Abstract
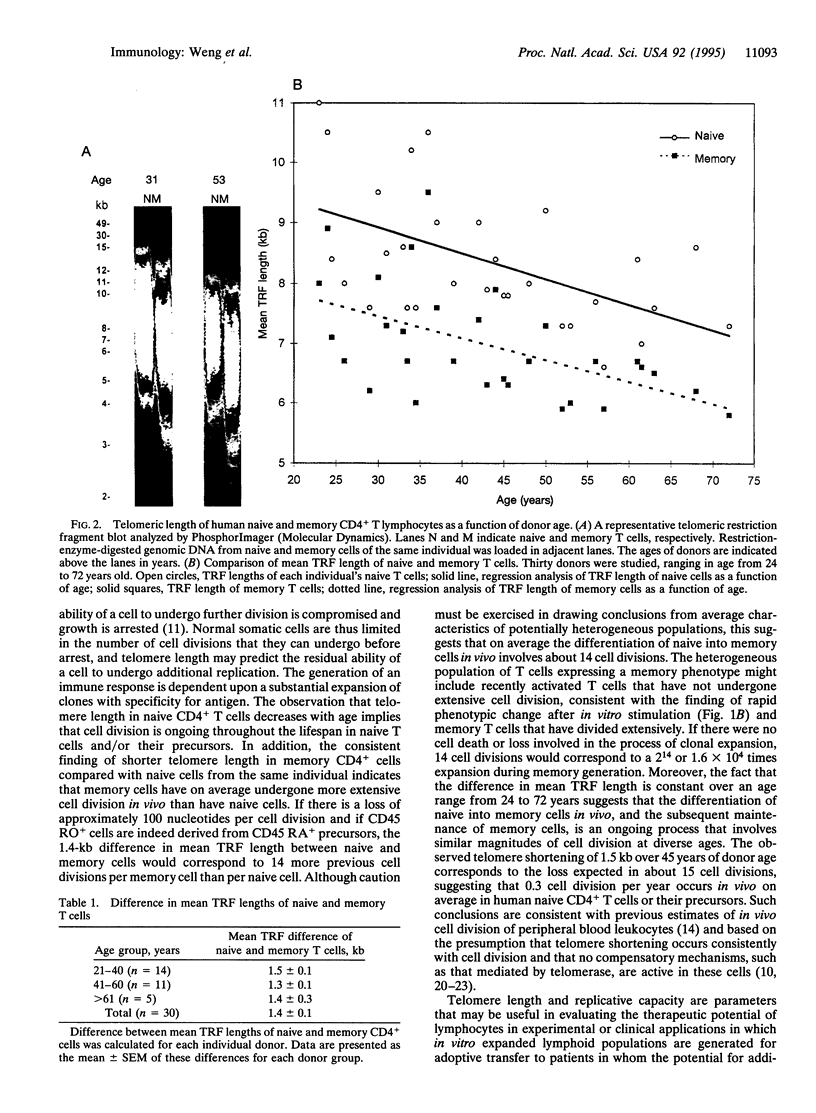
The present study has assessed the replicative history and the residual replicative potential of human naive and memory T cells. Telomeres are unique terminal chromosomal structures whose length has been shown to decrease with cell division in vitro and with increased age in vivo for human somatic cells. We therefore assessed telomere length as a measure of the in vivo replicative history of naive and memory human T cells. Telomeric terminal restriction fragments were found to be 1.4 +/- 0.1 kb longer in CD4+ naive T cells than in memory cells from the same donors, a relationship that remained constant over a wide range of donor age. These findings suggest that the differentiation of memory cells from naive precursors occurs with substantial clonal expansion and that the magnitude of this expansion is, on average, similar over a wide range of age. In addition, when replicative potential was assessed in vitro, it was found that the capacity of naive cells for cell division was 128-fold greater as measured in mean population doublings than the capacity of memory cells from the same individuals. Human CD4+ naive and memory cells thus differ in in vivo replicative history, as reflected in telomeric length, and in their residual replicative capacity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allsopp R. C., Vaziri H., Patterson C., Goldstein S., Younglai E. V., Futcher A. B., Greider C. W., Harley C. B. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counter C. M., Avilion A. A., LeFeuvre C. E., Stewart N. G., Greider C. W., Harley C. B., Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992 May;11(5):1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counter C. M., Gupta J., Harley C. B., Leber B., Bacchetti S. Telomerase activity in normal leukocytes and in hematologic malignancies. Blood. 1995 May 1;85(9):2315–2320. [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985 Dec;43(2 Pt 1):405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Futcher A. B., Greider C. W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990 May 31;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Villeponteau B. Telomeres and telomerase in aging and cancer. Curr Opin Genet Dev. 1995 Apr;5(2):249–255. doi: 10.1016/0959-437x(95)80016-6. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Dempster M., Dunlop M. G., Thompson A. M., Green D. K., Allshire R. C. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990 Aug 30;346(6287):866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- Holbeck S. L., Nepom G. T. Exon-specific oligonucleotide probes localize HLA-DQ beta allelic polymorphisms. Immunogenetics. 1986;24(4):251–258. doi: 10.1007/BF00364529. [DOI] [PubMed] [Google Scholar]
- Kim N. W., Piatyszek M. A., Prowse K. R., Harley C. B., West M. D., Ho P. L., Coviello G. M., Wright W. E., Weinrich S. L., Shay J. W. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994 Dec 23;266(5193):2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Levine B. L., Ueda Y., Craighead N., Huang M. L., June C. H. CD28 ligands CD80 (B7-1) and CD86 (B7-2) induce long-term autocrine growth of CD4+ T cells and induce similar patterns of cytokine secretion in vitro. Int Immunol. 1995 Jun;7(6):891–904. doi: 10.1093/intimm/7.6.891. [DOI] [PubMed] [Google Scholar]
- Michie C. A., McLean A., Alcock C., Beverley P. C. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature. 1992 Nov 19;360(6401):264–265. doi: 10.1038/360264a0. [DOI] [PubMed] [Google Scholar]
- Powrie F., Mason D. Phenotypic and functional heterogeneity of CD4+ T cells. Immunol Today. 1988 Sep;9(9):274–277. doi: 10.1016/0167-5699(88)91309-6. [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Shaw S. Human naive and memory T cells: reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol Today. 1988 Jul-Aug;9(7-8):195–199. doi: 10.1016/0167-5699(88)91212-1. [DOI] [PubMed] [Google Scholar]
- Slagboom P. E., Droog S., Boomsma D. I. Genetic determination of telomere size in humans: a twin study of three age groups. Am J Hum Genet. 1994 Nov;55(5):876–882. [PMC free article] [PubMed] [Google Scholar]
- Tedder T. F., Cooper M. D., Clement L. T. Human lymphocyte differentiation antigens HB-10 and HB-11. II. Differential production of B cell growth and differentiation factors by distinct helper T cell subpopulations. J Immunol. 1985 May;134(5):2989–2994. [PubMed] [Google Scholar]
- Vaziri H., Dragowska W., Allsopp R. C., Thomas T. E., Harley C. B., Lansdorp P. M. Evidence for a mitotic clock in human hematopoietic stem cells: loss of telomeric DNA with age. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9857–9860. doi: 10.1073/pnas.91.21.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri H., Schächter F., Uchida I., Wei L., Zhu X., Effros R., Cohen D., Harley C. B. Loss of telomeric DNA during aging of normal and trisomy 21 human lymphocytes. Am J Hum Genet. 1993 Apr;52(4):661–667. [PMC free article] [PubMed] [Google Scholar]




