Abstract
Fentanyl-induced cough (FIC) should be effectively prevented in patients requiring stable induction of general anesthesia. We reviewed available randomized-controlled trials (RCTs) that focused on the pre-emptive fentanyl to prevent FIC, and preformed this meta-analysis to clarify the efficacy and to recommend a specific application. The PubMed, EMBASE, Cochrane Central Register of Controlled Trials, and Chinese BioMedical Literature Database were searched for relevant RCTs without restriction on the year or language of the publications. All of the published RCTs that assessed the efficacy of pre-emptive fentanyl on preventing FIC were selected. A total of seven studies were identified for inclusion. Meta-analysis showed that a priming fentanyl dose of 0.5 μg/kg decreased the FIC incidence (RR = 0.29, 95% CI: 0.17-0.49) and severity (WMD = -0.46, 95% CI -0.70 - -0.23) of FIC; however, a priming fentanyl dose of 1.0 μg/kg (RR = 0.26, 95% CI 0.04-1.70; WMD = -0.60, 95% CI -1.33-0.14) or 1.5 μg/kg (RR = 0.94; 95% CI: 0.77-1.15; WMD = -0.08, 95% CI -0.33-0.17) had no effect on FIC. Our meta-analysis demonstrated that pre-emptive low dose of fentanyl could effectively prevent FIC, and the dose of 0.5 μg/kg was recommended.
Keywords: Pre-emptive fentanyl, fentanyl induced cough, anesthesia, meta-analysis
Introduction
Fentanyl is one of the most widely used opioids for analgesia because of its rapid onset of action, intense analgesic effect and cardiovascular stability [1,2]. Fentanyl is also often used in general anesthesia, because it can facilitate tracheal intubation [3] and suppress tube-induced cough [4]. Interestingly, fentanyl itself can cause cough and the incidence of fentanyl-induced cough (FIC) can even reach 18%-80%, according to some previous reports [5-7]. Currently, the exact mechanism underlying FIC remains unclear; however, several hypotheses have been proposed, including: (1) histamine and neuropeptides released after intravenous fentanyl administration contribute to FIC, (2) pulmonary chemoreflex mediated by either irritant receptors or vagal C-fiber receptors near the pulmonary vessels might play a role in FIC, and (3) fentanyl-induced muscle rigidity might induce FIC [8,9].
FIC has been reported to be independently associated with aging, premedication with benzodiazepines, cigarette smoking, a prior epidural injection of lidocaine, a priming dose of vecuronium, as well as injection time of fentanyl and dilution of fentanyl, but unaffected by gender, the presence of either bronchial asthma or chronic obstructive pulmonary disease, or prior use of atropine [6,10,11]. To data, FIC has received little attention because it seldom causes serious effects in most ASA I and II patients undergoing surgery. However, FIC is accompanied by high intrathoracic pressure and can increase intracranial, intraocular, and intra-abdominal pressures [6,12] and, therefore, should be effectively prevented in patients requiring a stable induction at the beginning of general anesthesia, especially in those with increased intracranial pressure, penetrating eye injury, acute glaucoma, serious airway responsiveness, dissecting aneurysm, pneumothorax, full stomach and so on [8,13]. Furthermore, severe FIC can lead to life-threatening upper airway obstruction [14] and aspiration pneumonia [15], which require immediate intervention.
Therefore, some studies have been designed to explore effective interventions to prevent FIC and found that pretreatment with certain agents (e.g. dexmedetomidine [16], clonidine [17], lidocaine [17], ketamine [18], dezocine [19], and so on) could reduce the incidence of FIC. Recently, several studies have evaluated the effects of pre-emptive small dose of fentanyl on the incidence and severity of FIC; however, its efficacy on FIC remains unclear because the results from different studies are conflicting. For example, Jung et al. [20] demonstrated that priming small dose of fentanyl did not reduce the incidence or severity of FIC while some studies [21-24] affirmed positive effects.
Therefore, we critically reviewed available randomized-controlled trials (RCTs) and conducted a meta-analysis to evaluate the efficacy of pre-emptive small doses of fentanyl on FIC.
Materials and methods
There is no protocol for our current meta-analysis. This meta-analysis was conducted according to the guidelines of the PRISMA statement [25,26].
Information sources
We searched PubMed (updated to Oct 2013), EMBASE (updated to Oct 2013), Cochrane Central Register of Controlled Trials (updated to Oct 2013), and Chinese BioMedical Literature Database (1978 to Oct 2013) without language restriction. The keyword string used for searching was (“pre-emptive” OR “preemptive” OR “priming”) AND (“fentanyl”) AND (“cough” OR “tussis”). The reference lists of the original research articles, reviews, letters to the editor, and case reports were also scanned.
Eligibility criteria
The selection criteria of this meta-analysis were as follows: (1) study design: RCTs; (2) participants: patients receiving intravenous fentanyl; (3) interventions: administration of pre-emptive a small dose of fentanyl vs. placebo or no intervention; and (4) outcome: the incidence or severity of FIC.
Research articles were excluded if: (1) they recruited patients with cough or high airway responsiveness before receiving fentanyl; (2) employed any other anesthetic or muscle relaxant with influence on cough that was administered before counting cough frequency; and (3) group size was less than 10.
Study selection
Full texts of all articles identified through the literature search were independently reviewed by two authors to determine the eligibility according to the selection and exclusion criteria. Any disagreement was adjudicated among all authors.
Data extraction process
A computerized spreadsheet was pre-designed for data extraction. Two coauthors independently extracted relevant information from the original studies. Disagreements were resolved by consultation with the third coauthor.
Data items
The primary outcome was the incidence of cough with intervention compared with the control. Tussis mentioned in RCTs was considered equivalent to cough. The severity of cough, which was graded as mild, moderate and severe in the original articles based on the frequencies of cough after fentanyl administration, was also extracted as a secondary outcome.
In addition, extracted information also included primary author, publication year, sample size, age, weight, ASA status, premedication, and fentanyl regimen.
Risk of bias in individual studies
The risk of bias in the eligible studies was assessed independently by two authors. For each study, we assessed the risk of bias according to the guidelines recommended by the Cochrane collaboration [27]. Six points were used to evaluate the risk of bias, which included sequence generation, allocation, blinding, incomplete outcome data, selective outcome reporting, and other sources of bias. Each of the above points was judged using a 3-point scale of low, unclear, and high risk of bias. Any disagreement was settled through discussion with all authors.
Statistical analysis
Data from individual studies were pooled and the relative risk (RR) with 95% confidence interval (95% CI) was computed for dichotomous outcomes extracted as the presence or absence of FIC. To evaluate the severity of FIC, we applied a 4-point rating scale (none = 0, mild = 1, moderate = 2, and severe = 3) to facilitate further comparisons and used the weighted mean difference (WMD) as a measure of effect [28]. The dose-responsiveness of priming fentanyl on FIC prevention was also assessed.
Heterogeneity among the included studies was assessed using the Q-test. A random-effects model was employed in the case of significant heterogeneity (P < 0.10 and I2 > 50%); otherwise, a fixed-effect model was used.
Sensitivity analysis of the primary endpoint was performed to assess the stability of the results. Individual studies included in the meta-analysis were sequentially omitted to determine contribution of individual data sets to the pooled RR. The potential publication bias regarding our primary variable RR was evaluated using funnel plot, Begg’s rank correlation test and Egger’s linear regression test. P < 0.05 implies the existence of publication bias.
Statistical analysis was conducted using Review Manager Version 5.2 (Cochrane Collaboration, Oxford, UK) and Stata 12.0 (Stata Corp, College Station, TX, USA) software. A P value of < 0.05 was considered statistically significant.
Results
Study selection
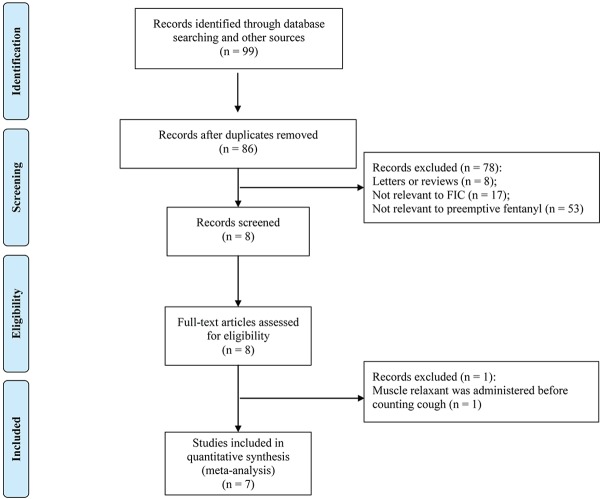
Based on our search strategy, a total of 99 potentially relevant studies were retrieved. However, the majority of these publications were excluded because they did not meet the inclusion criteria. Eventually, seven valid trials [20-22,24,29-31] were included in the meta-analysis. The detailed steps of our literature search and the study selection are shown in Figure 1.
Figure 1.
Flow chart of systematic research.
The methodological quality of included studies
Of the seven included trials, four [20,22,24,30] provided a sequence generation for randomization. Most trials except one [22] did not show sufficient information regarding allocation concealment and one study [20] had high risk. Four studies [20-22,24] were double-blinded. None of the studies had incomplete outcomes and all reported each endpoint mentioned in the methods section. The risk of bias for each trial is summarized (Figure 2).
Figure 2.
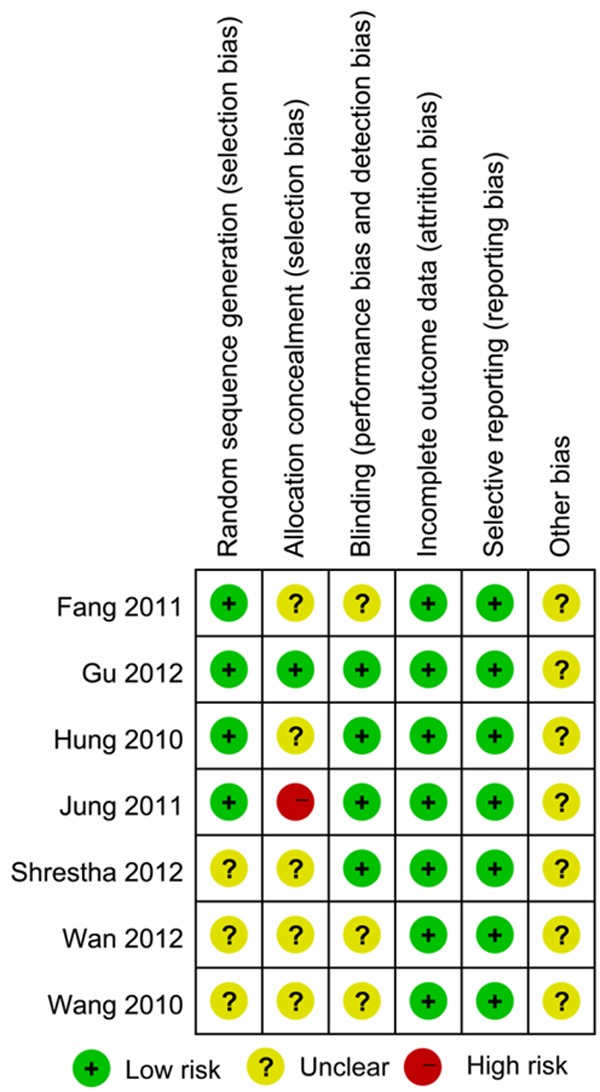
Summary of the risk of bias in the included studies.
Study characteristics
The characteristics of all included studies are shown in Table 1. The eligible studies included 120-800 patients and were published from 2010 to 2012 in two languages: English (four studies [20-22,24]) and Chinese (three studies [29-31]). All included studies were conducted in ASA I-II patients undergoing general anesthesia. The mean patient age of individual studies ranged from 39.1 to 46.5 years. Smokers were excluded from the patient population in two studies [22,31]. Phenobarbital sodium was administrated as premedication in two studies, [22,29] while lorazepam was used in one study [21]. The range of fentanyl dose was 2.0-4.9 μg/kg, which was calculated by the mean weight of all participants in individual studies. Pre-emptive fentanyl was withdrawn from the total dose in six studies [20-22,24,30,31] and was additional in four studies [21,24,29,30]. In most studies, the pre-emptive fentanyl was injected about 1 min before the larger dose, while one study [20] took into account the different intervals between the priming and second fentanyl doses. Two studies [22,31] evaluated the effect of different priming doses of fentanyl on FIC.
Table 1.
Characteristics of the selected randomized controlled trials
| Reference | Premedication | Dose of Fentanyl | Comparison ( ) = Number of Analyzed Patients | ASA status | Mean weight (kg) | Age (y) |
|---|---|---|---|---|---|---|
| Jung et al. [20] | NA | 2 µg/kg | 1. Saline + fentanyl 2 µg/kg i.v. (200) | I, II | 62.1 | 18-75 |
| 2. Preemptive fentanyl 0.5 µg/kg i.v + fentanyl 1.5 µg/kg i.v after 1 min (200) | ||||||
| 3. Preemptive fentanyl 0.5 µg/kg i.v + fentanyl 1.5 µg/kg i.v after 2 min (200) | ||||||
| 4. Preemptive fentanyl 0.5 µg/kg i.v + fentanyl 1.5 µg/kg i.v after 3 min (200) | ||||||
| Shrestha et al. [21] | lorazepam 0.04 mg/kg + pantoprazole 40 mg po | 150 µg | 1. Saline + fentanyl 150 µg i.v. (50) | I, II | 56.7 | 18-75 |
| 2. Preemptive fentanyl 25 µg + fentanyl 125 µg i.v (50) | ||||||
| 3. Preemptive fentanyl 25 µg + fentanyl 150 µg i.v (50) | ||||||
| Gu et al. [22] | phenobarbital sodium 0.1 g + atropine 0.5 mg i.m. | 2.5 µg/kg | 1. Saline + fentanyl 2.5 ug/kg i.v. (100) | I, II | 61.3 | 22-70 |
| 2. Preemptive fentanyl 0.5 µg/kg + fentanyl 2.0 µg/kg i.v. (100) | ||||||
| 3. Preemptive fentanyl 1.0 µg/kg + fentanyl 1.5 µg/kg i.v. (100) | ||||||
| 4. Preemptive fentanyl 1.5 µg/kg + fentanyl 1.0 µg/kg i.v. (100) | ||||||
| Hung et al. [24] | NA | 150 µg | 1. Saline + fentanyl 150 µg i.v. (200) | I, II | 65.1 | 18-75 |
| 2. Preemptive fentanyl 25 µg + fentanyl 125 µg i.v. (200) | ||||||
| 3. Preemptive fentanyl 25 µg + fentanyl 150 µg i.v. (200) | ||||||
| Wang et al. [29] | phenobarbital sodium 0.1 g + atropine 0.5 mg i.m. | 300 µg | 1. Saline + fentanyl 300 µg i.v. (60) | I, II | 60.6 | 25-45 |
| 2. Preemptive fentanyl 25 µg + fentanyl 300 µg i.v. (60) | ||||||
| Fang et al. [30] | NA | 175 µg | 1. Saline + fentanyl 175 µg i.v. (40) | I, II | 57.9 | 18-60 |
| 2. Preemptive fentanyl 25 µg + fentanyl 150 µg i.v. (40) | ||||||
| 3. Preemptive fentanyl 25 µg + fentanyl 175 µg i.v. (40) | ||||||
| Wan et al. [31] | NA | 200 µg | 1. Saline + fentanyl 200 µg i.v. (40) | I, II | 54.1 | 21-70 |
| 2. Preemptive fentanyl 25 µg + fentanyl 175 µg i.v. (40) | ||||||
| 3. Preemptive fentanyl 50 µg + fentanyl 150 µg i.v. (40) |
i.v. = intravenous injection; po = per os; im = intramuscular injection; NA = not available.
Quantitative review and meta-analysis
Primary outcome
For seven studies that assessed the effects of preemptive small dose of fentanyl on the incidence of FIC, a Q-test revealed possible heterogeneity (I2 = 69%, P < 0.1). In overall analysis, preemptive fentanyl significantly decreased the incidence of FIC when the studies were pooled in a random-effects model (RR = 0.39, 95% CI 0.28-0.54) (Figure 3).
Figure 3.
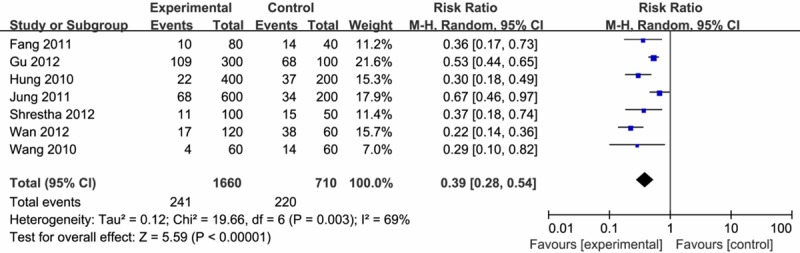
Effect of pre-emptive fentanyl on the incidence of FIC.
To assess the dose-responsiveness of the priming fentanyl, we standardized the measurements to μg/kg by the average weight of all participants in each study. According to the pre-emptive fentanyl dose, three groups were included: about 0.5 μg/kg (0.38-0.5 μg/kg) group, about 1.0 μg/kg (0.92-1.0 μg/kg) group, and 1.5 μg/kg group. It appeared that pre-emptive fentanyl was able to significantly reduce the incidence of FIC at the dose of 0.5 μg/kg (RR = 0.29, 95% CI: 0.17-0.49). However, there was no significant effect of priming 1.0 μg/kg (RR = 0.26, 95% CI: 0.04-1.70) or 1.5 μg/kg (RR = 0.94; 95% CI: 0.77-1.15) fentanyl on reducing FIC (Figure 4). The test of subgroups interaction indicated significant difference among the three doses (P < 0.05).
Figure 4.
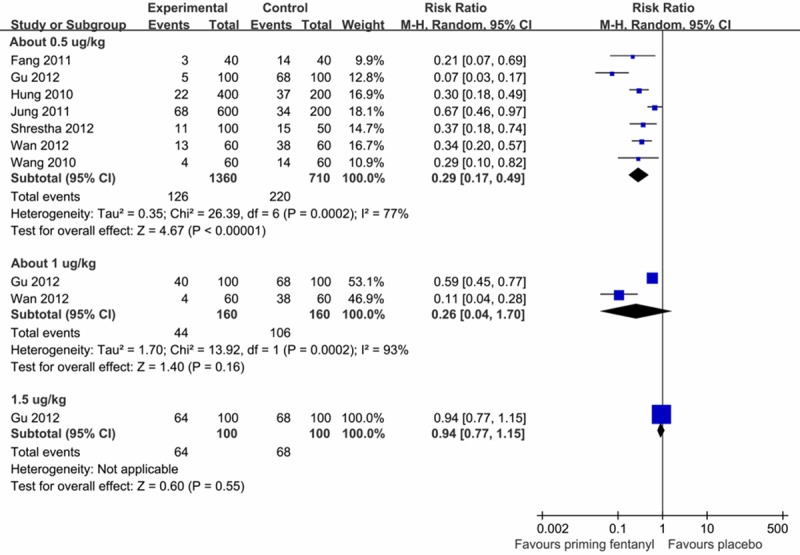
Dose-responsiveness of pre-emptive fentanyl on the incidence of FIC.
Secondary outcome
Pre-emptive small dose of fentanyl also alleviated the severity of FIC, for which the WMD was -0.38 (95 % CI: -0.53 - -0.22), indicating a significant effect, but with considerable heterogeneity (P < 0.1; I2 = 81%), as shown in Figure 5.
Figure 5.
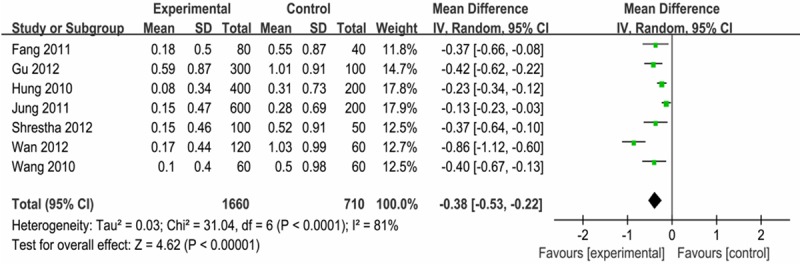
Effect of pre-emptive fentanyl on the severity of FIC.
Dose-responsiveness of the priming fentanyl was also tested. The subgroup analysis showed that a priming fentanyl dose of 0.5 μg/kg (WMD = -0.46, 95% CI: -0.70 - -0.23) could alleviate the severity of FIC, while 1.0 μg/kg (WMD = -0.60, 95% CI: -1.33-0.14) or 1.5 μg/kg (WMD = -0.08, 95% CI: -0.33-0.17) showed no significant effect (Figure 6).
Figure 6.
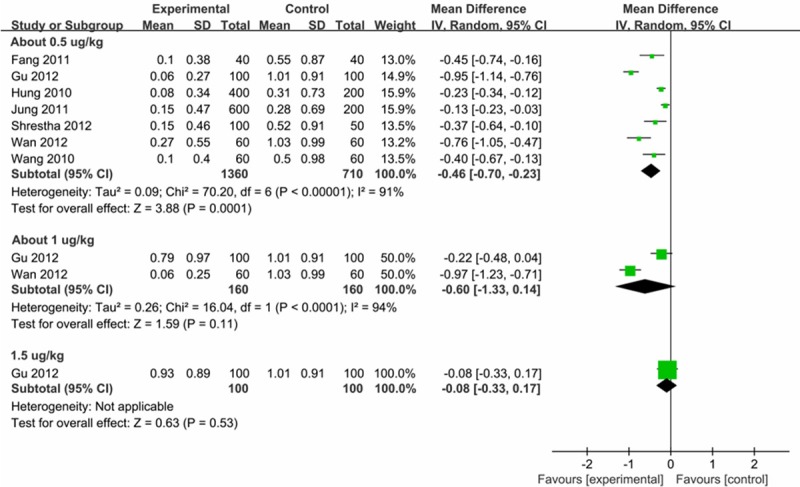
Dose-responsiveness of pre-emptive fentanyl on the severity of FIC.
Sensitivity analysis
Sensitivity analysis was performed by sequential omission of each study to evaluate the influence of each data set on the pooled RR in the meta-analysis. Pooled RR was not significantly altered by removal of any one specific data set (Figure 7).
Figure 7.
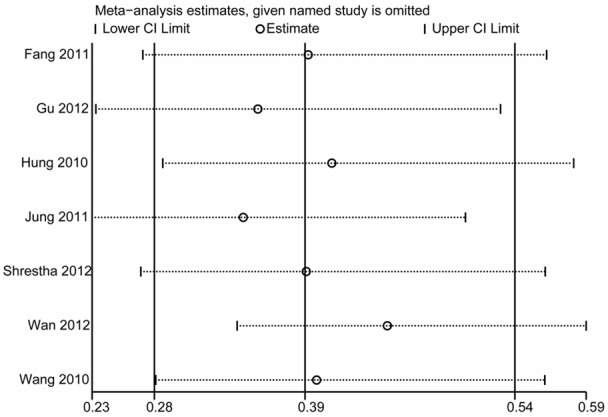
Sensitivity analysis of the effect of pre-emptive fentanyl on the incidence of FIC.
Publication bias
Funnel plots of all studies investigating the incidence of FIC are shown in Figure 8. Results of the Begg’s test (P = 0.368) and Egger’s test (P = 0.137) indicated no significant presence of publication bias.
Figure 8.
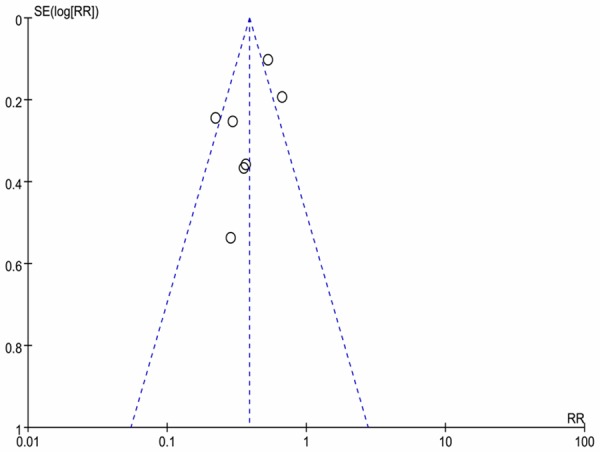
Funnel plot for all trials investigating the incidence of FIC.
Discussion
FIC is a common adverse event of fentanyl in the induction of general anesthesia and considered to be transient and self-limiting for most patients but, at times, is undesirable and should be effectively avoided [13]. The identification of functional intervention may provide insight into the mechanisms of FIC and facilitate preventable measures by anesthesiologists. Therefore, this meta-analysis was designed to assess the effect of priming small fentanyl doses on FIC by comparing the incidence and the severity of FIC in the experimental and control patients.
This meta-analysis included a total of 2370 patients from seven independent trials, of whom 1660 were allocated to priming fentanyl group and 710 to the control group. On average, 31.0% (220/710) of patients without priming fentanyl experienced FIC. The pooled results indicated that priming fentanyl could significantly decrease the incidence and attenuate the severity of FIC. A subsequent analysis of the dose-responsiveness was conducted, which showed that a priming fentanyl dose of 0.5 μg/kg could effectively reduce the incidence of FIC. According to the test results for subgroup interaction, there was a significant difference among the varied priming fentanyl doses, which demonstrated that 0.5 μg/kg more effectively prevented FIC than 1.0 μg/kg or 1.5 μg/kg. One possible explanation was that a priming fentanyl dose of 1.0 μg/kg or more was sufficient to induce coughing [22,32]. The present meta-analysis demonstrated that a priming low dose of fentanyl (about 0.5 μg/kg) will help to effectively prevent FIC.
The trigger of FIC is likely dependent on the relationship between plasma concentration and the threshold for FIC. From a pharmacological viewpoint, additional preconditioning with low fentanyl dose should result in a larger peak plasma concentration. Nevertheless, four studies [21,24,29,30] included in our meta-analysis all demonstrated that additional low dose of fentanyl could also reduce the incidence of cough caused by the primary dose of fentanyl administered subsequently. This finding proposes another possibility that FIC might be associated with a relative fluctuation in plasma fentanyl concentration [24]. In addition, a small priming dose could result in the depletion of neurotransmitters in peripheral nerve fibers [33], which may be another plausible explanation.
Cough is a well-integrated reflex, which includes the afferent limb consisting of receptors and afferent nerves, the cough center in the brainstem, and the efferent limb consisting of motor nerves and the relevant muscles [34]. Interventions that have effects on any element of the cough mechanism may prevent the onset of FIC. However, we must take into account the applicability and side effects of these interventions. Intravenous lidocaine administration can augment the cardiovascular depression of induction agents [35]. Pretreatment with ephedrine will increase heart rate and blood pressure [7], thus such pretreatment may be not suitable for patients with coronary artery disease or severe hypertension. Clonidine [17] and propofol [7] have been associated with a higher risk of hypotension. Ketamine is not recommended to for use in patients with increased intracranial pressure, penetrating eye injury, or acute glaucoma, for whom FIC should be avoided, because ketamine administration itself could raise intracranial [36] and intraocular pressure [37]. An interesting huffing maneuver [34], which includes a period of forced expiration and requires the patient cooperation, may not be applicable to patients with consciousness disorders or under sedation, although it was considered to be a feasible, simple, and convenient method to prevent FIC. However, prolonged injection time and dilution of fentanyl are worthy to be considered because either could decrease the incidence of FIC while avoiding the use of multiple drugs [6,10,38].
Recently, a systematic review [39] just mentioned, but did not focus on, the effect of priming fentanyl on FIC; especially, the severity of FIC and the dose-responsiveness were not evaluated. Our meta-analysis recruited three additional studies published in Chinese to provide more precise evidence and proposed more specific and useful recommendation. In this meta-analysis, we performed sensitivity analysis and the results were not significantly affected by removal of any one specific data set, which indicated that the results of this meta-analysis were statistically robust.
We performed this meta-analysis according to the PRISMA guidelines, but there were still several limitations. Firstly, considerable heterogeneity existed in our meta-analysis. The included studies had different doses of the agents and patient populations, which might cause clinical heterogeneity. Despite performing subgroup analyses according to the different doses of priming fentanyl, acceptable heterogeneity was still not reached; therefore, the random-effects model was adopted. Secondly, the number of the included studies was small, although we conducted a comprehensive search. Thirdly, all eligible studies were conducted in Asia, which might lead to limited generalizability.
To our best knowledge, this is the first meta-analysis focusing on the effect of pre-emptive fentanyl on FIC and some recommendations were raised for further research. We recommend further high-quality trials, which should be ideally randomized and double-blind with a reasonable number of patients or among different populations to provide further evidence on the efficacy and the optimal dose of pre-emptive fentanyl. More attention should be paid to the risk factors of FIC in future studies, because FIC sensitivity and efficacy of the interventions may differ between high-risk and low-risk patients.
Conclusion
In conclusion, our meta-analysis demonstrated that a pre-emptive low dose of fentanyl (about 0.5 μg/kg) could effectively decreased incidence and alleviated the severity of FIC. Further high-quality studies are warranted to confirm the efficacy of this recommended low fentanyl dose.
Disclosure of conflict of interest
None.
References
- 1.Mireskandari SM, Abulahrar N, Darabi ME, Rahimi I, Haji-Mohamadi F, Movafegh A. Comparison of the effect of fentanyl, sufentanil, alfentanil and remifentanil on cardiovascular response to tracheal intubation in children. Iran J Pediatr. 2011;21:173–180. [PMC free article] [PubMed] [Google Scholar]
- 2.Smith H. A comprehensive review of rapid-onset opioids for breakthrough pain. CNS Drugs. 2012;26:509–535. doi: 10.2165/11630580-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inomata S, Maeda T, Shimizu T, Satsumae T, Tanaka M. Effects of fentanyl infusion on tracheal intubation and emergence agitation in preschool children anaesthetized with sevoflurane. Br J Anaesth. 2010;105:361–367. doi: 10.1093/bja/aeq168. [DOI] [PubMed] [Google Scholar]
- 4.Yoo YC, Na S, Jeong JJ, Choi EM, Moon BE, Lee JR. Dose-dependent attenuation by fentanyl on cough during emergence from general anesthesia. Acta Anaesthesiol Scand. 2011;55:1215–1220. doi: 10.1111/j.1399-6576.2011.02529.x. [DOI] [PubMed] [Google Scholar]
- 5.Tang Q, Qian Y, Zhang Q, Yang J, Wang Z. Effects of different priming doses of propofol on fentanyl-induced cough during anesthesia induction: a preliminary randomized controlled study. Ups J Med Sci. 2010;115:121–124. doi: 10.3109/03009730903291034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin JA, Yeh CC, Lee MS, Wu CT, Lin SL, Wong CS. Prolonged injection time and light smoking decrease the incidence of fentanyl-induced cough. Anesth Analg. 2005;101:670–674. doi: 10.1213/01.ANE.0000159161.31276.DB. [DOI] [PubMed] [Google Scholar]
- 7.Lin CS, Sun WZ, Chan WH, Lin CJ, Yeh HM, Mok MS. Intravenous lidocaine and ephedrine, but not propofol, suppress fentanyl-induced cough. Can J Anaesth. 2004;51:654–659. doi: 10.1007/BF03018421. [DOI] [PubMed] [Google Scholar]
- 8.Yu J, Lu Y, Dong C, Zhu H, Xu R. Premedication with intravenous dexmedetomidine-midazolam suppresses fentanyl-induced cough. Ir J Med Sci. 2012;181:517–520. doi: 10.1007/s11845-012-0807-8. [DOI] [PubMed] [Google Scholar]
- 9.Ai Q, Hu Y, Wang Y, Wu S, Qin Z, Wang J, Wang G, Zhang J, An M. Pentazocine pretreatment suppresses fentanyl-induced cough. Pharmacol Rep. 2010;62:747–750. doi: 10.1016/s1734-1140(10)70333-9. [DOI] [PubMed] [Google Scholar]
- 10.Yu H, Yang XY, Zhang X, Li Q, Zhu T, Wang Y, Liu B. The effect of dilution and prolonged injection time on fentanyl-induced coughing. Anaesthesia. 2007;62:919–922. doi: 10.1111/j.1365-2044.2007.05147.x. [DOI] [PubMed] [Google Scholar]
- 11.Oshima T, Kasuya Y, Okumura Y, Murakami T, Dohi S. Identification of independent risk factors for fentanyl-induced cough. Can J Anaesth. 2006;53:753–758. doi: 10.1007/BF03022790. [DOI] [PubMed] [Google Scholar]
- 12.McCool FD. Global physiology and pathophysiology of cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:48S–53S. doi: 10.1378/chest.129.1_suppl.48S. [DOI] [PubMed] [Google Scholar]
- 13.Uvelin A, Rakic G. Guidelines for prevention of fentanyl-induced cough. Acta Anaesthesiol Scand. 2009;53:1228–1229. doi: 10.1111/j.1399-6576.2009.02041.x. [DOI] [PubMed] [Google Scholar]
- 14.Ambesh SP, Singh N, Srivastava K. Fentanyl induced coughing caused life-threatening airway obstruction in a patient with arteriovenous malformation of tongue and hypopharynx. Internet J Anesthesiol. 2013;20:7. [Google Scholar]
- 15.Lim KJ, Lee SK, Lee HM, Park EY, Kim MH, Kim YS, Kang MH. Aspiration pneumonia caused by fentanyl-induced cough -a case report. Korean J Anesthesiol. 2013;65:251–253. doi: 10.4097/kjae.2013.65.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He L, Xu JM, Dai RP. Dexmedetomidine reduces the incidence of fentanyl-induced cough: a double-blind, randomized, and placebo-controlled study. Ups J Med Sci. 2012;117:18–21. doi: 10.3109/03009734.2011.629749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horng HC, Wong CS, Hsiao KN, Huh BK, Kuo CP, Cherng CH, Wu CT. Pre-medication with intravenous clonidine suppresses fentanyl-induced cough. Acta Anaesthesiol Scand. 2007;51:862–865. doi: 10.1111/j.1399-6576.2007.01335.x. [DOI] [PubMed] [Google Scholar]
- 18.Yeh CC, Wu CT, Huh BK, Lee MS, Lin SL, J Sheen M, Wong CS. Premedication with intravenous low-dose ketamine suppresses fentanyl-induced cough. J Clin Anesth. 2007;19:53–56. doi: 10.1016/j.jclinane.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Sun ZT, Yang CY, Cui Z, Zhang J, Han XP. Effect of intravenous dezocine on fentanyl-induced cough during general anesthesia induction: a double-blinded, prospective, randomized, controlled trial. J Anesth. 2011;25:860–863. doi: 10.1007/s00540-011-1237-x. [DOI] [PubMed] [Google Scholar]
- 20.Jung HJ, Kim JB, Im KS, Cho HJ, Kim JW, Lee JM. Effects of a priming dose of fentanyl during anaesthesia on the incidence and severity of fentanyl-induced cough in current, former and non-smokers. J Int Med Res. 2011;39:2379–2384. doi: 10.1177/147323001103900638. [DOI] [PubMed] [Google Scholar]
- 21.Shrestha SK, Bhattarai B, Shah RS. Preemptive use of Small Dose Fentanyl Suppresses Fentanyl Induced Cough. Kathmandu Univ Med J (KUMJ) 2012;10:16–19. doi: 10.3126/kumj.v10i4.10988. [DOI] [PubMed] [Google Scholar]
- 22.Gu C, Zhou M, Wu H, Li F, Tang Q. Effects of different priming doses of fentanyl on fentanyl-induced cough: a double-blind, randomized, controlled study. Pharmacol Rep. 2012;64:321–325. doi: 10.1016/s1734-1140(12)70771-5. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Yao JH, Zhu JJ, Liu B, Zhu JG, Zhou DC. [Effect of optimizing anesthetic injecting sequence during induction on fentanyl-induced coughing] . Zhonghua Yi Xue Za Zhi. 2010;90:921–923. [PubMed] [Google Scholar]
- 24.Hung KC, Chen CW, Lin VC, Weng HC, Hsieh SW. The effect of pre-emptive use of minimal dose fentanyl on fentanyl-induced coughing. Anaesthesia. 2010;65:4–7. doi: 10.1111/j.1365-2044.2009.06109.x. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins JP, Green S, Collaboration C. Wiley Online Library. 2008. Cochrane handbook for systematic reviews of interventions. [Google Scholar]
- 28.Sun L, Guo R, Sun L. The impact of prophylactic intravenous lidocaine on opioid-induced cough: a meta-analysis of randomized controlled trials. J Anesth. 2013 doi: 10.1007/s00540-013-1732-3. published online 31 October 2013. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Pan Y, Zhu HL. Effects of the priming low dose of fentanyl on the fentayl induced cough. Shanghai Med J. 2010;33:768–769. [Google Scholar]
- 30.Fang NN, Zhang ZP, Gu MR, Sun GH, Gao H. Impact of preemptive small dose fentanyl on the fentanyl induced coughing. J Pract Med. 2011;27:2426–2427. [Google Scholar]
- 31.Wan CH, Shen TT. Pre-emptive small dose of fentanyl suppresses fentanyl induced couh. Modern Med. 2012;40:456–458. [Google Scholar]
- 32.Han JI, Lee H, Kim CH, Lee GY. The frequency of fentanyl-induced cough in children and its effects on tracheal intubation. J Clin Anesth. 2010;22:3–6. doi: 10.1016/j.jclinane.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 33.Knoll J. Two kinds of opiate receptor. Pol J Pharmacol Pharm. 1977;29:165–175. [PubMed] [Google Scholar]
- 34.Ambesh SP, Singh N, Gupta D, Singh PK, Singh U. A huffing manoeuvre, immediately before induction of anaesthesia, prevents fentanyl-induced coughing: a prospective, randomized, and controlled study. Br J Anaesth. 2010;104:40–43. doi: 10.1093/bja/aep333. [DOI] [PubMed] [Google Scholar]
- 35.Schlimp CJ, Wiedermann FJ. Does fentanyl-induced cough justify pre-treatment with iv lidocaine 2 mg. kg-1. Can J Anaesth. 2005;52:207. doi: 10.1007/BF03027731. [DOI] [PubMed] [Google Scholar]
- 36.Michalczyk K, Sullivan JE, Berkenbosch JW. Pretreatment with midazolam blunts the rise in intracranial pressure associated with ketamine sedation for lumbar puncture in children. Pediatr Crit Care Med. 2013;14:e149–155. doi: 10.1097/PCC.0b013e3182720459. [DOI] [PubMed] [Google Scholar]
- 37.Kovalcuka L, Birgele E, Bandere D, Williams DL. The effects of ketamine hydrochloride and diazepam on the intraocular pressure and pupil diameter of the dog’s eye. Vet Ophthalmol. 2013;16:29–34. doi: 10.1111/j.1463-5224.2012.01015.x. [DOI] [PubMed] [Google Scholar]
- 38.Chen YM, Chen WT, Liang SW, Gu MN. [Intravenous injection rate and site of fentanyl affect the incidence and onset time of fentanyl-induced cough] . Nan Fang Yi Ke Da Xue Xue Bao. 2009;29:339–340. [PubMed] [Google Scholar]
- 39.Kim JE, Min SK, Chae YJ, Lee YJ, Moon BK, Kim JY. Pharmacological and nonpharmacological prevention of fentanyl-induced cough: a meta-analysis. J Anesth. 2014;28:257–266. doi: 10.1007/s00540-013-1695-4. [DOI] [PubMed] [Google Scholar]