Abstract
Background: Pseudomonas aeruginosa may cause severe or even fatal infection in hosts with immunodeficiency. Interleukin-17 (IL-17) is a newly discovered pro-inflammatory cytokine, which promotes the recruitment and activation of neutrophils in the respiratory tract by inducing release of chemokine C-X-C. Objective: This study was conducted to explore the role of IL-17 in host defense against acute pseudomonas aeruginosa infection in lungs. Methods: The expression of IL-17 and its downstream effectors (IL-1β, MIP-2 and G-CSF) were detected in mouse lungs with acute pseudomonas aeruginosa infection; 48 h after intratracheal administration of justice plasmid, mice were infected with pseudomonas aeruginosa again, and the bacterial clearance rate and the expression of downstream effectors of IL-17, as well as the mice death rate, were determined 6 h later. Results: The expression of IL-17 and its downstream effectors (IL-1β, MIP-2 and G-CSF) significantly increased in mouse lungs with acute pseudomonas aeruginosa infection. After intratracheal administration of justice plasmid expressing IL-17, the expression of IL-17 and its downstream effectors significantly increased, accompanied by increase in neutrophil count. The justice plasmid expressing IL-17 was intratracheally administered before acute pseudomonas aeruginosa lung infection, which significantly increased the expression of IL-17 and its downstream effectors (IL-1β, MIP-2 and G-CSF) in the respiratory tract, leading to increasing clearance rate of bacteria and survival rate. Conclusion: IL-17 may recruit neutrophil to the infected areas in the early phase of pseudomonas aeruginosa lung infection.
Keywords: IL-17, pseudomonas aeruginosa, pneumonia infection, gene transfection, host defense
Introduction
Pseudomonas aeruginosa (PA), a conditioned pathogen, hardly causes disease in people with normal immunity, but severe and even fetal infection may be caused in hosts with immunodeficient conditions such as HIV, organ transplant and tumors [1-4]. The extensive application of antibiotics in recent years increases the tolerance of PA, making it one of the most important bacteria causing refractory infection [5,6]. Interleukin-17 (IL-17) is a newly discovered cytokine and named because it is generated by Th17 cells. IL-17 is a powerful pro-inflammatory cytokine that can promote the recruitment and activation of neutrophils in the respiratory tract by inducing the release of C-X-C chemokines [7-10]. A variety of studies have shown that IL-17 plays important roles in some inflammatory diseases of the airways like asthma and chronic obstructive pulmonary diseases [9,11-15]. This study was undertaken to investigate the role of IL-17 in acute PA lung infection, and explore whether the high IL-17 expression can increase the local expression of vital immune defense factors like interleukin-1β (IL-1β), macrophage inflammatory protein-2 (MIP-2) and granulocyte colony-stimulating factor G (G-CSF) in the lung, and whether IL-17 benefits the attenuation of acute PA lung infection and the survival of PA infected mice. Our findings may provide theoretical and practical evidence for the treatment of PA lung infection.
Materials and methods
Construction and transfection of justice plasmids expressing IL-17
Mouse IL-17 sequence was obtained from NCBI GeneBank, and restriction analysis and primer design were carried out. Total RNA was extracted from the lungs and then subjected to RT-PCR for amplification. The products were spliced to plasmid pcDNA3.1 (+) with gene clone; bacteria transformation and enzyme electrophoresis were conducted to identify and check order. All were designed and synthesized in the Invitrogen. 60 μg of justice plasmid expressing IL-17 was intratracheally administered to mice, and the lungs were collected 48 h later and processed for sectioning. The airway epithelial cells of these sections were observed under a fluorescence microscope.
Animals
Specific pathogen free C57BL/6 mice (male, 6-8 weeks, weighing 18-20 g) were purchased from the Experimental Animal Center of Wuhan University and housed independently in the Experimental Animal Center of Tongji Hospital of Huazhong University of Science and Technology. Animals were given ad libitum access to food and water. PA strain PAO1 was kindly provided by the Inspection Division of Microbial Room of Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology.
Modeling of lung infection
Intraperitoneal anesthesia with chloral hydrate was conducted in C57BL/6 mice, which were then fixed on a sterilized operation table. After a midline incision was made at the neck, the trachea was exposed, and 50 μL of PA suspension or control suspension was slowly injected into the trachea with a 1-ml syringe. The amount of bacteria instilled was 2.5 × 106 CFU per mouse. On day 1, 2 and 3, 8 mice were sacrificed, respectively, and 0.7 ml of blood was collected from the heart into an anticoagulant syringe followed by centrifugation at 1200 r/min for 10 min at 4°C. The supernatant was collected and stored at -80°C. The lungs were collected as a whole, and then the one of leaf lobe of the right lung was ligated, and lavaged with PBS solution twice (0.7 mL/time). The bronchoalveolar lavage fluid (BALF) after the first lavaging was centrifugated at 1200 rpm/min for 10 min at 4°C; and the supernatant was collected and stored at -80°C for the detection of cytokines. The cells of BALF were mixed with BALF after the second lavaging, and then the total number of cells and differential cells was determined. Left lung tissue was harvested and homogenized in 1 ml of cold sterile saline. The homogenate was 10-fold serially diluted, and then 10 μL of homogenate at different concentrations were inoculated to LB agar coated plate. Incubation was done for 24 h at 37°C and the colonies were counted. The right lung was embedded in paraffin, and the rest leaf lobes were stored at -80°C for RNA extraction and subsequent real-time fluorescence quantitative PCR [16,17].
To induce PA lung infection, 72 C57BL/6 mice were randomly assigned into normal group, PBS group and PA group. On days 1, 2d and 3 after PA infection, the IL-17 mRNA expression in the lung was detected with RT–PCR, IL-17 content of the BALF and serum was measured with ELISA, and the neutrophils of BALF were counted. To over-express IL-17 in the lung, 72 C57BL/6 mice were divided into normal group, pcDNA3.1 (+) blank plasmid group and IL-17 plasmid group. After intratracheal administration of PBS/plasmid, IL-17 mRNA expressions in the lung and IL-17 content of the BALF as well as content of IL-17 downstream effectors (IL-1β, MIP-2 and G-CSF) and the number of neutrophils in BALF were determined on days 1, 2 and 3. To investigate the effect of IL-17 on early infection, 64 mice were divided to IL-17 justice plasmid group and blank plasmid group in which mice were intratracheally treated with IL-17 at 48 h before PA treatment. At 6 h after PA treatment, the level of IL-17 and its downstream effectors (IL-1β, MIP-2 and G-CSF), and the amount of bacteria in the lung homogenate were determined. In addition, 36 mice were randomly divided into PA group, IL-17 justice plasmid group and blank plasmid group. Mice were intratracheally treated with IL-17 plasmid, pcDNA3.1 (+) plasmid in the later two groups, respectively. At 48 h after treatment, mice were infected with PA and then observed once every 24 h and the survival rate was determined [18].
ELISA detection
IL-17 ELISA kit was purchased from the eBioscience, while the ELISA kits for IL-1β, MIP-2 and G-CSF were from Raybio. In brief, specimens and standards at different concentrations were added into wells followed by incubation for 2.5 h at room temperature. After washing, the biotinylated antibody was added followed by incubation for 60 min. The plate was washed again and avidin - peroxidase complex (Streptavidin) was added followed by incubation for 45 min. The plate was washed, and disclosing solution was added followed by incubation in dark for 30 min. The reaction was stopped and the absorbance was measured at 450 nm with an automatic microplate reader Elx800. The standard curve was delineated and the content of samples was determined according to this standard curve.
Real time PCR
Primers were synthesized by the Sangon Engineering Company, and Real time PCR and reverse transcription kit were bought from TAKARA. Total RNA was extracted from the lung, followed by reverse transcription and Real Time-PCR according to manufacturer’s instructions. The Ct value was used to calculate relative expression with 2-ΔΔCt method.
Statistical analysis
Data were expressed as mean ± standard deviation. One way analysis of variance was employed for comparisons of means among groups, t-test between two groups, and survival rate was compared with time sequence log-rank. A value of P<0.05 was considered statistically significant.
Results
After PA infection, mice had a significantly higher IL-17 content in BALF and higher IL-17 mRNA expression in the lung than PBS treated mice in 3 consecutive days (P<0.05), while no IL-17 was detected in the uninfected mice. In the uninfected mice, PBS treated mice and PA infected mice, IL-17 was undetectable in the serum. Data are listed in Figures 1 and 2. Moreover, the infected mice had more neutrophils than PBS treated mice within 3 days (P<0.05), and the number of neutrophils peaked on the second day. The neutrophil count in normal group was 0.05 × 104/mL, as shown in Figure 3.
Figure 1.
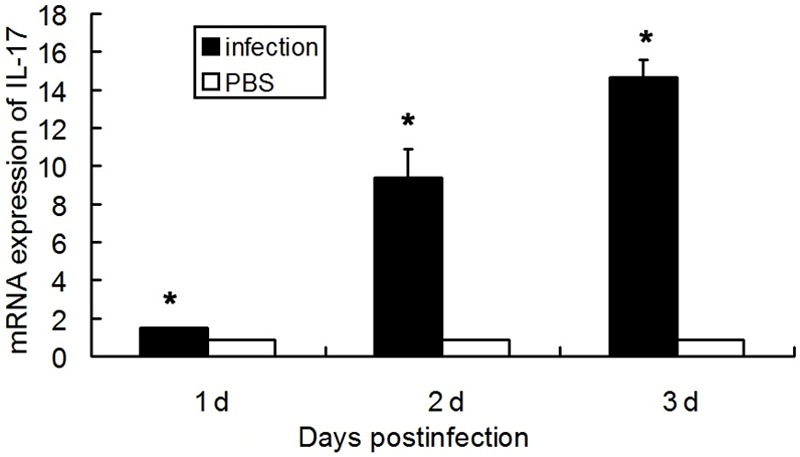
IL-17 mRNA expression in the lung homogenate after PA infection (n=8, *p<0.05).
Figure 2.
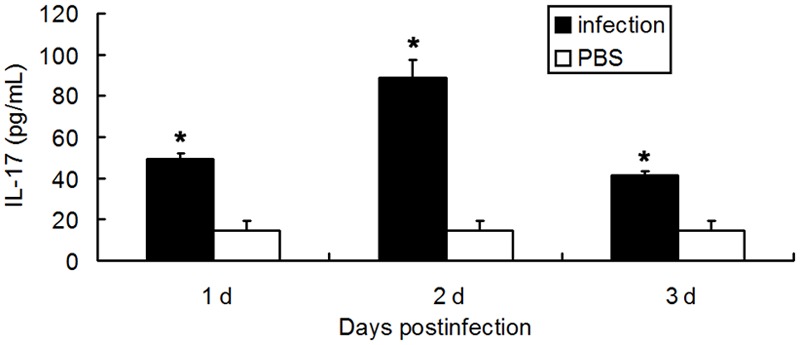
IL-17 content of the BALF after PA infection. (n=8, *p<0.05).
Figure 3.
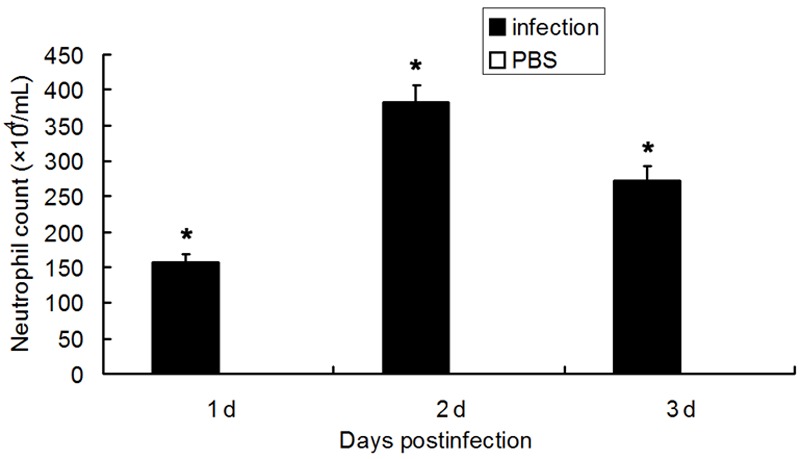
Neutrophil count of the BALF after PA infection (n=8, *p<0.05).
After intratracheal administration of justice plasmid expressing IL-17, the IL-17 mRNA expression in the lung on day 3 in IL-17 plasmid group was significantly higher than that in blank plasmid group (P<0.05). The BALF level of IL-17 and its downstream effectors (IL-1β, MIP-2 and G-CSF) was markedly higher than that in blank plasmid group (P<0.05). The IL-17 and its downstream effectors were not detected in normal group, nor was IL-17 expression in the serum. Data are listed in Figure 4. On day 3, the neutrophil count in IL-17 plasmid group was significantly higher than that in blank plasmid group (P<0.05), and peaked on the second day, as shown in Figure 5.
Figure 4.
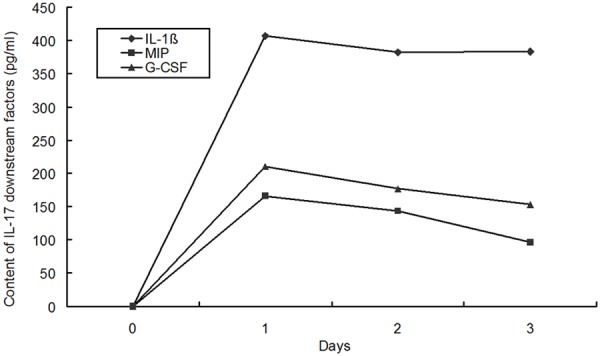
Contents of IL-1β, MIP-2 and G-CSF in the BALF after intratracheal administration of pcDNA3.1-IL-17 (n=8).
Figure 5.
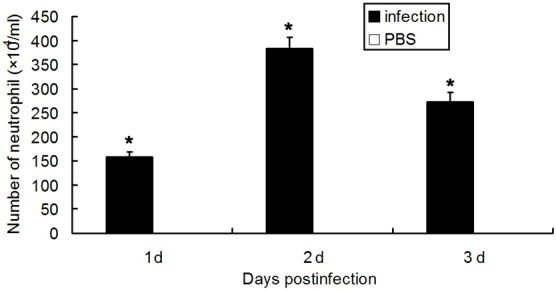
Neutrophil count of the BALF after intratracheal administration of pcDNA3.1-IL-17 (n=8, *P<0.05).
6 h after IL-17 plasmid’s intervening PA lung infection, the contents of IL-17 downstream effectors (IL-1β, MIP-2 and G-CSF) in IL-17 plasmid treated mice were significantly higher than those in PA infected mice which were not treated and treated with blank plasmid (P< 0.05), as shown in Figure 6. In IL-17 plasmid group, the bacteria count (4.7 log10 CFU) was significantly lower than that in infection group (6.22 log10 CFU) and blank plasmid group (6.26 log10 CFU) (P<0.05), as shown in Figure 7. In IL-17 plasmid group, the 3-day survival rate was 67%, while that was 41% in infection group and blank plasmid group, significant difference was noted between IL-17 plasmid group and infection group/blank plasmid group (P<0.05), as shown in Figure 8.
Figure 6.
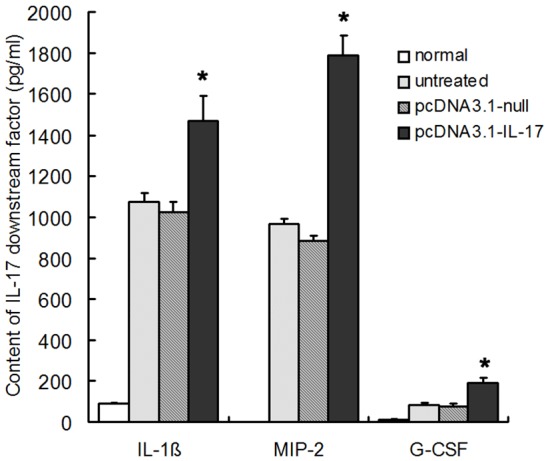
PcDNA3.1-IL-17 pretreatment increased the contents of IL-1β, MIP-2 and G-CSF after PA challenge (n=8, *P<0.05).
Figure 7.
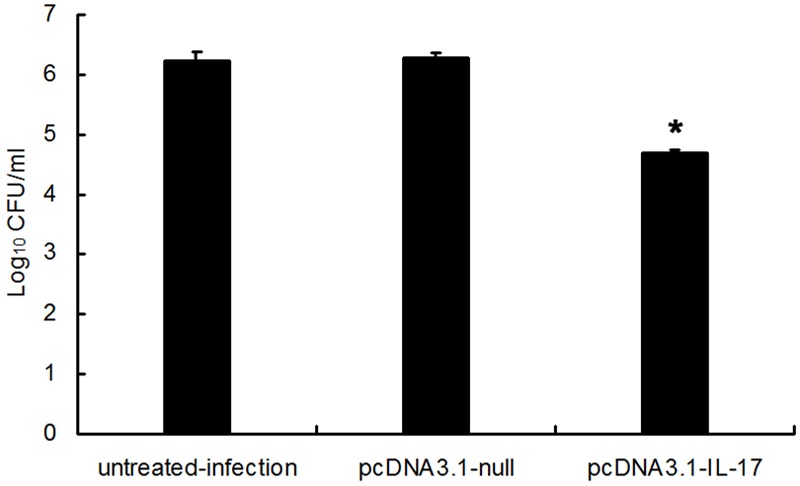
PcDNA3.1-IL-17 pretreatment augmented the clearance of PA in the lung (n=8, *P<0.05).
Figure 8.
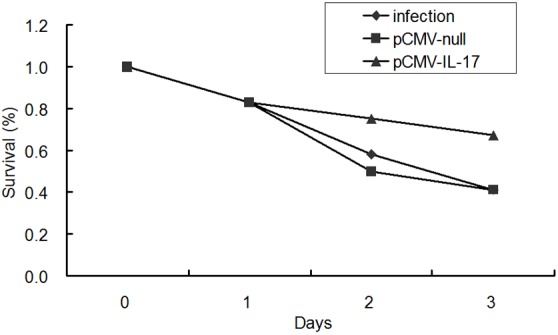
PcDNA3.1-IL-17 pretreatment increased early survival rate after PA challenge (n=12).
Discussion
In both developing and developed countries, bacterial pneumonia is the major cause of death and has a high mortality, especially in host with immunodeficiency such as HIV, organ transplant and cancers. PA is a conditioned pathogen and may cause severe or even fatal infection. Although great progress has been achieved in the antibacterial treatment, the wide application of broad-spectrum antibiotics increases the drug tolerance of bacteria [19,20]. Therefore, a better treatment is required to increase the immunity against bacterial infection.
IL-17 is a newly discovered cytokine, being a homodimeric glycoprotein consisting of 155 amino acids with 35 kD in molecular weight. Besides IL-17A, IL-17 includes IL-17B, IL-17C, IL-17D and IL-17F [21-24]. It is produced by not only Th17 cells but also other cells like thymocytes, epithelial cell and vascular endothelial cells. IL-17 functions via binding to IL-17 receptor (IL-17R) on cells in vivo. IL-17 receptor is distributed widely and expressed in almost every kind of tissues and cells [25]. IL-17 is a powerful pro-inflammatory cytokine and promotes the recruitment and activation of neutrophils in the respiratory tract via inducing the release of C-X-C chemokines, by which it increases the clearance of pathogens, such as mycoplasma and gram-negative bacteria [8-10,26-30]. These results also indicate the important role of IL-17 in the host defense [10,31-34]. Miyamoto et al [30] found that, 6 h after endotoxin’s partial airway administration, the IL-17 content of BALF significantly increased and the anti-IL-17 antibody completely inhibited the recruitment of neutrophils into respiratory tract. Shellito et al found when the mice treated with alcohol developed klebsiella pneumonia lung infection, the IL-17 was inhibited in lung tissues, the recruitment of neutrophils was compromised and the death rate increased [10]. Mice with IL-17R deficiency had shortened survival time after Klebsiella pneumonia infection, and increased lung bacteria, and the infection was easy to spread, which was related to the delayed recruitment of neutrophils and reduced neutrophils in the lungs, as well as to the down-regulated expression of G-CSF and MIP-2. At 48 h after Klebsiella pneumonia infection, mice with IL-17R deficiency had a mortality of 100%, but the mortality in wild-type mice was 40% [31]. Increasing evidence shows that the early recruitment of neutrophils is essential for the resistance to bacterial infection. Therefore, the increased early recruitment of neutrophils may benefit the treatment of PA lung infection [29]. In this study, the role of IL-17 in the immune response was investigated in mice with acute PA lung infection. Our results indicated that IL-17 mRNA expression was significantly up-regulated after acute PA lung infection, and the IL-17 content of BALF was significantly higher than that in control group (P<0.05). With the increase in IL-17 content, the neutrophil count of BALF increased correspondingly, but IL-17 expression was undetectable in the serum of infected mice. Therefore, the up-regulated IL-17 may function on local immune response against acute PA lung infection.
IL-17 induces the recruitment of neutrophils primarily by promoting the release of C-X-C chemokines (especially IL-8). Laan et al [28] found that the intratracheal administration of IL-17 in mice could lead to the recruitment of neutrophils into the lung, which could last at least 8 h. Thus, we speculated that intratracheal administration of justice plasmid expressing IL-17 could also cause a high IL-17 expression in the lung, and thus increase the production of IL-17’s downstream effectors (G-CSF, IL-1β and MIP-2), which may lead to the recruitment of neutrophils into the lung, increasing the clearance of bacteria. Consequently, the justice plasmid expressing IL-17 was administered to the airway, and the IL-17 plasmid was used to transfect the airway epithelial cells. Results showed the IL-17 mRNA expression in the lung of mice treated with IL-17 plasmid was higher than that in blank plasmid group (P<0.05), and the contents of IL-17 and its downstream effectors (G-CSF, IL-1β and MIP-2) in the BALF also increased markedly. Meanwhile, the BALF neutrophil count correspondingly increased. When compared with blank plasmid group, statistically significant difference was observed (P<0.05). As a result, the intratracheal transfection of IL-17 plasmid was successful in mice.
Neutrophil functions dually in bacterial pneumonia. Early neutrophil recruitment is important in counting bacterial infection. In later phase, it will generate a great deal of cytokines/chemokines, reactive oxygen species and proteases, thus causing damage to tissues [26,35]. Peng et al found that the intratracheal administration of IL-17 recombinant adenovirus (AdIL-17) into the lungs of mice before Klebsiella pneumonia infection to induce the over-expression of IL-17, leading to the production of TNF-α, IL-1β, MIP-2 and G-CSF in some parts, recruitment of neutrophil aggregation, increased clearance of Klebsiella pneumonia and the elevated defense against infection demonstrated by increased survival rate [36]. In our study, at 6 h after infection, the IL-17 content in IL-17 plasmid group was higher than that in both infected group and blank plasmid group. The bacterial count in IL-17 plasmid group was significantly lower than that in the infected group and blank plasmid group. In addition, the 3-day survival rate in IL-17 plasmid group (67%) was markedly higher than that in the infected group and blank plasmid group (41%). Hence, we postulate that the increased early neutrophil recruitment may contribute to the treatment of acute PA lung infection.
In summary, our results demonstrate that the up-regulation of IL-17 may increase the bacterial clearance and survival rate through increasing neutrophil recruitment via IL-17’s downstream effectors, and playing protective effect in early phase of acute PA lung infection in mice. If similar mechanisms exist in humans, it seems that IL-17 may be a potential target for the treatment of acute PA lung infection, especially for those with resistant bacterial infection.
Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of China (No. 30770946, 81300041), the fund from the Natural Science Foundation of the Anhui Higher Education Institutions of China (No. KJ2012Z184), the fund for the academic backbone of the excellent young and middle-age people of Anhui medical university, and the fund from First Affiliated Hospital of Anhui Medical University for Fostering Talents of the National Natural Science Foundation of China (No. 2011KJ05).
Disclosure of conflict of interest
None.
References
- 1.Rello J, Diaz E. Pneumonia in the intensive care unit. Crit Care Med. 2003;31:2544–2551. doi: 10.1097/01.CCM.0000089928.84326.D2. [DOI] [PubMed] [Google Scholar]
- 2.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 3.Chastre J, Trouillet JL. Problem pathogens (Pseudomonas aeruginosa and Acinetobacter) Semin Respir Infect. 2000;15:287–298. doi: 10.1053/srin.2000.20944. [DOI] [PubMed] [Google Scholar]
- 4.Afessa B, Green B. Bacterial pneumonia in hospitalized patients with HIV infection: the Pulmonary Complications, ICU Support, and Prognostic Factors of Hospitalized Patients with HIV (PIP) Study. Chest. 2000;117:1017–1022. doi: 10.1378/chest.117.4.1017. [DOI] [PubMed] [Google Scholar]
- 5.Talbot GH, Bradley J, Edwards JE Jr, Gilbert D, Scheld M, Bartlett JG. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis. 2006;42:657–668. doi: 10.1086/499819. [DOI] [PubMed] [Google Scholar]
- 6.Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med. 2005;171:1209–1223. doi: 10.1164/rccm.200408-1044SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoshino H, Laan M, Sjostrand M, Lotvall J, Skoogh BE, Linden A. Increased elastase and myeloperoxidase activity associated with neutrophil recruitment by IL-17 in airways in vivo. J Allergy Clin Immunol. 2000;105:143–149. doi: 10.1016/s0091-6749(00)90189-1. [DOI] [PubMed] [Google Scholar]
- 8.Semik-Orzech A, Barczyk A, Pierzchala W. [The role of interleukin 17A in inducing neutrophilic inflammation in the pulmonary tract] . Pol Merkur Lekarski. 2007;22:163–168. [PubMed] [Google Scholar]
- 9.Linden A, Laan M, Anderson GP. Neutrophils, interleukin-17A and lung disease. Eur Respir J. 2005;25:159–172. doi: 10.1183/09031936.04.00032904. [DOI] [PubMed] [Google Scholar]
- 10.Shellito JE, quan Zheng M, Ye P, Ruan S, Shean MK, Kolls J. Effect of alcohol consumption on host release of interleukin-17 during pulmonary infection with Klebsiella pneumoniae. Alcohol Clin Exp Res. 2001;25:872–881. [PubMed] [Google Scholar]
- 11.Gaffen SL. An overview of IL-17 function and signaling. Cytokine. 2008;43:402–407. doi: 10.1016/j.cyto.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellings PW, Kasran A, Liu Z, Vandekerckhove P, Wuyts A, Overbergh L, Mathieu C, Ceuppens JL. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am J Respir Cell Mol Biol. 2003;28:42–50. doi: 10.1165/rcmb.4832. [DOI] [PubMed] [Google Scholar]
- 13.Barczyk A, Pierzchala W, Sozanska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir Med. 2003;97:726–733. doi: 10.1053/rmed.2003.1507. [DOI] [PubMed] [Google Scholar]
- 14.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, Olivenstein R, Elias J, Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108:430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 15.Laan M, Lotvall J, Chung KF, Linden A. IL-17-induced cytokine release in human bronchial epithelial cells in vitro: role of mitogen-activated protein (MAP) kinases. Br J Pharmacol. 2001;133:200–206. doi: 10.1038/sj.bjp.0704063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moraes TJ, Martin R, Plumb JD, Vachon E, Cameron CM, Danesh A, Kelvin DJ, Ruf W, Downey GP. Role of PAR2 in murine pulmonary pseudomonal infection. Am J Physiol Lung Cell Mol Physiol. 2008;294:L368–377. doi: 10.1152/ajplung.00036.2007. [DOI] [PubMed] [Google Scholar]
- 17.Morris AE, Liggitt HD, Hawn TR, Skerrett SJ. Role of Toll-like receptor 5 in the innate immune response to acute P. aeruginosa pneumonia. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1112–1119. doi: 10.1152/ajplung.00155.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu Q, Zuo P, Shao B, Yang S, Xu G, Lan F, Lu X, Xiong W, Xu Y, Xiong S. Administration of nonviral gene vector encoding rat beta-defensin-2 ameliorates chronic Pseudomonas aeruginosa lung infection in rats. J Gene Med. 2010;12:276–286. doi: 10.1002/jgm.1435. [DOI] [PubMed] [Google Scholar]
- 19.Parkins MD, Gregson DB, Pitout JD, Ross T, Laupland KB. Population-based study of the epidemiology and the risk factors for Pseudomonas aeruginosa bloodstream infection. Infection. 2010;38:25–32. doi: 10.1007/s15010-009-9145-9. [DOI] [PubMed] [Google Scholar]
- 20.Girou E, Schortgen F, Delclaux C, Brun-Buisson C, Blot F, Lefort Y, Lemaire F, Brochard L. Association of noninvasive ventilation with nosocomial infections and survival in critically ill patients. JAMA. 2000;284:2361–2367. doi: 10.1001/jama.284.18.2361. [DOI] [PubMed] [Google Scholar]
- 21.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Chen J, Huang A, Stinson J, Heldens S, Foster J, Dowd P, Gurney AL, Wood WI. Cloning and characterization of IL-17B and IL-17C, two new members of the IL-17 cytokine family. Proc Natl Acad Sci U S A. 2000;97:773–778. doi: 10.1073/pnas.97.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi Y, Ullrich SJ, Zhang J, Connolly K, Grzegorzewski KJ, Barber MC, Wang W, Wathen K, Hodge V, Fisher CL, Olsen H, Ruben SM, Knyazev I, Cho YH, Kao V, Wilkinson KA, Carrell JA, Ebner R. A novel cytokine receptor-ligand pair. Identification, molecular characterization, and in vivo immunomodulatory activity. J Biol Chem. 2000;275:19167–19176. doi: 10.1074/jbc.M910228199. [DOI] [PubMed] [Google Scholar]
- 24.Hymowitz SG, Filvaroff EH, Yin JP, Lee J, Cai L, Risser P, Maruoka M, Mao W, Foster J, Kelley RF, Pan G, Gurney AL, de Vos AM, Starovasnik MA. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J. 2001;20:5332–5341. doi: 10.1093/emboj/20.19.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 26.Wagner JG, Roth RA. Neutrophil migration mechanisms, with an emphasis on the pulmonary vasculature. Pharmacol Rev. 2000;52:349–374. [PubMed] [Google Scholar]
- 27.Jones CE, Chan K. Interleukin-17 stimulates the expression of interleukin-8, growth-related oncogene-alpha, and granulocyte-colony-stimulating factor by human airway epithelial cells. Am J Respir Cell Mol Biol. 2002;26:748–753. doi: 10.1165/ajrcmb.26.6.4757. [DOI] [PubMed] [Google Scholar]
- 28.Laan M, Cui ZH, Hoshino H, Lotvall J, Sjostrand M, Gruenert DC, Skoogh BE, Linden A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347–2352. [PubMed] [Google Scholar]
- 29.Ivanov S, Linden A. Th-17 cells in the lungs? Expert Rev Respir Med. 2007;1:279–293. doi: 10.1586/17476348.1.2.279. [DOI] [PubMed] [Google Scholar]
- 30.Miyamoto M, Prause O, Sjostrand M, Laan M, Lotvall J, Linden A. Endogenous IL-17 as amediator of neutrophil recruitment caused by endotoxin exposure in mouse airways. J Immunol. 2003;170:4665–4672. doi: 10.4049/jimmunol.170.9.4665. [DOI] [PubMed] [Google Scholar]
- 31.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson KR, Napper JM, Denvir J, Sollars VE, Yu HD. Defect in early lung defence against Pseudomonas aeruginosa in DBA/2 mice is associated with acute inflammatory lung injury and reduced bactericidal activity in naive macrophages. Microbiology. 2007;153:968–979. doi: 10.1099/mic.0.2006/002261-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dubin PJ, Kolls JK. IL-23 mediates inflammatory responses to mucoid Pseudomonas aeruginosa lung infection in mice. Am J Physiol Lung Cell Mol Physiol. 2007;292:L519–528. doi: 10.1152/ajplung.00312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol. 2003;170:2106–2112. doi: 10.4049/jimmunol.170.4.2106. [DOI] [PubMed] [Google Scholar]
- 35.Reiniger N, Lee MM, Coleman FT, Ray C, Golan DE, Pier GB. Resistance to Pseudomonas aeruginosa chronic lung infection requires cystic fibrosis transmembrane conductance regulator-modulated interleukin-1 (IL-1) release and signaling through the IL-1 receptor. Infect Immun. 2007;75:1598–1608. doi: 10.1128/IAI.01980-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]


