Abstract
At excitatory synapses of hippocampal neurons, the multi-PDZ domain scaffolding protein, MUPP1, assembles the NR2B subunit of the NMDA receptor (NMDAR), Ca2+–calmodulin kinase (CamKII), and the α 1 isoform of the postsynaptic density GTPase activating protein, SynGAP (SynGAPα). In order to evaluate the role of this complex in excitatory synaptic neurotransmission we specifically disrupted MUPP1–SynGAPα interactions in CA1 neurons of acute hippocampal slices using intracellular perfusion with peptides derived from SynGAPα –MUPP1 binding domains. Disruption of the interaction between MUPP1 and SynGAPα with two complementary peptides derived from SynGAP and MUPP1 mutual binding sites resulted in enhancement of excitatory postsynaptic currents (EPSCs). This potentiation did not occlude pairing-induced long-term potentiation (LTP); indeed the amplitude of postsynaptic responses of activity-potentiated synapses was further increased. Pre-potentiation of excitatory synapses with theta burst stimulations did not modify the MUPP1–SynGAPα-dependent enhancement of EPSCs. Our data suggest that MUPP1–SynGAPα complex dissociation triggers a mechanism for AMPAR enhancement that is distinct from activity-induced LTP.
Keywords: NMDA receptor, SynGAP, LTP, CaMKII, MUPP1
Introduction
NMDAR-dependent LTP of glutamatergic synapses is a model system for the understanding of memory and learning. Synaptic GTPase activating protein (SynGAP; Chen et al., 1998; Kim et al., 1998) is a potential-signal transduction intermediate between NMDAR activation and LTP. SynGAP is phosphorylated via NMDAR-dependent CaMKII, (Krapivinsky et al., 2004; Oh et al., 2004), the enzyme critical for NMDA-dependent LTP (Malinow et al., 1989; Silva et al., 1992). In the CNS, SynGAP splice variants (Li et al., 2001) encode 5 distinct SynGAP proteins. Four of these isoforms (α1, α2, β, and γ) have unique C-terminal tails; α1 contains a C-terminal PDZ-binding domain and indirectly binds to CaMKII via MUPP1 (Krapivinsky et al., 2004) while β-isoforms lacking the PDZ-binding motif directly bind the non-phosphorylated form of CaMKII (Li et al., 2001). In vivo disruption of the interaction between MUPP1 and SynGAPα by peptides derived from MUPP1–SynGAPα binding domains, resulted in SynGAP dephosphorylation, inactivation of p38 MAP kinase, and an increase of the frequency and amplitude of the AMPA-receptor-mediated mEPSCs (Krapivinsky et al., 2004). The magnitudes of the mEPSCs frequency and amplitude changes resembled those observed during long lasting potentiation of mEPSCs induced by synaptic NMDAR activation in dissociated hippocampal cultures (Lu et al., 2001), called “LTP of mEPSCs”. Finally, mice in which one allele of SynGAP had been deleted, exhibited decreased activity-dependent LTP (Kim et al., 2003; Komiyama et al., 2002). Taken together, the evidence suggests that SynGAPα is part of the NMDAR-mediated Ca2+ signal transduction cascade that controls excitatory neurotransmission.
Here we examine whether the MUPP1/SynGAPα complex is involved in NMDA-dependent long-term potentiation of EPSCs in CA1 pyramidal neurons. We find that disruption of the MUPP1/SynGAPα complex using two complementary peptides derived from SynGAP and MUPP1 mutual binding sites effectively potentiated EPSCs. This potentiation did not occlude pairing-induced LTP, but rather increased LTP amplitude. LTP of excitatory synapses induced by theta burst stimulation of Schaffer’s collaterals did not occlude EPSC potentiation caused by MUPP1/SynGAPα complex dissociation. These data suggest that the MUPP1 complex effectively regulates the AMPAR EPSCs, but is not itself the signal mediating NMDAR potentiation of AMPARs during LTP.
Results
MUPP1–SynGAPα complex disruption increases EPSC amplitudes
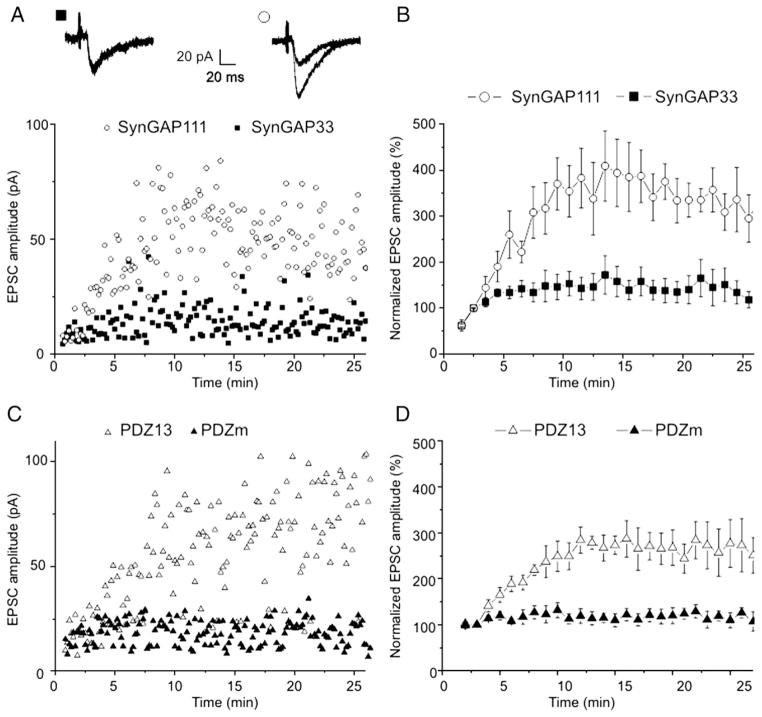
Previous experiments demonstrated that peptides dissociating the MUPP1/SynGAPα-complex potentiated mEPSCs in cultured hippocampal neurons (Krapivinsky et al., 2004). Exogenous molecules in the culture media, however, might influence the regulation of signaling pathways in primary neuronal cultures. Therefore, the initial experiments in this study were designed to examine whether peptide interference would modify the properties of AMPAR-mediated neuro-transmission in acute hippocampal slices. For this reason, we analyzed the properties of EPSCs in CA1 neurons evoked by stimulation of the CA2 pyramidal layer. Neurons were internally perfused (via the patch pipette) with SynGAP111, a peptide encoding the last 111 amino acids of SynGAP111α. This peptide specifically disrupts native MUPP1/SynGAPα complexes (Krapivinsky et al., 2004). SynGAP33, a truncated SynGAP that neither binds MUPP1 nor disrupts the SynGAP/MUPP1 complex (Krapivinsky et al., 2004) served as the negative control for peptide specificity. Consistent with potentiation of mEPSCs observed in dissociated neuronal cultures, intraneuronal SynGAP111 peptide perfusion increased EPSC amplitudes >2-fold in slice CA1 neurons over neurons dialyzed with SynGAP33 peptide (Figs. 1A, B). Differences in EPSC amplitudes became statistically significant 6 min after the start of whole-cell recording. Steady-state levels were achieved 8–10 min after initiation of whole-cell recording.
Fig. 1.
Peptides SynGAP111 and PDZ13 increase EPSC amplitudes evoked in CA1 neurons of rat acute hippocampal slices. A,C. Scatterplots of single experiments in which the indicated peptides were included in the patch pipette. The inset in A shows superimposed average traces of 5 consecutive EPSCs recorded 3 and 20 min after whole-cell formation with SynGAP33 (left traces) and SynGap111 (right traces). B,D. Normalized mean values from experiments performed with the indicated peptides in the patch pipette (5 experiments under each condition).
Additional experiments using PDZ13 peptide determined if specific MUPP1/SynGAPα complex disruption, rather than SynGAP111 interaction with other proteins, potentiated EPSCs amplitudes. PDZ13 is a peptide that encodes the 13th PDZ domain of MUPP1. The only common feature of SynGAP111 and PDZ13 is their ability to dissociate the MUPP1/SynGAPα complex. A homologous peptide that did not dissociate the MUPP1/SynGAPα complex acted as the control (mutated PDZ domain; PDZm, (Krapivinsky et al., 2004), see Experimental methods). Intraneuronal perfusion with PDZ13, not PDZm, potentiated EPSCs (Figs. 1C, D). Differences in EPSC amplitudes became statistically significant 5 min after the start of whole-cell recording. Steady-state levels were reached 8–10 min after initiation of whole-cell recording.
Although the two EPSC-potentiating peptides, SynGAP111 and PDZ13, could potentially interact with other molecules, it is unlikely that disruption of unrelated SynGAP interactions and unrelated MUPP1 interactions would coincidentally result in similar changes of EPSCs. We conclude that disruption of the interaction between MUPP1 and SynGAPα potentiated the amplitudes of EPSCs in CA1 neurons of acute hippocampal slices.
MUPP1–SynGAPα complex disruption does not abolish pairing-induced LTP
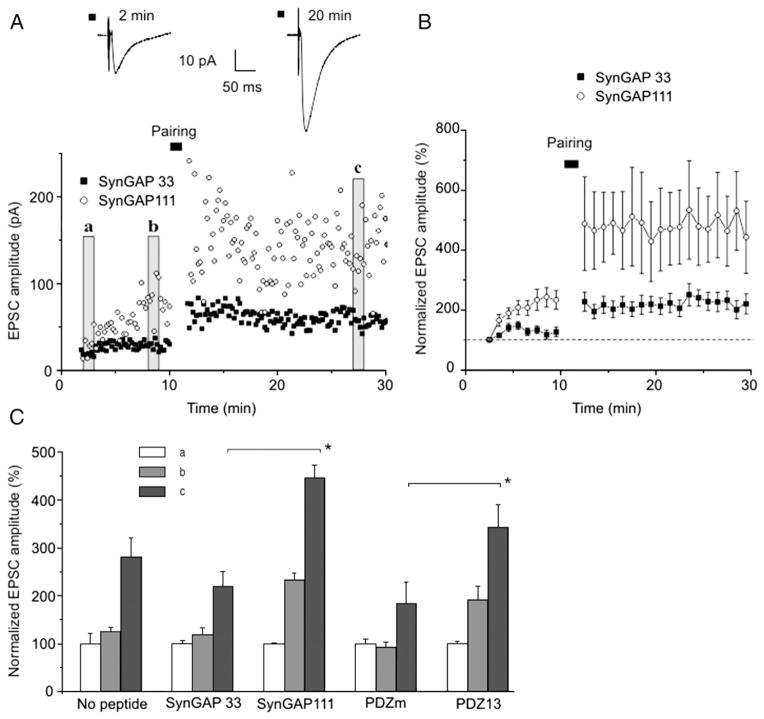
MUPP1–SynGAPα complex disruption potentiated synaptic transmission. If the mechanism for this potentiation is the same as synaptic activity-induced LTP, then SynGAP111 and PDZ13 peptides would occlude activity-induced LTP. To examine this possibility, we compared pairing-induced LTP of AMPARs in neurons perfused with control peptides and neurons perfused with AMPAR-potentiating SynGAP111 and PDZ13 peptides. After the increase in peptide-evoked current reached a plateau, application of the pairing protocol enhanced synaptic transmission by ~2-fold in both sets of experiments (Fig. 2). Thus, disruption of the MUPP1–SynGAPα complex did not occlude activity-induced LTP. In neurons in which the MUPP1–SynGAPα complex had been disrupted, the amplitude of pairing-evoked response was even higher than in neurons perfused with control peptides (Fig. 2C). These results suggest that the MUPP1–SynGAPα complex potentiates AMPAR currents via pathways that are distinct from those employed in activity-induced LTP.
Fig. 2.
Binding peptides do not occlude pairing-induced LTP. A. Scatterplot of EPSC amplitudes as a function of time in single experiments with SynGAP33 or SynGAP111 in the patch pipette as indicated. Vertical bars indicate sections analyzed in C. B. Normalized mean values of EPSCs from experiments performed with SynGAP33 (n=14) or SynGAP111 (n=7) in the patch pipette. C. Normalized EPSCs amplitudes measured with the indicated peptides in the patch pipette: no peptide (n=7), SynGAP 33 (n=14), SynGAP111 (n=7), PDZm (n=4), PDZ13 (n=11). Mean EPSCs values were obtained during the time intervals a, b, c as illustrated by vertical bars (with corresponding letters in A and normalized to values at the beginning of experiment; section a). Asterisks indicate significant differences (p<0.05).
Pre-potentiation of hippocampal neurons by theta bursts did not occlude MUPP1/SynGAPα-dependent AMPA current activation
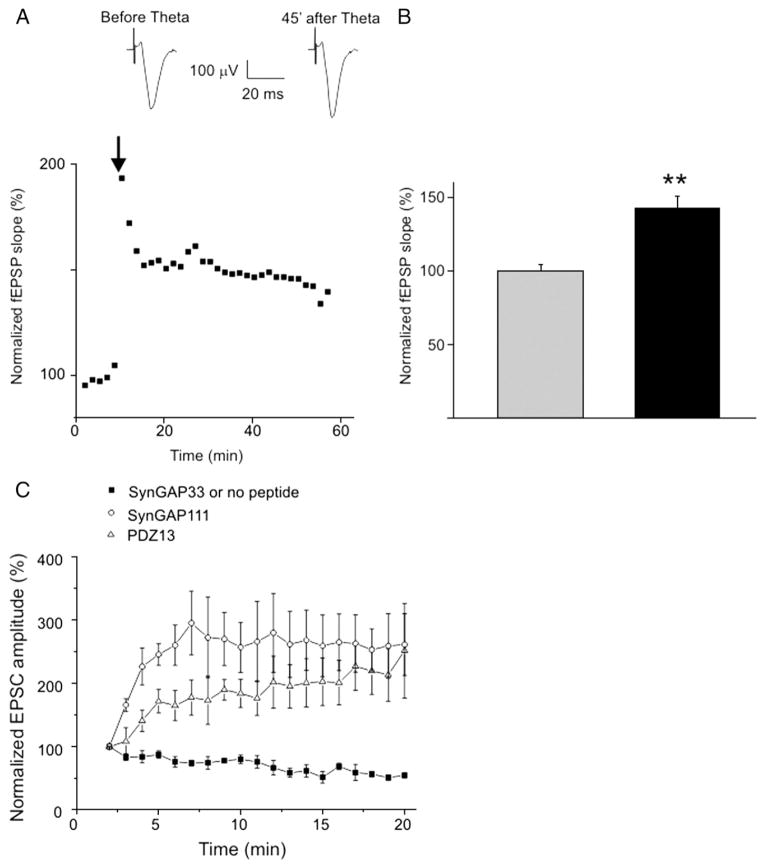
A complementary method to test the role of MUPP1/SynGAPα in LTP is to measure SynGAP111-mediated EPSC potentiation after induction of LTP. If the MUPP1/SynGAPα complex is required for LTP, then activity-evoked synaptic pre-potentiation should occlude the peptide-dependent increase of EPSC amplitudes. LTP was induced in CA1 neurons by theta burst stimulation of the CA2–CA1 neuronal layer. The increased slopes of the extracellular field potential responses in the CA1 layer verify LTP induction (Figs. 3A, B). Fifty minutes after LTP induction, EPSCs were recorded in whole-cell configuration from a CA1 neuron. EPSCs were evoked using the same stimulation electrode and stimulus (i.e. EPSCs were recorded from pre-potentiated synapses). EPSCs from neurons perfused with SynGAP111 or PDZ13 were significantly increased compared to those neurons perfused with SynGAP33 (Fig. 3C). Thus, pre-potentiation of the CA1 neurons with high-frequency theta bursts did not occlude the SynGAP111 or PDZ13 -dependent increase of EPSC amplitudes.
Fig. 3.
LTP does not occlude peptide-dependent potentiation of EPSC amplitudes. A. LTP of field EPSPs recorded from the CA1 region of hippocampal slices. The arrow indicates the onset of theta burst stimulation of Schaffer collaterals. B. Mean values of fEPSP slopes before (grey column) and 45 min after theta burst stimulation (black column, n=10, p<0.01). C. EPSCs recorded from CA1 neurons pre-potentiated with theta burst stimulation as shown in A. SynGAP33, n=8; SynGAP111, n=4; PDZ13, n=5.
Discussion
Several previous observations suggested that the MUPP1/SynGAPα complex might contribute to the regulation of AMPAR-mediated synaptic responses and NMDAR-dependent LTP. SynGA-Pα is phosphorylated in a CaMKII-dependent manner and activation of NMDAR results in SynGAPα dephosphorylation (Krapivinsky et al., 2004). Ca2+/CaM binding to CaMKII dissociated CaMKII from the MUPP1–SynGAPα complex and potentiated mEPSCs (Krapivinsky et al., 2004). The SynGAP-regulated small GTPase, Rap1 (Krapivinsky et al., 2004), participates in the control of synaptic AMPAR trafficking (Zhu et al., 2002). Finally, mice in which one allele of SynGAP had been deleted, exhibited decreased LTP (Kim et al., 2003; Komiyama et al., 2002). One possibility suggested by these experiments is that SynGAPα tethered by MUPP1 to NMDAR and CaMKII, is required to relay the NMDAR-mediated Ca2+ signal to the mechanism underlying LTP.
To examine MUPP1/SynGAPα in LTP, we dissociated SynGAP from MUPP1 in vivo, using peptides derived from the SynGAPα and MUPP1-binding domains (Krapivinsky et al., 2004). The use of binding peptides is an alternate approach to genetic modifications of the level of SynGAP and/or MUPP1. Whole proteins may participate in multiple interactions and their elimination or overexpression may affect multiple pathways. A peptide that only disrupts specific binding sites is more selective (and acute when perfused) than elimination or overexpression of the whole molecule. Although each peptide might interact with proteins other than SynGAPα and MUPP1, it is less likely that disruption of unrelated SynGAP interactions and unrelated MUPP1 interactions would coincidentally result in similar changes in AMPAR properties.
Dissociation of SynGAPα from MUPP1 using binding peptides resulted in SynGAPα dephosporylation, mimicking NMDAR’s action (Krapivinsky). Like LTP (Liao et al., 2001; Lu et al., 2001), SynGAP–MUPP1 complex disruption also increased the number of GluR1 and GluR2/3 neuronal clusters and induced potentiation of mEPSCs in primary neuronal cultures (Krapivinsky et al., 2004). We therefore hypothesized that SynGAPα might be involved in synaptic activity-induced LTP of excitatory neurotransmission.
Here we have shown that disruption of SynGAPα –MUPP1 interactions with a specific binding peptide rapidly augmented the amplitude of CA1 neuron EPSCs in acute slices and further increased AMPAR currents in CA1 neurons in which LTP had been induced with a pairing protocol. If SynGAP were a required intermediate in the signaling cascade between NMDAR and LTP of the AMPAR current, then activation of the SynGAP-dependent mechanism should not only have activated the AMPAR currents, but also occluded activity-stimulated LTP.
Modifications of molecules that are hypothetically involved in LTP (“mediators”; Sanes and Lichtman, 1999) decrease LTP magnitude: inhibition of such molecules (for example, CaMKII (Malinow et al., 1989), Ras (Zhu et al., 2002), ERK (Sweatt, 2001), and PI3K (Man et al., 2003) attenuated LTP, while expression of the constitutively active forms of CaMKII (Hayashi et al., 2000), Ras (Zhu et al., 2002), and PI3K (Man et al., 2003) potentiated EPSCs and occluded LTP.
In our experiments, prior theta burst-induced LTP (pre-potentiation of the synapses by electrical stimuli) did not occlude SynGAPα/MUPP1-mediated AMPAR potentiation. Our finding that SynGAP-dependent AMPAR activation did not occlude LTP suggests that the AMPAR activation mechanism initiated by MUPP1–SynGAPα complex dissociation is not a required intermediate in the signal transduction cascade leading from NMDAR activation to AMPAR LTP. Thus, activity-induced AMPAR activation and SynGAPα-dependent AM-PAR activation are independent processes. An important task of future studies will be to determine the physiological and experimental conditions under which NMDAR-mediated Ca2+ influx activates SynGAPα.
Experimental methods
All experiments with animals were performed under the guidelines of the Animal Care and Use Committee of INSERM (Institut National de la Santé et de la Recherche Médicale).
Preparation of hippocampal slices
Hippocampal slices were obtained from 12–16 d old Wistar rats. Prior to decapitation, the animals were anaesthetized using 7% chloral hydrate. The brain was removed quickly and submerged in ice-cold oxygenated (95% O2/5% CO2), choline-replaced artificial CSF (ACSF) cutting solution, containing (in mM):110 choline chloride, 2.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 7 MgCl2, 25 NaHCO3, 7 D-glucose, pH 7.4. Transverse slices (300 μm) from the middle portion of each hippocampus were obtained with a vibrating microtome (Leica VT 1000S, Germany) and were incubated at room temperature in ACSF that contained (in mM): 119 NaCl, 2.5 KCl, 4 MgCl2, 26 NaHCO3, 2 NaH2PO4, 4 CaCl2 and 10 glucose. Slices were allowed to recover for at least 90 min before recording. The slice was transferred to the recording chamber and submerged beneath continuously perfusing ACSF that had been saturated with 95% O2/5% CO2, containing bicuculline (10 μM) and glycine (10 μM). The CA3 region was cut to prevent epileptic discharges.
Electrophysiological recordings
Whole-cells recordings were performed at a holding potential (Vh) of −60 mV (HEKA EPC 9/2 amplifier with Pulse v8.74 software; Heka Electronik, Lambrecht/Pfalz, Germany) using an experimental paradigm described previously (Daoudal et al., 2002). The intracellular solution for whole-cell recordings contained (in mM): 110 Cs gluconate, 20 CsCl, 10 HEPES, 2 MgCl2, 10 EGTA, 10 Na-phosphocreatine, 4 ATP-Mg and 0.4 GTP-Na. The pH of the intracellular solutions was adjusted to 7.2 and the osmolarity to 280–290 mOsm l−1. Pipettes resistances were 5–8 MΩ when filled with this solution. The access resistance (15–30 MΩ) and input resistance (300–600 MΩ) were continuously monitored throughout the experiment. Occasional recordings with higher access resistance, lower input resistance, or those that displayed more than 20% changes in access and input resistances were discarded from analysis.
EPSCs were evoked at a frequency of 0.1 Hz using bipolar electrodes placed in the stratum radiatum (CA2 region, just after the incision blocking CA3 input). Stimulation intensity was adjusted during the first min of whole-cell recording to evoke 40–50 pA responses.
LTP induction in acute slices
LTP was induced using pairing protocols applied 10 min after formation of the whole-cell configuration (120 pulses at 2 Hz, −20 mV). Field LTP was induced by theta burst stimulation (TBS; 4 trains of 5 pulses delivered at 100 Hz with a 250 ms interburst interval). Field potentials were recorded using a DAM80 Amplifier (World Precision Instruments, USA) with a 1–3000 Hz bandpass filter. Field recordings were performed with glass micropipettes filled with ACSF (10–20 MΩ). Amplitudes of synaptic responses were normalized to the mean peak value of 6 consecutive EPSCs measured between the 2nd and 3rd minutes of whole-cell recordings. In summary plots, the reported EPSCs are 1 min averages.
Binding peptides
The peptides comprising the C-terminal 33 amino acids of SynGAPα, 6×His-HA-SynGAP33, the C-terminal 111 amino acids, 6×His-HASyn-GAP111 (denoted SynGAP33 and SynGAP111), GST fusions of 13th PDZ domain of the MUPP1 (amino acids 1973–2070) and mutated PDZ domain with impaired binding activity (denoted PDZ13 and PDZm) were characterized and prepared as described previously (Krapivinsky et al., 2004). Briefly, PCR fragments of appropriate regions of SynGAPα (accession # AF058790) MUPP1 (accession # NP_003820) were subcloned under a T7 promoter in frame with the 6×His-HA and GST tags, correspondingly. Constructs were expressed in BL21 bacteria (Novagen) and proteins were purified using immobilized Ni and glutathione, correspondingly. Purified proteins were equilibrated with buffer containing 10 mM K-HEPES and 150 mM KCl (pH 7.6) at final concentrations of 200–500 μM. For electrophysiology experiments, peptides were dissolved into the intracellular solution to a final concentration 5 μM. Aliquots were stored at −20 °C.
Statistical analysis
All population data were expressed as mean±SEM. The Student’s t test was employed to examine the statistical significance of the differences between groups of data. Significantly, different values (p<0.05) are indicated in figures as marked by asterisks.
Acknowledgments
We are grateful to Yehezkel Ben-Ari, Nik Otmakhov and Jean-Luc Gaiarsa for helpful discussion, critical reading and comments on the manuscript. This study was supported by grants from INSERM (to I.M.) and the French Foundation for Medical Research (to. S.R.).
Footnotes
MUPP1, SynGAP and LTP.
References
- Chen HJ, Rojas-Soto M, Oguni D, Kennedy MB. A synaptic Ras-GTPase activating protein (p135 SynGAP) inhibited by CaM kinase II. Neuron. 1998;20:895–904. doi: 10.1016/s0896-6273(00)80471-7. [DOI] [PubMed] [Google Scholar]
- Daoudal G, Hanada Y, Debanne D. Bidirectional plasticity of excitatory postsynaptic potential (EPSP)-spike coupling in CA1 hippocampal pyramidal neurons. Proc Natl Acad Sci U S A. 2002;99:14512–14517. doi: 10.1073/pnas.222546399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Kim JH, Liao D, Lau LF, Huganir RL. SynGAP: a synaptic RasGAP that associates with the PSD-95/SAP90 protein family. Neuron. 1998;20:683–691. doi: 10.1016/s0896-6273(00)81008-9. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee HK, Takamiya K, Huganir RL. The role of synaptic GTPase-activating protein in neuronal development and synaptic plasticity. J Neurosci. 2003;23:1119–1124. doi: 10.1523/JNEUROSCI.23-04-01119.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama NH, Watabe AM, Carlisle HJ, Porter K, Charlesworth P, Monti J, Strathdee DJ, O’Carroll CM, Martin SJ, Morris RG, O’Dell TJ, Grant SG. SynGAP regulates ERK/MAPK signaling, synaptic plasticity, and learning in the complex with postsynaptic density 95 and NMDA receptor. J Neurosci. 2002;22:9721–9732. doi: 10.1523/JNEUROSCI.22-22-09721.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapivinsky G, Medina I, Krapivinsky L, Gapon S, Clapham DE. SynGAP-MUPP1-CaMKII synaptic complexes regulate p38 MAP kinase activity and NMDA receptor-dependent synaptic AMPA receptor potentiation. Neuron. 2004;43:563–574. doi: 10.1016/j.neuron.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Li W, Okano A, Tian QB, Nakayama K, Furihata T, Nawa H, Suzuki T. Characterization of a novel synGAP isoform, synGAP-beta. J Biol Chem. 2001;276:21417–21424. doi: 10.1074/jbc.M010744200. [DOI] [PubMed] [Google Scholar]
- Liao D, Scannevin RH, Huganir R. Activation of silent synapses by rapid activity-dependent synaptic recruitment of AMPA receptors. J Neurosci. 2001;21:6008–6017. doi: 10.1523/JNEUROSCI.21-16-06008.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29:243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Malinow R, Schulman H, Tsien RW. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989;245:862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- Man HY, Wang Q, Lu WY, Ju W, Ahmadian G, Liu L, D’Souza S, Wong TP, Taghibiglou C, Lu J, Becker LE, Pei L, Liu F, Wymann MP, MacDonald JF, Wang YT. Activation of PI3-kinase is required for AMPA receptor insertion during LTP of mEPSCs in cultured hippocampal neurons. Neuron. 2003;38:611–624. doi: 10.1016/s0896-6273(03)00228-9. [DOI] [PubMed] [Google Scholar]
- Oh JS, Manzerra P, Kennedy MB. Regulation of the neuron-specific Ras GTPase-activating protein, synGAP, by Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 2004;279:17980–17988. doi: 10.1074/jbc.M314109200. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Can molecules explain long-term potentiation? Nat Neurosci. 1999;2:597–604. doi: 10.1038/10154. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Stevens CF, Tonegawa S, Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]