Abstract
Background
Liver transplantation has become an established treatment for cirrhotic patients with hepatocellular carcinoma (HCC), and the Milan criteria are now widely accepted and applied as an indication for deceased donor liver transplantation (DDLT) in Western countries. In contrast, however, due to the severe organ shortage, living donor liver transplantation (LDLT) is mainstream in Japan and in other Asian countries.
Summary
Unlike DDLT, LDLT is not limited by the restrictions imposed by the nationwide allocation system, and the indication for LDLT in patients with HCC often depends on institutional or case-by-case considerations, balancing the burden on the donor, the operative risk, and the overall survival benefit for the recipient. Accumulating data from a nationwide survey as well as individual center experience indicate that extending the Milan criteria is warranted.
Key Messages
While the promotion of DDLT should be intensified in Japan and other Asian countries, LDLT will continue to be a mainstay for the treatment of HCC in cirrhotic patients.
Key Words: Deceased donor liver transplantation, Hepatocellular carcinoma, Living donor liver transplantation, Living donors, Recurrence
Introduction
Hepatocellular carcinoma (HCC) is the seventh most common cancer overall and the third most common cause of cancer-related death worldwide [1, 2]. HCC usually coexists with liver cirrhosis, which is most commonly secondary to hepatitis C virus (HCV) or hepatitis B virus (HBV) infection or other diseases, such as alcoholic liver disease and autoimmune disease. Liver transplantation (LT) is now widely accepted as an effective treatment modality for HCC, especially in patients with cirrhosis, which often precludes conventional locoregional treatment [3, 4, 5, 6, 7].
Early reports of LT as a treatment for HCC were associated with poor outcomes [8, 9], reflecting the advanced HCC status of the recipients indicated for LT. The landmark study by Mazzaferro et al. [10], however, demonstrated that survival rates after LT among selected HCC patients were equivalent to those of patients transplanted for non-malignant liver disease. In that study, 48 LT recipients having a single tumor smaller than 5 cm in diameter or up to three tumors smaller than 3 cm in diameter with no vascular invasion or extra-hepatic disease, as determined by preoperative imaging studies, had actuarial 4-year disease-free and overall survival rates of 83% and 75%, respectively. These criteria, called the Milan criteria, are the gold standard indication for LT in patients with HCC. Recently, Mazzaferro and associates [11] reported that the Milan criteria comprise an independent prognostic factor for long-term outcome after LT for HCC based on a systematic review of the literature encompassing 15 years of experience and including 3949 LT recipients. At a recent international conference of expert panels, the Milan criteria were concluded to be the gold standard indication for LT in recipients with HCC and the basis for comparison with other investigated criteria [12].
On the other hand, however, there has been ongoing debate as to whether the Milan criteria are too strict, thereby precluding patients with HCC from LT who could otherwise benefit from LT, and many investigators have performed studies extending the Milan criteria with satisfactory results. The issue of extending the criteria for patients with HCC is a crucial topic for cadaveric LT in Western countries [13].
In Asian countries, living donor liver transplantation (LDLT) makes up the majority of LT cases, and thus the situation differs from that of Western countries [14, 15, 16]. Grafts from living donors are not limited by restrictions imposed by the organ allocation system, meaning that the relation of the graft and recipient is usually one-on-one. Consequently, selection criteria based on the tumor burden, such as the tumor size and tumor number, can be considered relative on a case-by-case basis, taking into account the presence of risk factors for recurrence and the chance of survival, as well as the wishes of the donor. In fact, many highvolume LT centers in Asia already perform LDLT for patients with HCC based on extended Milan criteria [7]. The present review covers recent topics regarding LT for HCC with special reference to LDLT for HCC in Japan and other Asian countries.
Factors Associated with HCC Recurrence after LT
Despite every effort to minimize recurrence by the careful selection of HCC patients for LT, HCC recurrence after LT remains a clinically important problem. Based on the literature, HCC recurrence after LT uniformly occurs with an incidence of 10-20% [17]. In one study of 60 LT recipients, median overall survival after recurrence was 10.5 months (range 1-136 months), and only late recurrence and eligibility for surgical resection were positively correlated with survival [18].
Well-recognized predictors of recurrence include tumor size and number, bilobar disease, tumor differentiation, presence of macro- and microvascular invasion and tumor satellites, and tumor-specific biomarkers such as alpha-fetoprotein (AFP) levels before LT [10, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29]. Gross features of HCC, including the tumor size and number, which are part of the Milan criteria, are critical. A recent meta-analysis of 74 studies involving 22,432 patients revealed that assessment of the diameter of the largest nodule or the total diameter of nodules was the best predictor of outcome, and that a total tumor size (sum of diameters) greater than 10 cm was associated with a fourfold higher risk of recurrence [30]. Another meta-analysis of 1198 patients indicated that the presence of vascular invasion, poor differentiation, tumor diameter greater than 5 cm, and tumor status beyond the Milan criteria were independent risk factors for HCC recurrence [31]. Although tumor differentiation and the presence of microvascular invasion are recognized as important risk factors for HCC recurrence after LT, these features are usually not able to be determined until after explantation of the liver. Saborido and colleagues [32] reported a higher recurrence rate among patients who underwent tumor biopsy before LT. Currently, pre-transplant tumor biopsy is not required in cirrhotic patients considered for LT who have high-quality dynamic computed tomography (CT) or magnetic resonance image (MRI) findings typical for HCC [12]. Biomarkers such as AFP and des-gamma carboxy prothrombin (DCP) are reported to correlate with post-LT recurrence of HCC [21, 28, 33, 34]. A recent study using the Organ Procurement and Transplant Network database recommends adding AFP level greater than 400 ng/ml to total tumor volume as a predictor of outcome after LT for HCC [28]. A French group also proposed that the prediction of HCC recurrence after LT is significantly improved by applying modified Milan criteria that incorporate the AFP level [21]. Recently, micro-RNA clusters were extensively investigated as a biomarker representing the biologic behavior of HCC [35, 36] in association with recurrence.
Another important issue regarding risk factors for HCC recurrence following LDLT is that LDLT itself could be a risk factor for recurrence compared with deceased donor liver transplantation (DDLT). A large multicenter cohort study from Japan [37] and Korea [38] demonstrated that application of the Milan and University of California, San Francisco, (UCSF) criteria to LDLT yielded an equivalent long-term outcome to that for DDLT, but some authors [39, 40] recently reported a higher incidence of HCC recurrence in LDLT recipients compared with DDLT recipients.
Eligibility of Extended Criteria for DDLT
Reports of proposed extended Milan criteria, which are in some cases already commonly used, are summarized in table 1 [19, 27, 28, 41, 42, 43, 44]. Among these, the UCSF criteria, initially reported by Yao et al. in 2001 [27], are well-recognized extended Milan criteria that have been applied to clinical practice; the UCSF criteria are patients with a solitary tumor ≤ 65 mm in diameter, or two or three tumors, each with a diameter ≤ 45 mm and a total tumor diameter ≤ 80 mm. According to Yao et al., patients meeting the UCSF criteria had an overall survival rate of 90% and 73% at 1 and 5 years after LT, respectively [27]. Although the initial UCSF criteria were reported based on pathologic examination of the explant, the same authors validated their eligibility based on pre-LT imaging studies. Subsequent studies validated the UCSF criteria in a larger cohort.
Table 1.
Reports comparing outcomes between extended criteria and Milan criteria in the DDLT setting
| Reference (year) | Eligibility criteria | Tumor characteristics and the number of cases included | Overall survival (%) |
||
|---|---|---|---|---|---|
| 1 year | 3 years | 5 years | |||
| Yao et al. (2001) [26] | Solitary tumor with diameter ≤6.5 cm | Within extended criteria n=60 | 90 | 75 | |
| UCSF criteria | Tumors up to three nodules with maximum diameter ≤4.5 cm, and total tumor diameter ≤8 cm | Within Milan n=51 | 91 | 72 | |
| Mazzaferro (2009) [43] | Sum of the number of nodules and diameter of the largest nodule (in cm) ≤7 | Within extended criteria, beyond Milan, and without microvascular invasion n=283 | 66 | 71 | |
| Up-to-seven criteria | Within extended criteria, beyond Milan, and with microvascular invasion n=116 | 60 | 47 | ||
| Within Milan and without microvascular invasion n=362 | 82 | 76 | |||
| Within Milan and with microvascular invasion n=44 | 77 | 72 | |||
| DuBay (2011) [18] | Unrestricted tumor size or number | Within extended criteria and beyond Milan n=105 | 70 | ||
| Toronto criteria | Not poorly differentiated histology on biopsy (tumors beyond Milan only) | Within Milan n=189 | 72 | ||
| Herrero et al. (2008) [40] | Solitary tumor with diameter ≤6 cm | Within extended criteria and beyond Milan n=26 | 88 | 72 | 68 |
| Tumors up to 3 nodules with maximum diameter ≤5 cm | Within Milan n=59 | 88 | 73 | 66 | |
| Toso et al. (2008) [27] | Total tumor volume ≤115 cm3 | Within extended criteria n=251 | 80 | ||
| Within Milan n=157 | 82 | ||||
| Silva et al. (2008) [41] | Tumors up to 3 nodules with maximum diameter ≤5 cm, and total tumor diameter ≤10 cm | Within extended criteria and beyond Milan n=26 | 92 | 79 | 69 |
| Within Milan n=231 | 82 | 68 | 69 | ||
| Zheng et al. (2008) [42] | Total tumor diameter ≤8 cm | Within extended criteria n=99 | 93 | 71 | 71 |
| Total tumor diameter ≤8 cm, with grade I or II tumor on biopsy and AFP ≤400 ng/ml | Within Milan n=72 | 94 | 78 | 78 | |
Recently, Mazzaferro and colleagues [44] introduced the “Up-to-seven” criteria, based on a multicenter study of 1556 LT recipients: patients with the total number of tumor nodules added to the diameter of the largest nodule (in cm) not exceeding 7 had a 5-year survival rate of 70%, equivalent to that of the Milan criteria.
DuBay and associates [19] reported the Toronto criteria, which incorporate tumor biopsy: patients with HCC beyond the Milan criteria are still eligible for LT provided that those with a poorly differentiated tumor are excluded. Using this approach, the 5-year overall survival and disease-free survival rates were 72% and 70%, respectively, in those within the Milan criteria, and 70% and 66%, respectively, in those not limited by tumor number or size [19].
To date, although many expanded criteria have been proposed, as shown in table 1, only the Milan criteria have been widely validated and accepted as a gold standard worldwide. The main problem associated with expansion or modification of the Milan criteria is that tumor characteristics, such as microvascular invasion and tumor differentiation, cannot currently be evaluated reliably prior to LT [12]. Any expansion must be balanced with its effect, in terms of organ allocation, on the survival of candidates for LT who do not have HCC [16, 45].
LDLT for HCC in Japan
The Japanese Ministry of Health, Labour, and Welfare considers LT indicated for patients with HCC within the Milan criteria, stating that (1) the decision of whether the patient satisfies the Milan criteria should be based on dynamic CT or MRI taken within 1 month before LT, (2) a pre-LT diagnosis of HCC means that the tumor demonstrates the classical pattern, low-high-low density on dynamic contrast-enhanced CT, (3) and in patients who undergo locoregional treatment prior to LT, at least a 3-month interval between the last treatment and LT is mandatory (tumors judged as totally necrotic need not be counted). All Japanese institutions, however, allow patients whose tumor status is beyond the Milan criteria to undergo LT according to the institution's criteria, as described previously, as an uninsured treatment, provided that there is no contraindication such as macroscopic vascular invasion or extrahepatic metastases [46].
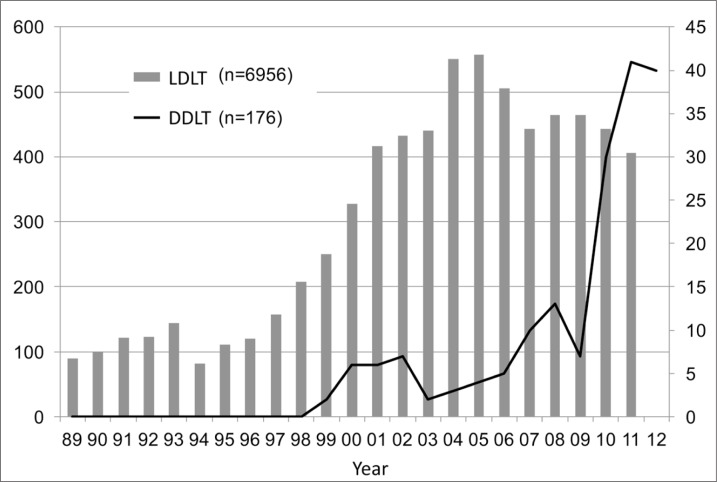
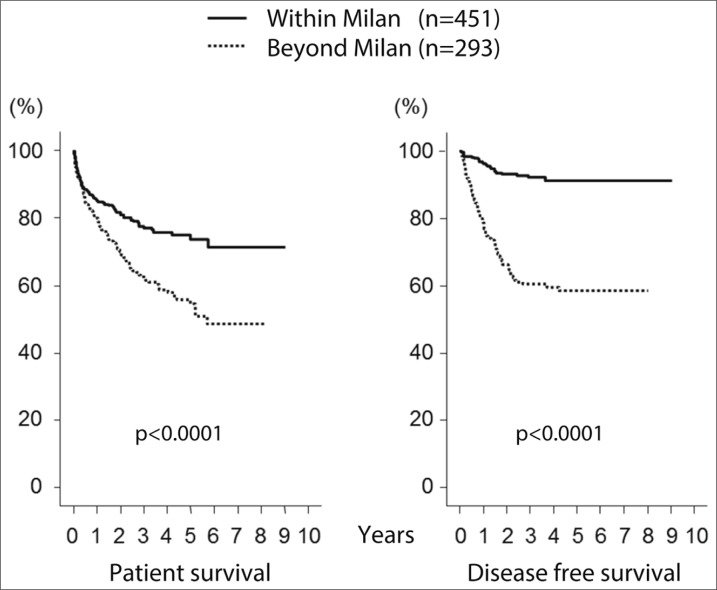
In Japan, even though the brain-death law was passed in 1997, there remains a crucial shortage of deceased donors. Only 176 DDLTs had been performed by the end of 2012, whereas 6956 LDLTs were performed within the same period (fig. 1). LDLT is widely accepted and applied for the treatment of HCC, and it is also incorporated within the Japanese guidelines [47] for HCC management, just as DDLT is in Western countries [4]. According to a report from the Japanese Liver Transplantation Society Registry [48], a total of 6097 LDLTs had been performed in Japan by the end of 2010. Of these, 3873 (64%) were performed in adult patients (over 18 years old), and 1225 (32%) were indicated for HCC, which was the most common indication in adult patients. The 1-, 3-, 5-, and 10-year cumulative survival rates of LDLT for HCC are 85%, 74%, 69%, and 60%, respectively. When stratified by the Milan criteria, there is a significant difference between those within the Milan criteria and those beyond the Milan criteria (fig. 2). These findings are comparable with those found in the DDLT databases of the United States [49] and Europe [50]. Todo and colleagues [51] performed a detailed survey using the same database (up to the end of 2005) of 653 patients who had undergone LDLT for HCC in Japan. HCV infection was the leading cause of HCC, accounting for 385 (59%) recipients. At 1, 3, and 5 years, overall patient survival was 83%, 73%, and 69%, and disease-free survival was 77%, 65%, and 61%, respectively. Based on preoperative imaging studies, 62% of these 653 patients were within the Milan criteria and 38% were beyond the Milan criteria, with 5-year recurrence-free survival rates of 90% and 61%, respectively (p<0.001). The tumor recurred in 92 (14%) LT recipients, with a cumulative recurrence rate at 1, 3, and 5 years of 9%, 20%, and 22%, respectively. In a multivariate analysis, preoperative AFP and DCP levels were determined to be independent risk factors for HCC recurrence.
Fig. 1.
Changes in the number of liver transplant cases in Japan.
Fig. 2.
Patient survival and recurrence-free survival stratified by the Milan criteria.
Despite insurance coverage for LT for HCC being limited to patients who fulfill the Milan criteria in Japan, each center has developed and proposed expanded selection criteria based on institutional and regional experience, as mentioned above. The proposed extended criteria from three major transplant centers in Japan are summarized in table 2 [52,53,54].
Table 2.
Reports of LDLT for HCC from high-volume Asian centers
| Reference (year) | Eligibility criteria | Tumor characteristics and the number of cases included | Overall survival (%) |
||
|---|---|---|---|---|---|
| 1 year | 3 years | 5 years | |||
|
Reports from Japan | |||||
| Present report | Tumors up to 5 nodules with maximum diameter ≤5 cm | Within extended criteria n=118 | 89 | 81 | 80 |
| Tokyo | Within Milan n=109 | 88 | 79 | 78 | |
| Kaido et al. (2013) [52] | Tumor with diameter ≤5 cm, up to 10 nodules, and DCP ≤400 mAU/ml | Within extended criteria n=147 | 82 | ||
| Kyoto | Within Milan n=118 | 76 | |||
| Shirabe et al. (2011) [53] | Tumor with diameter ≤5 cm or DCP ≤300 mAU/ml | Within extended criteria n=89 | 92 | 86 | 83 |
| Kyushu | Unrestricted tumor number | Within Milan n=36 | 100 | 83 | 83 |
|
Reports from other Asian countries | |||||
| Lee et al. (2008) [57] | Tumors up to 6 nodules with maximum diameter ≤5 cm | Within extended criteria n=186 | 76 | ||
| Korea | Within Milan n=164 | 76 | |||
| Concejero et al. (2008) [55] | Milan criteria | Within Milan n=35 | 98 | 96 | 90 |
| Taiwan | |||||
| Ng et al. (2010) [56] | UCSF criteria | Within UCSF and beyond Milan n=33 | 100 | 73 | 65 |
| Hong Kong | Within Milan n=50 | 96 | 83 | 72 | |
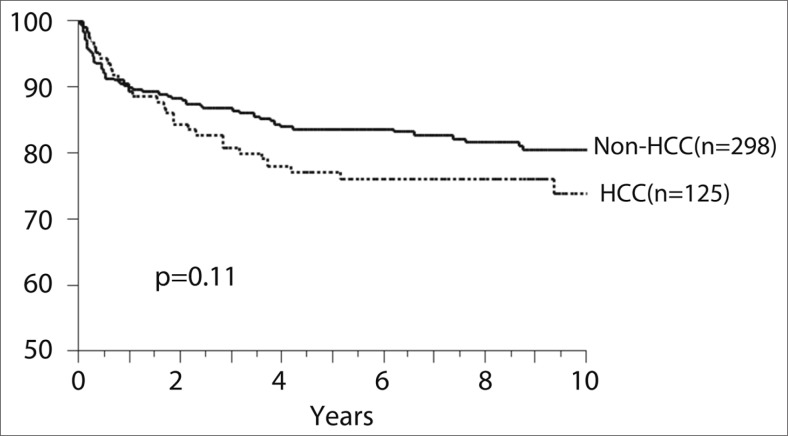
At our institution, the University of Tokyo Hospital, a total of 423 adult recipients had undergone LDLT by the end of 2012. Among them, 125 (30%) patients had HCC. The principle criterion for LDLT for HCC at our center is “up to five nodules with a maximum tumor diameter of 5 cm,” which we call the five-five rule [52]. Of the 125 HCC patients, 118 (94%) were within the five-five rule criteria and 109 (87%) were within the Milan criteria. Overall survival of the 125 recipients at 1, 3, and 5 years was 88%, 82%, and 76%, respectively, with a median follow-up period of 8 years. There was no difference in the overall survival rate between patients with HCC and those without HCC at our institution (fig. 3). A total of 11 (9%) patients developed HCC recurrence with a cumulative recurrence rate at 1, 3, and 5 years of 6%, 9%, and 11%, respectively. In the multivariate analysis for HCC recurrence, tumors not within the five-five rule, AFP level >400 ng/ml, and DCP level >200 mAU/ml were independent risk factors within our cohort.
Fig. 3.
Patient survival after LDLT at the University of Tokyo Hospital.
The Kyoto group [53] proposed extending the selection criteria to “Within 10 nodules, all tumor diameters within 5 cm, and DPC levels under 400 mAU/ml.” A total of 198 HCC patients underwent LDLT, and among those, 147 (82%) were within the Kyoto criteria, whereas 118 (76%) met the Milan criteria. The 5-year survival rate of those within the Kyoto criteria was 82%, while that of those beyond the Kyoto criteria was 42% (p<0.001). In contrast, the 5-year survival rate for those within the Milan criteria was 76%, which was not statistically different from the 65% figure for those beyond the Milan criteria (p=0.3).
The Kyushu group [54] proposed extending the criteria to “A maximum diameter of the tumor of less than 5 cm without limiting the number of nodules, and DCP levels under 300 mAU/ml.” A total of 109 HCC patients underwent LDLT, and among those, 103 patients (94%) were within the Kyushu criteria, whereas 55 (50%) met the Milan criteria. The 5-year recurrence-free survival of patients who met the Kyushu criteria was 71%, whereas all six patients beyond the Kyushu criteria developed recurrent HCC within 2 years. The 5-year recurrence-free survival of those within the Kyushu, but beyond the Milan criteria (n=48), was 80%, while that of those beyond both the Kyushu and Milan criteria (n=6) was 0%.
LDLT for HCC in Asian Counties
In other Asian countries, as in Japan, the majority of LT for HCC patients are LDLT, and these account for 96% of LT for HCC [14]. Apart from the predominance of hepatitis B-related HCC [7, 55], the situation in other Asian countries is similar to that in Japan. The Taiwan group adopted the Milan criteria as an indication for LDLT [56], while the Hong Kong group adopted the UCSF criteria [57]. The Asan group of Korea, just like Japanese institutions, advocates its own criteria, specifically, “the tumor number should be up to six nodules and the maximum diameter of the tumor is limited to within 5 cm” [58]. The reports from these centers are summarized in table 2.
Notably, all Asian expanded criteria restrict the maximum diameter of the tumor to 5 cm for the indication for LDLT, whereas there is a large discrepancy regarding the maximum number of tumors. Two large historical retrospective studies [59, 60] revealed that tumors greater than 5 cm in diameter result in a high recurrence rate after LT, mainly due to the association between tumor size and vascular invasion/poor differentiation. Recently, the association between vascular invasion and the size of the nodule was confirmed: a study found that microscopic vascular invasion was present in 20% of tumors smaller than 2 cm in diameter, in 30-60% of tumors of 2-5 cm, and in up to 60-90% of nodules greater than 5 cm in diameter [61]. These findings support the basis for keeping the maximum tumor size at 5 cm while expanding the limit for the maximum number of tumors in Asian countries.
Conclusions
The high prevalence of viral infection and subsequent high incidence of HCC combined with the low organ donation rate from deceased donors in Japan and in other Asian countries have led to the need for unique indications and strategies for application of LT in the region. While the promotion of DDLT should be intensified in Japan and other Asian countries, LDLT will continue to be a mainstay treatment of HCC in cirrhotic patients. Expansion of the criteria for the indication of LT for HCC patients is a matter of debate regarding LDLT in Asian countries and DDLT in Western countries.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut 2014; doi:10.1136/gutjnl-2013-306627. [DOI] [PMC free article] [PubMed]
- 4.Bruix J, Sherman M. American Association for the Study of Liver Diseases: Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang S, Ha TY, Ahn CS, Moon DB, Kim KH, Song GW, Jung DH, Park GC, Namgoong JM, Jung SW, Yoon SY, Sung KB, Ko GY, Cho B, Kim KW, Lee SG. Reconstruction of inferior right hepatic veins in living donor liver transplantation using right liver grafts. Liver Transpl. 2012;18:238–247. doi: 10.1002/lt.22465. [DOI] [PubMed] [Google Scholar]
- 6.Belghiti J, Fuks D. Liver resection and transplantation in hepatocellular carcinoma. Liver Cancer. 2012;1:71–82. doi: 10.1159/000342403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan SC. Liver transplantation for hepatocellular carcinoma. Liver Cancer. 2013;2:338–344. doi: 10.1159/000343849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwatsuki S, Gordon RD, Shaw BW, Jr, Starzl TE. Role of liver transplantation in cancer therapy. Ann Surg. 1985;202:401–407. doi: 10.1097/00000658-198510000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ringe B, Pichlmayr R, Wittekind C, Tusch G. Surgical treatment of hepatocellular carcinoma: experience with liver resection and transplantation in 198 patients. World J Surg. 1991;15:270–285. doi: 10.1007/BF01659064. [DOI] [PubMed] [Google Scholar]
- 10.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 11.Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17:S44–S57. doi: 10.1002/lt.22365. [DOI] [PubMed] [Google Scholar]
- 12.Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A, OLT for HCC Consensus Group Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13:e11–e22. doi: 10.1016/S1470-2045(11)70175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koschny R, Schmidt J, Ganten TM. Beyond Milan criteria—chances and risks of expanding transplantation criteria for HCC patients with liver cirrhosis. Clin Transplant. 2009;23:49–60. doi: 10.1111/j.1399-0012.2009.01110.x. [DOI] [PubMed] [Google Scholar]
- 14.de Villa V, Lo CM. Liver transplantation for hepatocellular carcinoma in Asia. Oncologist. 2007;12:1321–1331. doi: 10.1634/theoncologist.12-11-1321. [DOI] [PubMed] [Google Scholar]
- 15.Hwang S, Lee SG, Belghiti J. Liver transplantation for HCC: its role: Eastern and Western perspectives. J Hepatobiliary pancreat Sci. 2010;17:443–448. doi: 10.1007/s00534-009-0241-0. [DOI] [PubMed] [Google Scholar]
- 16.Cheah YL, Chow PKH. Liver transplantation for hepatocellular carcinoma: an appraisal of current controversies. Liver Cancer. 2012;1:183–189. doi: 10.1159/000343832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welker MW, Bechstein WO, Zeuzem S, Trojan J. Recurrent hepatocellular carcinoma after liver transplantation - an emerging clinical challenge. Transpl Int. 2013;26:109–118. doi: 10.1111/j.1432-2277.2012.01562.x. [DOI] [PubMed] [Google Scholar]
- 18.Kornberg A, Küpper B, Tannapfel A, Katenkamp K, Thrum K, Habrecht O, Wilberg J. Long-term survival after recurrent hepatocellular carcinoma in liver transplant patients: clinical patterns and outcome variables. Eur J Surg Oncol. 2010;36:275–280. doi: 10.1016/j.ejso.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 19.DuBay D, Sandroussi C, Sandhu L, Cleary S, Guba M, Cattral MS, McGilvray I, Ghanekar A, Selzner M, Greig PD, Grant DR. Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann Surg. 2011;253:166–172. doi: 10.1097/sla.0b013e31820508f1. [DOI] [PubMed] [Google Scholar]
- 20.Vivarelli M, Cucchetti A, La Barba G, Ravaioli M, Del Gaudio M, Lauro A, Grazi GL, Pinna AD. Liver transplantation for hepatocellular carcinoma under calcineurin inhibitors: reassessment of risk factors for tumor recurrence. Ann Surg. 2008;248:857–862. doi: 10.1097/SLA.0b013e3181896278. [DOI] [PubMed] [Google Scholar]
- 21.Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, Francoz C, Compagnon P, Van-lemmens C, Dumortier J, Dharancy S, Gugenheim J, Bernard PH, Adam R, Radenne S, Muscari F, Conti F, Hardwigsen J, Pageaux GP, Chazouillères O, Salame E, Hilleret MN, Lebray P, Abergel A, Debette-Gratien M, Kluger MD, Mallat A, Azoulay D, Cherqui D, Liver Transplantation French Study Group Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143:986–994. doi: 10.1053/j.gastro.2012.05.052. e3, quiz e14-e15. [DOI] [PubMed] [Google Scholar]
- 22.Jonas S, Bechstein WO, Steinmüller T, Herrmann M, Radke C, Berg T, Settmacher U, Neuhaus P. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080–1086. doi: 10.1053/jhep.2001.23561. [DOI] [PubMed] [Google Scholar]
- 23.Esnaola NF, Lauwers GY, Mirza NQ, Nagorney DM, Doherty D, Ikai I, Yamaoka Y, Regimbeau JM, Belghiti J, Curley SA, Ellis LM, Vauthey JN. Predictors of microvascular invasion in patients with hepatocellular carcinoma who are candidates for orthotopic liver transplantation. J Gastrointest Surg. 2002;6:224–232. doi: 10.1016/s1091-255x(01)00015-4. [DOI] [PubMed] [Google Scholar]
- 24.Cillo U, Vitale A, Bassanello M, Boccagni P, Brolese A, Zanus G, Burra P, Fagiuoli S, Farinati F, Rugge M, D'Amico DF. Liver transplantation for the treatment of moderately or well-differentiated hepatocellular carcinoma. Ann Surg. 2004;239:150–159. doi: 10.1097/01.sla.0000109146.72827.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onaca N, Davis GL, Jennings LW, Goldstein RM, Klintmalm GB. Improved results of transplantation for hepatocellular carcinoma: a report from the International Registry of Hepatic Tumors in Liver Transplantation. Liver Transpl. 2009;15:574–580. doi: 10.1002/lt.21738. [DOI] [PubMed] [Google Scholar]
- 26.Chan EY, Larson AM, Fix OK, Yeh MM, Levy AE, Bakthavatsalam R, et al. Identifying risk for recurrent hepatocellular carcinoma after liver transplantation: implications for surveillance studies and new adjuvant therapies. Liver Transpl. 2008;14:956–965. doi: 10.1002/lt.21449. [DOI] [PubMed] [Google Scholar]
- 27.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 28.Toso C, Asthana S, Bigam DL, Shapiro AM, Kneteman NM. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientific Registry of Transplant Recipients database. Hepatology. 2009;49:832–838. doi: 10.1002/hep.22693. [DOI] [PubMed] [Google Scholar]
- 29.Toso C, Trotter J, Wei A, Bigam DL, Shah S, Lancaster J, Grant DR, Greig PD, Shapiro AM, Kneteman NM. Total tumor volume predicts risk of recurrence following liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2008;14:1107–1115. doi: 10.1002/lt.21484. [DOI] [PubMed] [Google Scholar]
- 30.Germani G, Gurusamy K, Garcovich M, Toso C, Fede G, Hemming A, Suh KS, Weber A, Burroughs AK. Which matters most: number of tumors, size of the largest tumor, or total tumor volume? Liver Transpl. 2011;17:S58–S66. doi: 10.1002/lt.22336. [DOI] [PubMed] [Google Scholar]
- 31.Sotiropoulos GC, Molmenti EP, Lösch C, Beckebaum S, Broelsch CE, Lang H. Meta-analysis of tumor recurrence after liver transplantation for hepatocellular carcinoma based on 1,198 cases. Eur J Med Res. 2007;12:527–534. [PubMed] [Google Scholar]
- 32.Saborido BP, Díaz JC, de Los Galanes SJ, Segurola CL, de Usera MA, Garrido MD, Elola-Olaso AM, Sánz RG, Romero CJ, Garcia García I, González EM. Does preoperative fine needle aspiration-biopsy produce tumor recurrence in patients following liver transplantation for hepatocellularcarcinoma? Transplant Proc. 2005;37:3874–3877. doi: 10.1016/j.transproceed.2005.09.169. [DOI] [PubMed] [Google Scholar]
- 33.Shirabe K, Itoh S, Yoshizumi T, Soejima Y, Taketomi A, Aishima S, Maehara Y. The predictors of microvascular invasion in candidates for liver transplantation with hepatocellular carcinoma-with special reference to the serum levels of des-gamma-carboxy prothrombin. J Surg Oncol. 2007;95:235–240. doi: 10.1002/jso.20655. [DOI] [PubMed] [Google Scholar]
- 34.Fujiki M, Takada Y, Ogura Y, Oike F, Kaido T, Teramukai S, Uemoto S. Significance of des-gamma-carboxy prothrombin in selection criteria for living donor liver transplantation for hepatocellular carcinoma. Am J Transplant. 2009;9:2362–2371. doi: 10.1111/j.1600-6143.2009.02783.x. [DOI] [PubMed] [Google Scholar]
- 35.Barry CT, D'Souza M, McCall M, Safadjou S, Ryan C, Kashyap R, Marroquin C, Orloff M, Almudevar A, Godfrey TE. Micro RNA expression profiles as adjunctive data to assess the risk of hepatocellular carcinoma recurrence after liver transplantation. Am J Transplant. 2012;12:428–437. doi: 10.1111/j.1600-6143.2011.03788.x. [DOI] [PubMed] [Google Scholar]
- 36.Toffanin S, Hoshida Y, Lachenmayer A, Villanueva A, Cabellos L, Minguez B, Savic R, Ward SC, Thung S, Chiang DY, Alsinet C, Tovar V, Roayaie S, Schwartz M, Bruix J, Waxman S, Friedman SL, Golub T, Mazzaferro V, Llovet JM. MicroRNA-based classification of hepatocellular carcinoma and oncogenic role of miR-517a. Gastroenterology. 2011;140:1618–1628. doi: 10.1053/j.gastro.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Todo S, Furukawa H. Japanese Study Group on Organ Transplantation: Living donor liver transplantation for adult patients with hepatocellular carcinoma: experience in Japan. Ann Surg. 2004;240:451–459. doi: 10.1097/01.sla.0000137129.98894.42. discussion 459-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwang S, Lee SG, Joh JW, Suh KS, Kim DG. Liver transplantation for adult patients with hepatocellular carcinoma in Korea: comparison between cadaveric donor and living donor liver transplantations. Liver Transpl. 2005;11:1265–1272. doi: 10.1002/lt.20549. [DOI] [PubMed] [Google Scholar]
- 39.Park MS, Lee KW, Suh SW, You T, Choi Y, Kim H, Hong G, Yi NJ, Kwon CH, Joh JW, Lee SK, Suh KS. Living-donor liver transplantation associated with higher incidence of hepatocellular carcinoma recurrence than deceased-donor liver transplantation. Transplantation. 2014;97:71–77. doi: 10.1097/TP.0b013e3182a68953. [DOI] [PubMed] [Google Scholar]
- 40.Grant RC, Sandhu L, Dixon PR, Greig PD, Grant DR, McGilvray ID. Living vs. deceased donor liver transplantation for hepatocellular carcinoma: a systematic review and meta-analysis. Clin Transplant. 2013;27:140–147. doi: 10.1111/ctr.12031. [DOI] [PubMed] [Google Scholar]
- 41.Herrero JI, Sangro B, Pardo F, Quiroga J, Iñarrairaegui M, Rotellar F, Montiel C, Alegre F, Prieto J. Liver transplantation in patients with hepatocellular carcinoma across Milan criteria. Liver Transpl. 2008;14:272–278. doi: 10.1002/lt.21368. [DOI] [PubMed] [Google Scholar]
- 42.Silva M, Moya A, Berenguer M, Sanjuan F, López-Andujar R, Pareja E, Torres-Quevedo R, Aguilera V, Montalva E, De Juan M, Mattos A, Prieto M, Mir J. Expanded criteria for liver transplantation in patients with cirrhosis and hepatocellular carcinoma. Liver Transpl. 2008;14:1449–1460. doi: 10.1002/lt.21576. [DOI] [PubMed] [Google Scholar]
- 43.Zheng SS, Xu X, Wu J, Chen J, Wang WL, Zhang M, Liang TB, Wu LM. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation. 2008;85:1726–1732. doi: 10.1097/TP.0b013e31816b67e4. [DOI] [PubMed] [Google Scholar]
- 44.Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, De Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Van Hoek B, Majno P, Metroticket Investigator Study Group Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 45.Volk ML, Vijan S, Marrero JA. A novel model measuring the harm of transplanting hepatocellular carcinoma exceeding Milan criteria. Am J Transplant. 2008;8:839–846. doi: 10.1111/j.1600-6143.2007.02138.x. [DOI] [PubMed] [Google Scholar]
- 46.Tamura S, Sugawara Y, Kokudo N. Living donor liver transplantation for hepatocellular carcinoma: the Japanese experience. Oncology. 2011;81:111–115. doi: 10.1159/000333270. [DOI] [PubMed] [Google Scholar]
- 47.Kokudo N, Makuuchi M. Evidence-based clinical practice guidelines for hepatocellular carcinoma in Japan: the J-HCC guidelines. J Gastroenterol. 2009;44:119–121. doi: 10.1007/s00535-008-2244-z. [DOI] [PubMed] [Google Scholar]
- 48.Inomata Y, Umeshita K, Uemoto S. Liver transplantation in Japan: Registry by the Japanese Liver Transplantation Society. Ishoku. 2011;46:524–536. doi: 10.1111/hepr.12676. [DOI] [PubMed] [Google Scholar]
- 49.Singal AK, Guturu P, Hmoud B, Kuo YF, Salameh H, Wiesner RH. Evolving frequency and outcomes of liver transplantation based on etiology of liver disease. Transplantation. 2013;95:755–760. doi: 10.1097/TP.0b013e31827afb3a. [DOI] [PubMed] [Google Scholar]
- 50.Adam R, Karam V, Delvart V, O'Grady J, Mirza D, Klempnauer J, Castaing D, Neuhaus P, Jamieson N, Salizzoni M, Pollard S, Lerut J, Paul A, Garcia-Valdecasas JC, Rodríguez FS, Burroughs A. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR) J Hepatol. 2012;57:675–688. doi: 10.1016/j.jhep.2012.04.015. All contributing centers ( www.eltr.org) European Liver and Intestine Transplant Association (ELITA) [DOI] [PubMed] [Google Scholar]
- 51.Todo S, Furukawa H, Tada M, Japanese Liver Transplantation Study Group Extending indication: role of living donor liver transplantation for hepatocellular carcinoma. Liver Transpl. 2007;13(Suppl 2):S48–S54. doi: 10.1002/lt.21334. [DOI] [PubMed] [Google Scholar]
- 52.Sugawara Y, Tamura S, Makuuchi M. Living donor liver transplantation for hepatocellular carcinoma: Tokyo University series. Dig Dis. 2007;25:310–312. doi: 10.1159/000106910. [DOI] [PubMed] [Google Scholar]
- 53.Kaido T, Ogawa K, Mori A, Fujimoto Y, Ito T, Tomiyama K, Takada Y, Uemoto S. Usefulness of the Kyoto criteria as expanded selection criteria for liver transplantation for hepatocellular carcinoma. Surgery. 2013;154:1053–1060. doi: 10.1016/j.surg.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 54.Shirabe K, Taketomi A, Morita K, Soejima Y, Uchiyama H, Kayashima H, Ninomiya M, Toshima T, Maehara Y. Comparative evaluation of expanded criteria for patients with hepatocellular carcinoma beyond the Milan criteria undergoing living-related donor liver transplantation. Clin Transplant. 2011;25:E491–E498. doi: 10.1111/j.1399-0012.2011.01463.x. [DOI] [PubMed] [Google Scholar]
- 55.Lee SG, Moon DB, Shin H, Kim KH, Ahn CS, Ha TY, Song GW, Jung DH, Park GC. Living donor liver transplantation for hepatocellular carcinoma: current status in Korea. Transplant Proc. 2012;44:520–522. doi: 10.1016/j.transproceed.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 56.Concejero A, Chen CL, Wang CC, Wang SH, Lin CC, Liu YW, Yang CH, Yong CC, Lin TS, Jawan B, Huang TL, Cheng YF, Eng HL. Living donor liver transplantation for hepatocellular carcinoma: a single-center experience in Taiwan. Transplantation. 2008;85:398–406. doi: 10.1097/TP.0b013e3181622ff8. [DOI] [PubMed] [Google Scholar]
- 57.Ng KK, Lo CM, Chan SC, Chok KS, Cheung TT, Fan ST. Liver transplantation for hepatocellular carcinoma: the Hong Kong experience. J Hepatobiliary pancreat Sci. 2010;17:548–554. doi: 10.1007/s00534-009-0165-8. [DOI] [PubMed] [Google Scholar]
- 58.Jeng LB, Li PC, Yang MD, Lee CC, Chang CL, Wu RS. Artificial vascular graft for inferior vena cava reconstruction in living donor liver transplantation: a case report. Transplant Proc. 2008;40:2527–2528. doi: 10.1016/j.transproceed.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 59.Iwatsuki S, Starzl TE, Sheahan DG, Yokoyama I, Demetris AJ, Todo S, Tzakis AG, Van Thiel DH, Carr B, Selby R, et al. Hepatic resection versus transplantation for hepatocellular carcinoma. Ann Surg. 1991;214:221–228. doi: 10.1097/00000658-199109000-00005. discussion 228-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klintmalm GB. Liver transplantation for hepatocellular carcinoma: a registry report of the impact of tumor characteristics on outcome. Ann Surg. 1998;228:479–490. doi: 10.1097/00000658-199810000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ravaioli M, Grazi GL, Piscaglia F, Trevisani F, Cescon M, Ercolani G, Vivarelli M, Golfieri R, D'Errico Grigioni A, Panzini I, Morelli C, Bernardi M, Bolondi L, Pinna AD. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant. 2008;8:2547–2557. doi: 10.1111/j.1600-6143.2008.02409.x. [DOI] [PubMed] [Google Scholar]