm−1: 3344, 3291 (NH2), 3100 (CH arom.), 2966, 2881 (CH aliph.), 2200 (CN), 1699 (C=O). 1H (DMSO-d6, ppm): 1.6 [s, 3H, CH3], 2.4 [s, 3H, N–CH3], 6.5 [s, 2H, NH2, D2O-exchangeable], 7.0–8.0 [m, 6H, Ar–H + CH-pyrrole]. 13C (DMSO-d6, ppm): 13.9, 39.1, 101.2(2), 107.4, 110.4, 113.9(2), 116.2, 117.6(2), 119.1, 129.3(2), 138.1, 139.4, 161.2. Anal. Calcd. for C17H14N6O (318): C, 64.14; H, 4.43; N, 25.40. Found: C, 64.32; H, 4.67; N, 25.12.
3.2.6. Ethyl-3-amino-4-cyano-1-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-1Hpyrrole-2-carboxylate 7
Compound 2 (0.01 mol) was mixed with ethyl bromoacetate (0.01 mol) in dioxane (20 mL) containing 3 drops of triethylamine and refluxed for 5 h, the reaction mixture was cooled, poured onto ice water. The precipitated solid products were filtered and crystallized from methanol to give compound 7. Yield%: 89, m.p. = 143.4 °C, IR, cm−1: 3411, 3396 (NH2), 3100 (CH arom.), 2976, 2882 (CH aliph.), 2186 (CN), 1718, 1696 (2C=O). 1H (DMSO-d6, ppm): 1.2 [t, 3H, CH3–ester], 2.0 [s, 3H, CH3], 2.4 [s, 3H, N–CH3], 4.1 [q, 2H, CH2–ester], 7.3–8.0 [m, 7H, Ar–H + NH2], 8.7 [s, 1H, CH–pyrrole]. 13C (DMSO-d6, ppm): 12.6, 13.4, 39.0, 59.8, 98.7, 112.0, 114.3(2), 115.6, 118.4, 122.7, 126.3, 129.1(2), 138.1, 139.6, 141.2, 160.0, 161.2. Anal. Calcd. for C19H19N5O3 (365): C, 62.46; H, 5.24; N, 19.17. Found: C, 62.79; H, 5.57; N, 19.34.
3.2.7. 3-Amino-4-cyano-1-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-1H-pyrrole-2- carboxamide 8
Compound 2 (0.01 mol) was mixed with 2-chloroacetamide (0.01 mol) in dioxane (20 mL) containing 3 drops of triethylamine and refluxed for 5 h, the reaction mixture was cooled, poured onto ice water. The precipitated solid products were filtered and crystallized from methanol to give compound 8. Yield%: 76, m.p. > 350 °C, IR, cm−1: 3404, 3385, 3236 (NH2), 3066 (CH arom.), 2961, 2836 (CH aliph.), 2196 (CN), 1700, 1680 (2C=O). 1H (DMSO-d6, ppm): 1.6 [s, 3H, CH3], 2.3 [s, 3H, N–CH3], 7.2–8.0 [m, 9H, Ar–H + CONH2 + NH2], 8.5 [s, 1H, CH–pyrrole]. 13C (DMSO-d6, ppm): 11.9, 39.1, 99.2, 111.8, 114.3(2), 115.6, 118.2, 124.4, 129.0(2), 131.8, 135.7, 138.2, 153.2, 162.4, 166.0. Anal. Calcd. for C17H16N6O3 (336): C, 60.71; H, 4.79; N, 24.99. Found: C, 60.99; H, 4.49; N, 24.70.
3.2.8. 4-((4,6-Diamino-2-thioxopyrimidin-5(2H)-ylidene)methylamino)-1,5-dimethyl-1,2-phenyl-1,2-dihydropyrazol-3-one 9
A mixture of 2 (0.01 mol) and thiourea (0.01 mol) was refluxed for 5 h in ethanol containing sodium ethoxide (0.01 mol). The reaction mixture was cooled, poured onto ice water, acidified with dil. HCl, the precipitated solid product was filtered and crystallized from methanol to give 9. Yield%: 90, m.p. = 219.6 °C, IR, cm−1: 3410, 3362, 3350 (NH2), 3054 (CH arom.), 2922, 2860 (CH aliph.), 1675 (C=O), 1624 (C=N), 1317 (C=S). 1H (DMSO-d6, ppm): 2.2 [s, 3H, CH3], 3.1 [s, 3H, N–CH3], 7.3 [s, 4H, 2NH2, D2O-exchangeable], 7.5–7.9 [m, 5H, Ar–H], 9.4 [s, 1H, CH], 13.0 [s, 1H, NH, D2O-exchangeable]. 13C (DMSO-d6, ppm): 10.3, 39.3, 84.2, 109.1, 116.4(2), 124.2, 127.2(2), 129.1, 134.4(2), 160.2, 170.8(2), 211.3 (C=S). Anal. Calcd. for C16H17N7OS (355): C, 54.07; H, 4.82; N, 27.59. Found: C, 54.34; H, 4.65; N, 27.25.
3.3. General Procedure for Preparation of Compounds 10–15
A mixture of 2 (0.01 mol) and the appropriate aryl isothiocyanate (0.01 mol) was refluxed for 10 h in ethanol containing sodium ethoxide (0.01 mol). The reaction mixture was cooled, poured onto ice water, acidified with dil. HCl, the precipitated solid product was filtered and crystallized from methanol to give 10–15, respectively.
3.3.1. 1-(1,5-Dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-4-oxo-3-phenyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile 10
Yield%: 81, m.p. = 132.9 °C, IR, cm−1: 3100 (CH arom.), 2966, 2871 (CH aliph.), 2201 (CN), 1672, 1659 (2C=O), 1292 (C=S). 1H (DMSO-d6, ppm): 2.2 [s, 3H, CH3], 3.2 [s, 3H, N–CH3], 7.3 [s, 1H, CH–pyrimidine], 7.5–8.1 [m, 10H, Ar–H]. 13C (DMSO-d6, ppm): 14.4, 39.1, 104.6, 109.3, 114.1(2), 116.5, 118.1, 122.0(2), 126.3, 128.6(2), 129.1(2), 129.3, 134.5, 137.8, 139.1, 159.9, 160.5, 187.2 (C=S). Anal. Calcd. for C22H17N5O2S (415): C, 63.60; H, 4.12; N, 16.86. Found: C, 63.34; H, 4.32; N, 16.60.
3.3.2. 1-(1,5-Dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-3-(4-methoxyphenyl)-4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile 11
Yield%: 71, m.p. = 90.6 °C, IR, cm−1: 3046 (CH arom.), 2991, 2875 (CH aliph.), 2208 (CN), 1684, 1654 (2C=O), 1257 (C=S). 1H (DMSO-d6, ppm): 2.4 [s, 3H, CH3], 3.3 [s, 3H, N–CH3], 3.7 [s, 3H, OCH3], 6.8–7.5 [m, 10H, Ar–H + CH–pyrimidine]. 13C (DMSO-d6, ppm): 13.9, 39.1, 55.1, 95.2, 107.5, 113.5(2), 113.8(3), 123.3, 124.2(2), 124.9, 129.1(2), 130.8, 131.4, 143.6, 156.2, 156.6, 164.7, 188.0. Anal. Calcd. for C23H19N5O3S (445): C, 62.01; H, 4.30; N, 15.72. Found: C, 62.34; H, 4.09; N, 15.51.
3.3.3. 1-(1,5-Dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-3-(4-nitrophenyl)-4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile 12
Yield%: 79, m.p. = 190.9 °C, IR, cm−1: 3076 (CH arom.), 2991, 2908 (CH aliph.), 2188 (CN), 1700, 1689 (2C=O), 1597, 1380 (NO2), 1308 (C=S). 1H (DMSO-d6, ppm): 2.5 [s, 3H, CH3], 3.3 [s, 3H, N–CH3], 7.8–8.2 [m, 10H, Ar–H + CH–pyrimidine]. 13C (DMSO-d6, ppm): 14.8, 39.3, 89.6, 108.2, 112.3(3), 117.5, 120.9, 124.5(2), 124.9(2), 126.3(2), 129.0, 137.4(2), 142.8, 144.0, 155.6, 162.4, 187.6 (C=S). Anal. Calcd. for C22H16N6O4S (460): C, 57.38; H, 3.50; N, 18.25. Found: C, 57.69; H, 3.20; N, 18.56.
3.3.4. 3-(4-Bromophenyl)-1-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile 13
Yield%: 69, m.p. = 76.0 °C, IR, cm−1: 3100 (CH arom.), 2986, 2861 (CH aliph.), 2218 (CN), 1684, 1653 (2C=O), 1216 (C=S). 1H (DMSO-d6, ppm): 2.2 [s, 3H, CH3], 3.1 [s, 3H, N–CH3], 7.0–7.6 [m, 10H, Ar–H + CH–pyrimidine]. 13C (DMSO-d6, ppm): 13.9, 39.5, 92.4, 104.6, 113.8(2), 114.1, 116.6, 119.9, 121.0(2), 127.2(2), 129.0, 132.6, 133.4(2), 137.7, 138.6, 159.9, 160.1, 186.6 (C=S). Anal. Calcd. for C22H16BrN5O2S (493): C, 53.45; H, 3.26; N, 14.17. Found: C, 53.70; H, 3.11; N, 14.44.
3.3.5. 1-(1,5-Dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-3-(4-iodophenyl)-4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile 14
Yield%: 90, m.p. = 114.1 °C, IR, cm−1: 3092 (CH arom.), 2980, 2861 (CH aliph.), 2187 (CN), 1684, 1654 (2C=O), 1276 (C=S). 1H (DMSO-d6, ppm): 2.5 [s, 3H, CH3], 3.1 [s, 3H, N–CH3], 7.6–7.9 [m, 10H, Ar–H + CH–pyrimidine]. 13C (DMSO-d6, ppm): 13.9, 39.5, 88.8(2), 107.3, 114.6(2), 116.2, 120.2, 123.6(2), 129.0(3), 130.6, 137.2, 137.9(2), 139.1, 162.7, 166.1, 187.3. Anal. Calcd. for C22H16N5O2S (541): C, 48.81; H, 2.98; N, 12.94. Found: C, 48.69; H, 2.69; N, 12.72.
3.3.6. 4-(5-Cyano-3-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-6-oxo-2-thioxo-2,3-tetrahydropyrimidin-1(6H)-yl)benzensulfonamide 15
Yield%: 90, m.p. = 164.8 °C, IR, cm−1: 3041 (CH arom.), 2934, 2881 (CH aliph.), 2217 (CN), 1714, 1662 (2C=O), 1296 (C=S). 1H (DMSO-d6, ppm): 2.2 [s, 3H, CH3], 3.0 [s, 3H, N–CH3], 7.3–8.0 [m, 11H, Ar–H + SO2NH2], 8.1 [s, 1H, CH–pyrimidine]. 13C (DMSO-d6, ppm): 13.9, 39.4, 96.2, 104.6, 114.2(2), 116.4, 117.2, 121.6(2), 125.1(2), 127.3(2), 129.1, 134.2, 134.5, 139.6, 140.9, 160.2, 160.5, 187.6 (C=S). Anal. Calcd. for C22H18N6O4S2 (494): C, 53.43; H, 3.67; N, 16.99. Found: C, 53.69; H, 3.90; N, 16.34.
3.3.7. 3,3′-(4,4′-Sulfonylbis(4,1-phenylene))bis(1-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol- 4-yl)-4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carbonitrile) 16
A mixture of 2 (0.01 mol) and 1-(4-isothiocyanatophenylsulfonyl)-4-isothiocyanatobenzene (0.02 mol) was refluxed for 10 h in ethanol containing sodium ethoxide (0.01 mol). The reaction mixture was cooled, poured onto ice water, acidified with dil. HCl, the precipitated solid product was filtered and crystallized from methanol to give 16. Yield%: 81, m.p. = 140.6 °C, IR, cm−1: 3076 (CH arom.), 2981, 2861 (CH aliph.), 2219 (CN), 1661, 1643 (4C=O), 1322 (2C=S). 1H (DMSO-d6, ppm): 2.2 [s, 6H, 2CH3], 3.1 [s, 6H, 2N–CH3], 7.3–8.0 [m, 18H, Ar–H], 8.6 [s, 2H, 2CH–pyrimidine]. 13C (DMSO-d6, ppm): 13.8(2), 39.0(2), 92.6(2), 108.6(2), 113.5(4), 114.1(2), 118.4(2), 121.7(4), 127.0(4), 128.2(4), 129.1(2), 134.5(2), 136.2(2), 136.7(2), 142.4(2), 159.4(2), 160.2(2), 187.6(2). Anal. Calcd. for C44H32N10O6S3 (892): C, 59.18; H, 3.61; N, 15.69. Found: C, 59.40; H, 3.80; N, 15.48.
3.4. General Procedure for Preparation of Compounds 17 and 18
Compound 10 (0.01 mol) was mixed with either hydrazine hydrate or phenyl hydrazine (0.01 mol) in dioxane (20 mL) and refluxed for 5 h, the reaction mixture was cooled, poured onto ice water. The precipitated solid products were filtered and crystallized from methanol to give compounds 17 and 18, respectively.
3.4.1. (Z)-1-(1,5-Dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-2-hydrazono-4-oxo-3-phenyl-1,2,3,4-tetrahydropyrimidine-5-carbonitrile 17
Yield%: 68, m.p. = 78.0 °C, IR, cm−1: 3213, 3191 (NH2), 3047 (CH arom.), 2961, 2876 (CH aliph.), 2191 (CN). 1H (DMSO-d6, ppm): 2.5 [s, 3H, CH3], 3.2 [s, 3H, N–CH3], 4.5 [s, 2H, NH2, D2O-exchangeable], 7.1–7.5 [m, 11H, Ar–H + CH–pyrimidine]. 13C (DMSO-d6, ppm): 13.1, 39.0, 94.6, 106.2, 114.5(2), 116.7, 118.2, 120.6(2), 122.1, 127.6(2), 128.4(2), 129.7, 134.2, 137.7, 138.5, 156.2, 162.8, 168.9. Anal. Calcd. for C22H19N7O2 (413): C, 63.91; H, 4.63; N, 23.72. Found: C, 63.70; H, 4.91; N, 23.55.
3.4.2. (Z)-1-(1,5-Dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-4-oxo-3-phenyl-2-(2-phenylhydrazono)-1,2,3,4-tetrahydropyrimidine-5-carbonitrile 18
Yield%: 65, m.p. = 90.6 °C, IR, cm−1: 3381 (NH), 3074 (CH arom.), 2966, 2836 (CH aliph.), 2220 (CN), 1700, 1653 (2C=O). 1H (DMSO-d6, ppm): 2.4 [s, 3H, CH3], 3.2 [s, 3H, N–CH3], 7.0–8.0 [m, 15H, Ar–H], 8.4 [s, 1H, CH–pyrimidine], 9.5 [s, 1H, NH, D2O-exchangeable]. 13C (DMSO-d6, ppm): 14.1, 39.6, 95.3, 108.3, 113.8(2), 114.6, 115.8, 116.4(2), 118.9, 119.5(2), 122.2, 128.6(2), 129.0(2), 129.6, 129.8(2), 130.2, 139.2, 141.5, 144.6, 153.5, 155.7, 165.6. Anal. Calcd. for C28H23N7O2 (489): C, 68.70; H, 4.74; N, 20.03. Found: C, 68.92; H, 4.99; N, 20.33.
3.4.3. (Z)-4-(3-Amino-6-hydrazono-7-phenyl-6,7-dihydropyrazolo[3,4-d]pyrimidin-5-yl)-1,5-dimethyl-2-phenyl-1,2-dihydropyrazol-3-one 19
Compound 10 (0.01 mol) was mixed with hydrazine hydrate (0.02 mol) in dioxane (20 mL) and refluxed for 5 h, the reaction mixture was cooled, poured onto ice water. The precipitated solid products were filtered and crystallized from methanol to give compounds 19. Yield%: 73, m.p. = 222.6 °C, IR, cm−1: 3420, 3362 (NH2), 3050 (CH arom.), 2971, 2861 (CH aliph.), 1654 (C=O), 1617 (C=N). 1H (DMSO-d6, ppm): 2.2 [s, 3H, CH3], 3.2 [s, 3H, N–CH3], 5.4 [s, 2H, N–NH2, D2O-exchangeable], 6.5 [s, 2H, NH2, D2O-exchangeable], 7.0–8.1 [m, 11H, Ar–H + CH–pyrimidine]. 13C (DMSO-d6, ppm): 11.4, 39.0, 97.6, 107.3, 112.6(2), 114.3(2), 116.7, 118.6, 123.7, 128.6(2), 129.1, 129.4(2), 134.1, 139.8, 155.7, 162.3, 166.0(2). Anal. Calcd. for C22H21N9O (427): C, 61.81; H, 4.95; N, 29.49. Found: C, 62.03; H, 5.12; N, 29.67.
3.5. General Procedure for Preparation of Compounds 20 and 21
A solution of 4-aminoantipyrine 1 (0.001 mol) in either triethylorthoformate or triethylorthoacetate (0.001 mol) containing three drops of acetic anhydride was refluxed for 8 h, the reaction mixture was cooled and then poured onto cold water, the obtained solid was recrystallized from methanol to give compounds 20 and 21, respectively.
3.5.1. (E)-Ethyl-N-1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl-formimidate 20
Yield%: 90, m.p. = 196.9 °C, IR, cm−1: 3078 (CH arom.), 2976, 2912 (CH aliph.), 1661 (C=O), 1612 (C=N). 1H (DMSO-d6, ppm): 1.0 [t, 3H, CH3], 2.3 [s, 3H, CH3], 3.1 [s, 3H, N–CH3], 3.5 [q, 2H, CH2], 7.2–7.5 [m, 5H, Ar–H], 8.1 [s, 1H, CH]. 13C (DMSO-d6, ppm): 11.3, 18.5, 39.3, 56.0, 106.3, 107.0(2), 120.0, 129.0(2), 135.1, 151.6, 160.1, 164.7. Anal. Calcd. for C14H17N3O2 (259): C, 64.85; H, 6.61; N, 16.34. Found: C, 64.66; H, 6.34; N, 16.67.
3.5.2. (E)-Ethyl-N-1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl-acetimidate 21
Yield%: 88, m.p. = 81.6 °C, IR, cm−1: 3086 (CH arom.), 2936, 2817 (CH aliph.), 1658 (C=O), 1598 (C=N). 1H (DMSO-d6, ppm): 1.2 [t, 3H, CH3], 1.9 [s, 3H, N=C–CH3], 2.1 [s, 3H, CH3], 3.3 [s, 3H, N–CH3], 4.1 [q, 2H, CH2], 7.2–7.5 [m, 5H, Ar–H]. 13C (DMSO-d6, ppm): 10.1 (CH3–pyrazole), 14.0 (CH3–ethyl), 17.4 (N=C–CH3), 39.1 (N–CH3), 61.0 (CH2–ethyl), 112.6, 118.7(2), 122.8, 128.9(2), 135.4, 146.1, 160.0, 164.0 (C=O). Anal. Calcd. for C15H19N3O2 (273): C, 65.91; H, 7.01; N, 15.37. Found: C, 66.13; H, 7.34; N, 15.48.
3.6. In-Vitro Anticancer Screening
The human tumor cell line (MCF7) was available from the National Cancer Institute, Cairo, Egypt. The antitumor activity of the newly synthesized compounds against the MCF7 cells was measured using the Sulforhodamine B (SRB) assay by the method of Skehan et al. (1990) [23,24]. The cell lines were grown in RPMI 1640 medium containing 10% fetal bovine serum and 2 mM l-glutamine. Cells were plated in 96-multi-well plates (104 cells/well) and were incubated at 37 °C, 5% CO2 in a humidified atmosphere for 24 h to allow attachment prior to addition of compounds. The test compounds 3–14 were dissolved in DMSO and diluted with saline to the appropriate volume and maintained in RPMI 1640 medium. Different concentrations of the compounds under test were made: 5, 12.5, 25, 30 and 50 μM and were added to the cells. Triplicate wells were prepared for each individual dose. Cells were incubated with the compounds for 48 h at 37 °C, 5% CO2. After 48 h, cells were fixed in situ by the gentle addition of 50 μL of cold 30% (w/v) trichloroacetic acid (TCA) (final concentration, 10%) and incubated for 60 min at 4 °C. The supernatant was discarded and the plates were washed five times with tap water and air dried. Sulforhodamine B (SRB) solution (50 μL) at 0.4% (w/v) in 1% acetic acid was added to each well and plates were incubated for 20 min at room temperature. After staining, unbounded dye was removed by four washes with 1% acetic acid, and attached stain was recovered with Tris-EDTA buffer. Color intensity was measured at wave length 564 nm in an ELISA reader (Gmbh, Viesbaden, Germany). The relation between surviving fraction and drug concentration was plotted to get the survival curve from each compound after the specified time. The concentration required for 50% inhibition of cell viability (IC50) was calculated and compared with the reference drug doxorubicin and the results are given in Table 1.
4. Conclusions
The objective of the present study was to synthesize and investigate the anticancer activity of some novel pyrazole carrying a biologically active sulfonamide moieties. It was found that compounds 5, 13, 14, and 16–19 showed promising anticancer activity, higher than that of doxorubicin as reference drug against human breast cancer cell line (MCF7), while compounds 10, 12 are nearly as active as doxorubicin as positive control. Compound 8 exhibited a moderate activity and compounds 2–4, 6, 7, 9, and 20 showed a weak activity, while compounds 11, 15 and 21 revealed no activity. Further investigations on different probable mechanisms of action and dose-response studies should be helpful in identifying the specific site(s) of action and optimum doses of the synthesized antipyrine derivatives. These investigations would be crucial in discovering more potent and more selective anti-breast cancer agents.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University (Riyadh, Saudi Arabia) for its funding of this research through the Research Group Project No. RGP-VPP-302.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Author Contributions
M.M.G. suggested the research idea, contributed in the experimental work and in writing the paper. M.G.E.-G. contributed in experimental work and in writing the paper. M.S.A. contributed in the experimental work, biological activity and in writing the paper.
References
- 1.Himly M., Jahn-Schmid B., Pittertschatscher K., Bohle B., Grubmayr K., Ferreira F., Ebner H., Ebner C. Ig E-mediated immediate-type hypersensitivity to the pyrazolone drug propyphenazone. J. Allergy Clin. Immunol. 2003;111:882–888. doi: 10.1067/mai.2003.163. [DOI] [PubMed] [Google Scholar]
- 2.Gürsoy A., Demirayak S., Capan G., Erol K., Vural K. Synthesis and preliminary evaluation of new 5-pyrazolinone derivatives as analgesic agents. Eur. J. Med. Chem. 2000;35:359–364. doi: 10.1016/s0223-5234(00)00117-3. [DOI] [PubMed] [Google Scholar]
- 3.Teng Y., Liu R., Li C., Zhang H. Effect of 4-aminoantipyrine on oxidative stress induced by glutathione depletion in single human erythrocytes using a microfluidic device together with fluorescence imaging. J. Hazard. Mater. 2011;192:1766–1771. doi: 10.1016/j.jhazmat.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Turan-Zitouni G., Sivaci M., Kiliç F.S., Erol K. Synthesis of some triazolyl-antipyrine derivatives and investigation of analgesic activity. Eur. J. Med. Chem. 2001;36:685–689. doi: 10.1016/s0223-5234(01)01252-1. [DOI] [PubMed] [Google Scholar]
- 5.Lutsevich A.N., Bender K.I., Reshet’ko O.V. The relationship between antipyrine kinetics, the seromucoid content and the xanthine oxidase activity in the plasma of rats with acute and chronic inflammation. Eksp Klin. Farmakol. 1995;58:51–55. [PubMed] [Google Scholar]
- 6.Bondock S., Rabie R., Etman H.A., Fadda A.A. Synthesis and antimicrobial activity of some new heterocycles incorporating antipyrine moiety. Eur. J. Med. Chem. 2008;43:2122–2129. doi: 10.1016/j.ejmech.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Metwally M.A., Gouda M.A., Harmal A.N., Khalil A.M. Synthesis, antitumor, cytotoxic and antioxidant evaluation of some new pyrazolotriazines attached to antipyrine moiety. Eur. J. Med. Chem. 2012;56:254–262. doi: 10.1016/j.ejmech.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 8.Kakiuchi Y., Sasaki N., Satoh-Masuoka M., Murofushi H., Murakami-Murofushi K. A novel pyrazolone, 4,4-dichloro-1-(2,4-dichlorophenyl)-3-methyl-5-pyrazolone, as a potent catalytic inhibitor of human telomerase q. Biochem. Biophy. Res. Commun. 2004;320:1351–1358. doi: 10.1016/j.bbrc.2004.06.094. [DOI] [PubMed] [Google Scholar]
- 9.Sigroha S., Narasimhan B., Kumar P., Khatkar A., Ramasamy K., Mani V., Mishra R.K., Abdul Majeed A.B. Design, synthesis, antimicrobial, anticancer evaluation, and QSAR studies of 4-(substituted benzylidene-amino)-1,5-dimethyl-2-phenyl-1,2-dihydropyrazol-3-ones. Med. Chem. Res. 2012;21:3863–3875. [Google Scholar]
- 10.Chandrasekharan N.V., Dai H., Roos K.L.T., Evanson N.K., Tomsik J., Elton T.S. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: Cloning, structure, and expression. Proc. Natl. Acad. Sci. USA. 2002;99:13926–13931. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy M. Hypersensitivity to pyrazolones. Thorax. 2000;55:72–74. doi: 10.1136/thorax.55.suppl_2.S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Haiza M.A., El-Assiery S.A., Sayed G.H. Synthesis and potential antimicrobial activity of some new compounds containing the pyrazol-3-one moiety. Acta Pharm. 2001;51:251–261. [Google Scholar]
- 13.Lin R., Chiu G., Yu Y. Design, synthesis, and evaluation of 3,4-disubstituted pyrazole analogues as anti-tumor CDK inhibitors. Bioorg. Med. Chem. Lett. 2007;17:4557–4581. doi: 10.1016/j.bmcl.2007.05.092. [DOI] [PubMed] [Google Scholar]
- 14.Aly H.M., El-Gazzar M.G. Novel pyrazole derivatives as anticancer and radiosensitizing agents. Arzneimittelforschung. 2012;62:105–112. doi: 10.1055/s-0031-1297252. [DOI] [PubMed] [Google Scholar]
- 15.Ghorab M.M., Ragab F.A., Heiba H.I., Hanan A.Y., El-Gazzar M.G. Synthesis of novel pyrazole and pyrimidine derivatives bearing sulfonamide moiety as antitumor and radiosensitizing agents. Med. Chem. Res. 2012;21:1376–1383. doi: 10.1016/j.bmcl.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Ghorab M.M., Heiba H.I., Hassan A.A., Abd El-Aziz A.B., El-Gazzar M.G. Antimicrobial evaluation of novel pyrrole, pyrazole, pyrimidine and pyrrolo [2,3-d]-pyrimidine derivatives brearing sulfonamide moiety. J. Am. Sci. 2011;7:1064–1073. [Google Scholar]
- 17.Ghorab M.M., Ragab F.A., Heiba H.I., Youssef H.A., Galal M. Synthesis of some new pyrazole and pyrimidine derivatives carrying a sulfonamide moiety of expected antitumor activity and study of the synergistic effect of gamma-irradiation. Arzneimittelforschung. 2010;60:48–55. doi: 10.1055/s-0031-1296248. [DOI] [PubMed] [Google Scholar]
- 18.Ghorab M.M., Alsaid M.S., Nissan Y.M. Dapson in heterocyclic chemistry, part V: Synthesis, molecular docking and anticancer activity of some novel sulfonylbiscompounds carrying biologically active dihydropyridine, dihydroisoquinoline, 1,3-dithiolan, 1,3-dithian, acrylamide, pyrazole, pyrazolopyrimidine and benzochromenemoieties. Chem. Pharm. Bull. (Tokyo) 2012;60:1019–1028. doi: 10.1248/cpb.c12-00292. [DOI] [PubMed] [Google Scholar]
- 19.Mohareb R.M., Sherif S.M., Zohdi H.F. Heterocyclic synthesis with enamines: Convenient synthesis of polyfunctionally substituted pyrazole, pyridine, pyrimidine and pyrazolo[3,4-d]pyrimidine derivatives. J. Chin. Chem. Soc. 1993;40:181–187. [Google Scholar]
- 20.Mohamed A.M., El-Sayed W.A., Alsharari M.A., Al-Qalawi H.R., Germoush M.O. Anticancer activities of some newly synthesized pyrazole and pyrimidine derivatives. Arch. Pharm. Res. 2013;36:1055–1065. doi: 10.1007/s12272-013-0163-x. [DOI] [PubMed] [Google Scholar]
- 21.Ghorab M.M., Ragab F.A., Heiba H.I., Youssef H.A., El-Gazzar M.G. Synthesis of novel pyrrole and pyrrolo[2,3-d]pyrimidine derivatives bearing sulfonamide moiety for evaluation as anticancer and radiosensitizing agents. Bioorg. Med. Chem. Lett. 2010;20:6316–6320. doi: 10.1016/j.bmcl.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Al-Issa S.A. Synthesis and anticancer activity of some fused pyrimidines and related heterocycles. Saudi Pharm. J. 2013;21:305–316. doi: 10.1016/j.jsps.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skehan P., Storeng R., Scudiero D., Monks A., McMahon J., Vistica D., Warren J.T., Bokesch H., Kenney S., Boyd M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 24.Rubinstein L.V., Shoemaker R.H., Paull K.D., Simon R.M., Tosini S., Skehan P., Scudiero D.A., Monks A., Boyd M.R. Comparison of in vitro anticancer-drug-screening data generated with a tetrazolium assay vs. a protein assay against a diverse panel of human tumor cell lines. J. Natl. Cancer Inst. 1990;82:1113–1118. doi: 10.1093/jnci/82.13.1113. [DOI] [PubMed] [Google Scholar]