Abstract
Chlorpyriphos is one of the most widely used organophosphate (OP) insecticide in agriculture with potential toxicity. Current post-exposure treatments consist of anti-cholinergic drugs and oxime compounds. We studied the effects of intralipid and caffeic acid phenethyl ester (CAPE) on chlorpyriphos toxicity to compose an alternative or supportive treatment for OP poisoning. Methods: Forty-nine rats were randomly divided into seven groups. Chlorpyriphos was administered for toxicity. Intralipid (IL) and CAPE administered immediately after chlorpyriphos. Serum acetylcholinesterase (AChE) level, total oxidant status (TOS), total antioxidant response (TAR), and histologic examination of cerebellum and brain tissue with Hematoxylin-Eosin and immunohistochemical dyes were examined. Results: Serum enzym levels showed that chlorpyriphos and CAPE inhibited AChE while IL alone had no effect, chlorpyriphos and CAPE intensifies the inhibition effect. Significant difference at AChE levels between the chlorpyriphos+IL and chlorpyriphos+CAPE verified that IL has a protective effect on AChE inhibition. TAR levels were significantly increased in all groups except chlorpyriphos group, TOS levels revealed that CAPE and IL decrease the amount of oxidative stress. Histologic examination revealed that neuronal degeneration was slightly decreased at chlorpyriphos+IL group, but CAPE had a significant effect on protection of neuronal degeneration. Conclusion: The results of this study gave us three key points. 1) AChE activity is important for diagnosis of OP intoxication but it has no value for determining the neuro-degeneration. 2) CAPE inhibits AChE activity and may increase the muscarinic-nicotinic hyperactivation. Therefore it should not be used for treatment of OP intoxication. 3) IL decreases the severity of neurodegeneration and symptoms of OP intoxication and it can be used as a supportive agent.
Keywords: Chlorpyriphos, intralipid, CAPE, acetylcholinesterase, neurotoxicity
Introduction
Organophosphate compounds are the most widely used pesticides in agriculture. Commonly used organophosphates have included parathion, malathion, methyl parathion and chlorpyriphos.
Chlorpyriphos (O,O-diethyl O-3,5,6-trichloropy-ridin-2-yl-phosphorothioate) is a crystalline organophosphate insecticide, with potential for both acute toxicity at larger amounts and neurological effects in fetuses and children even at very small amounts. For acute effects, the EPA classifies chlorpyriphos as Class II: moderately toxic [1].
OPs are one of the most common causes of poisoning worldwide, and are frequently intentionally used in suicides in agrarian areas. There are around 1 million OP poisonings per year with several hundred thousand resulting in fatalities annually [2]. Organophosphate poisoning results from exposure to OPs, which cause the inhibition of AChE, leading to the accumulation of acetylcholine (ACh) in the body. Accumulation of ACh at motor nerves causes overstimulation of nicotinic expression at the neuromuscular junction. When this occurs symptoms such as muscle weakness, fatigue, muscle cramps, fasciculation, and paralysis can be seen [3]. Neurotoxic effects have also been linked to poisoning with OP pesticides causing cholinergic syndrome. Cholinergic syndrome occurs in acute poisonings with OP pesticides and is directly related to levels of AChE activity. Symptoms include miosis, sweating, lacrimation, gastrointestinal symptoms, respiratory difficulties, dyspnea, bradycardia, cyanosis, vomiting, diarrhea, as well as other symptoms. Along with these central effects can be seen and finally seizures, convulsions, coma, respiratory failure. If the person survives the first day of poisoning personality changes can occur, aggressive events, psychotic episodes, disturbances and deficits in memory and attention, as well as other delayed effects.
Reactive oxygen species (ROS) are produced in metabolic and physiological processes, and harmful oxidative reactions may occur in organisms which remove them via enzymatic and nonenzymatic antioxidative mechanisms. Under certain conditions, the increase in oxidants and decrease in antioxidants cannot be prevented, and the oxidative/antioxidative balance shifts towards the oxidative status [4]. Antioxidant molecules can prevent and/or inhibit these harmful reactions [5]. OPs, apart from inhibition of cholinesterase and presence of cholinergic effects, oxidative stress and hyperglycemi a has been reported by many authors as one of the adverse effects in poisoning by OP in both humans and animals. Oxidative stress induced by OP leads to disturbances in the function of different organs and tissues. In subchronic or chronic OP exposition induction of oxidative stress has been reported, by many authors, as the main mechanism of its toxicity [6].
In recent years, several reports have suggested that CAPE, active substance of propolis, is natural composite which have anti-inflammatory, antioxidant, immunomodulatory, antimycosis and anticarcinogenic effects [7]. The antioxidant activity of propolis extract is mainly attributed to its flavonoid content, that is capable of scavenging free radicals and thereby protection against lipid peroxidation. Propolis also induces the activation of antioxidant enzymes such as superoxide dismutase and catalase against free radicals [8].
Intravenous lipid emulsions (IDE) is always used in parenteral nutrition therapy. Recently, IDE was used to resuscitate severe local anesthetic drug toxicity [9]. While the exact mechanisms of IDE’s antidotal action are not clear, the “lipid sink” mechanism may give a potential explanation [10]. The theory included that infusing a large amount of lipids to the blood could move the fat-soluble drugs away from the site of toxicity and dissolved it in the plasma which will alleviate toxic effect of the fat-soluble drug. With the development of lipid emulsion therapy, Sirianni [11] found that lipid emulsion was also efficacious for the treatment of toxicity caused by ingested lipophilic medications.
In this study, effects of CAPE was investigated on oxidative stress and other toxic effects caused by chlorpyriphos intoxication. On the other hand, because of OP pesticides are highly lipophilic agents, poisoned patients might benefit from this antidotal therapy and the profit of intralipid treatment was studied.
Materials and method
Animals, care, and nutrition
A total forty-nine female Wistar Albino rats weighing 200-250 g were kept under laboratory conditions with a 12-hour light/dark cycle and a room temperature of 21 ± 3°C. The study was approved by the Necmettin Erbakan University Experimental Medical Research Center’s Experimental Animals Ethics Committee.
Animals and treatment
The forty-nine rats were randomly divided into seven groups as control group (C) (n=7), and other groups (n=7); chlorpyriphos (CPF) treated, intralipid (IL), CAPE, IL plus CPF treated (IL+CPF), CAPE plus CPF treated CPF treated (CAPE+CPF) and IL plus CAPE plus CPF treated (IL+CAPE+CPF). The rats were administered with chlorpyriphos (10 mg/kg oral) and intralipid (18.6 mL/kg oral) and with CAPE (10 μmol/kg intraperitoneal). Intralipid and CAPE were administered immediately after chlorpyriphos.
Biochemical analysis
The AChE activities in serum were determined with Roche Cobas Integra 800 autoanalyser by enzymatic colorimetric method using Roche brand commercial kits in 409/659 nm. The enzyme activities were expressed as U/L. Measurement range of the tests are 200-14.000 U/L (3.34-234.00 μkat/L) for AChE.
The total antioxidant response (TAR) of supernatant fractions was evaluated by using a novel, automated and colorimetric measurement method developed by Erel (5). Hydroxyl radicals, the most potent biological radicals were produced by this method. In the assay, the ferrous ion solution present in reagent 1 was mixed with hydrogen peroxide, which was present in reagent 2. The subsequently produced radicals, such as brown-colored dianisidinyl radical cations produced by the hydroxyl radicals, are also potent radicals. Using this method, the antioxidative effect of the sample was measured against the potent-free radical reactions initiated by the produced hydroxyl radicals. The assay has excellent precision values lower than 3%. The TAR results were expressed as nmol Trolox equivalent/mg protein.
The total oxidant status (TOS) of supernatant fractions was evaluated by using a novel, automated, and colorimetric measurement method developed by Erel (4). Oxidants present in the sample oxidize the ferrous ion-o-dianisidine complex to ferric ion. The oxidation reaction was increased by glycerol molecules, which were abundantly present in the reaction medium. The ferric ion makes a colored complex with xylenol orange in an acidic medium. The color intensity, which can be measured spectrophotometrically, was related to the total amount of oxidant molecules present in the sample. The assay was calibrated with hydrogen peroxide, and the results were expressed in terms of nmol H2O2 equivalent/mg protein.
Immunohistochemical procedures
Immunohistochemical examination was performed on a Leica Bond-Max automated IHC/ISH platform (Leica Microsystems Inc, Buffalo Grove, Illinois) (immunohistochemistry and in situ hybridization) according to the manufacturer’s protocol with a slight modification. Four micrometer paraffin sections were dewaxed in a Bond Dewax solution, rehydrated in alcohol and Bond Wash solution (Leica Microsystems). Antigen retrieval was performed using a high-pH (ER2) retrieval solution for 15 minutes followed by endogenous peroxidase blocking for 5 minutes on the machine. Standard method with the avidinbiotin -peroxidase complex (ABC Staining System, Santa Cruz Biotechnology Inc.) was used for detection of antibodies: Anti-mouse monoclonal antibody Bcl-2 (C-2: sc-7382, Santa Cruz Biotechnology, Inc. in dilution 1:200), anti-mouse monoclonal antibody Bax (B-9: sc-7480, Santa Cruz Biotechnology, Inc. in dilution 1:100) and anti-mouse caspase-3 (CPP32) monoclonal antibody (clone JHM62, Leica Biosystems Ltd, Newcastle) was applied at 1:50 dilution for 60 minutes at room temperature. Detection was performed using the Bond Polymer Refine Red Detection system (Leica Microsystems) with a 15 minute postprimary step followed by 25 minutes incubation with alkaline phosphatase-linked polymers. Detecting reaction was developed with red substrate provided in the Refine detection kit. Sections were then counterstained with hematoxylin on the machine, dehydrated in alcohols, and mounted with mounting medium (Sakura Finetek USA Inc, Torrance, California). Prepared tissues were observed by histopathologists unaware of the experimental study groups. The numbers of caspase-3, bcl-2 and Bax positive apopitotic cells were counted in ten randomly selected microscope fields under a 400× magnification in a blind fashion. We calculated the average number of stained neurons for each set of ten fields and expressed as the number of the positive cells/high-power field.
Histological examination
The brain and cerebellum specimens were individually immersed in 10% neutral buffered formalin, dehydrated in alcohol and embedded in paraffin. Sections of 4 μm were obtained, deparaffinized and stained with hematoxylin and eosin (H&E). The brain tissue was examined and evaluated in random order under blindfold conditions with standard light microscopy. Inflammation, oedema, congestion, degeneration, necrosis and necrobiosis were evaluated. After the results were graded, all of the groups were compared with eachother.
Statistical analysis
The data for the biochemical parameters were analyzed by ANOVA, followed by the post hoc Tukey test and Dunnet T3. All data was presented using SPSS Windows 20.0 (IBM SPSS Statistics Data editor). A value of p<0.05 was considered statistically significant.
Results
Biochemical results
Serum AChE levels: When IL group compared with the control group, there was no significant difference. But we found that CAPE and CPF group inhibited serum AChE enzym level. Comparing IL group with CPF+CAPE and CPF+IL with CPF+CAPE there was significant difference (*p<0.05). The result revealed that IL protects the inhibition effect of CAPE and CPF on AChE (Table 1).
Table 1.
Comparison of AChE enzyme levels in the groups
| (I) GROUPS | (J) GROUPS | Mean Difference (I-J) | Std. Error | Sig. |
|---|---|---|---|---|
| CAPE | 237.9 | 128.55044 | 0.073 | |
| IL | -4.06667 | 122.14171 | 0.974 | |
| Control | CPF | 133.38571 | 117.34996 | 0.264 |
| CPF+CAPE | 381.70000* | 128.55044 | 0.006 | |
| CPF+IL | 3.76667 | 122.14171 | 0.976 | |
| KP+İL+CAPE | 387.10000* | 137.60503 | 0.008 | |
| Control | -237.9 | 128.55044 | 0.073 | |
| IL | -241.96667 | 132.93908 | 0.078 | |
| CAPE | CPF | -104.51429 | 128.55044 | 0.422 |
| CPF+CAPE | 143.8 | 138.85035 | 0.308 | |
| CPF+IL | -234.13333 | 132.93908 | 0.087 | |
| CPF+IL+CAPE | 149.2 | 147.27303 | 0.318 | |
| IL | Control | 4.06667 | 122.14171 | 0.974 |
| CAPE | 241.96667 | 132.93908 | 0.078 | |
| CPF | 137.45238 | 122.14171 | 0.269 | |
| CPF+CAPE | 385.76667* | 132.93908 | 0.007 | |
| CPF+IL | 7.83333 | 126.75244 | 0.951 | |
| CPF+IL+CAPE | 391.16667* | 141.71354 | 0.009 | |
| Control | -133.38571 | 117.34996 | 0.264 | |
| CAPE | 104.51429 | 128.55044 | 0.422 | |
| CPF | IL | -137.45238 | 122.14171 | 0.269 |
| CPF+CAPE | 248.31429 | 128.55044 | 0.062 | |
| CPF+IL | -129.61905 | 122.14171 | 0.296 | |
| CPF+IL+CAPE | 253.71429 | 137.60503 | 0.074 | |
| Control | -381.70000* | 128.55044 | 0.006 | |
| CAPE | -143.8 | 138.85035 | 0.308 | |
| CPF+CAPE | IL | -385.76667* | 132.93908 | 0.007 |
| CPF | -248.31429 | 128.55044 | 0.062 | |
| CPF+IL | -377.93333* | 132.93908 | 0.008 | |
| CPF+IL+CAPE | 5.4 | 147.27303 | 0.971 | |
| Control | -3.76667 | 122.14171 | 0.976 | |
| CAPE | 234.13333 | 132.93908 | 0.087 | |
| CPF+IL | IL | -7.83333 | 126.75244 | 0.951 |
| CPF | 129.61905 | 122.14171 | 0.296 | |
| CPF+CAPE | 377.93333* | 132.93908 | 0.008 | |
| CPF+IL+CAPE | 383.33333* | 141.71354 | 0.011 | |
| Control | -387.10000* | 137.60503 | 0.008 | |
| CAPE | -149.2 | 147.27303 | 0.318 | |
| CPF+IL+CAPE | IL | -391.16667* | 141.71354 | 0.009 |
| CPF | -253.71429 | 137.60503 | 0.074 | |
| CPF+CAPE | -5.4 | 147.27303 | 0.971 | |
| CPF+IL | -383.33333* | 141.71354 | 0.011 |
The mean difference is significant at the 0.05 level.
TAR levels: In chlorpyriphos group, TAR levels were lower than control group. In the other groups, levels were significantly higher than control group. Comparing CPF+CAPE group with chlorpyriphos group indicated that CAPE increases TAR levels (Table 2).
Table 2.
TAR levels were significantly decreased in CPF group. In the other groups levels were increased. Comparing the levels in CPF group with CPF+CAPE revealed that CAPE increases the TAR level significantly
| Dependent | Variable | (I) GROUPS | (J) GROUPS | Mean Difference (I-J) | Std. Error | Sig. |
|---|---|---|---|---|---|---|
| TAR | Tukey | C | CPF | .44714* | 0.05972 | 0 |
| HSD | IL | 0.07571 | 0.05972 | 0.8 | ||
| CAPE | 0.07029 | 0.06216 | 0.866 | |||
| CPF+CAPE | 0.12069 | 0.05506 | 0.264 | |||
| CPF+CAPE+IL | -0.00071 | 0.05631 | 1 | |||
| CPF | C | -.44714* | 0.05972 | 0 | ||
| IL | -.37143* | 0.05972 | 0 | |||
| CAPE | -.37686* | 0.06216 | 0 | |||
| CPF+CAPE | -.32646* | 0.05506 | 0 | |||
| CPF+CAPE+IL | -.44786* | 0.05631 | 0 | |||
| IL | C | -0.07571 | 0.05972 | 0.8 | ||
| CPF | .37143* | 0.05972 | 0 | |||
| CAPE | -0.00543 | 0.06216 | 1 | |||
| CPF+CAPE | 0.04497 | 0.05506 | 0.963 | |||
| CPF+CAPE+IL | -0.07643 | 0.05631 | 0.751 | |||
| CAPE | C | -0.07029 | 0.06216 | 0.866 | ||
| CPF | .37686* | 0.06216 | 0 | |||
| IL | 0.00543 | 0.06216 | 1 | |||
| CPF+CAPE | 0.0504 | 0.0577 | 0.951 | |||
| CPF+CAPE+IL | -0.071 | 0.05889 | 0.831 | |||
| CPF+CAPE | C | -0.12069 | 0.05506 | 0.264 | ||
| CPF | .32646* | 0.05506 | 0 | |||
| IL | -0.04497 | 0.05506 | 0.963 | |||
| CAPE | -0.0504 | 0.0577 | 0.951 | |||
| CPF+CAPE+IL | -0.1214 | 0.05134 | 0.193 | |||
| CPF+CAPE+IL | C | 0.00071 | 0.05631 | 1 | ||
| CPF | .44786* | 0.05631 | 0 | |||
| IL | 0.07643 | 0.05631 | 0.751 | |||
| CAPE | 0.071 | 0.05889 | 0.831 | |||
| CPF+CAPE | 0.1214 | 0.05134 | 0.193 |
The mean difference is significant at the 0.05 level.
TOS levels: There was correlation between TAR and TOS levels. In chlorpyriphos group, TOS levels were significantly higher than other groups. The most significant result was the decrease of TOS levels at the CAPE+IL group. This result showed that CAPE+IL decreases the oxidative stress level caused by chlorpyriphos (Table 3).
Table 3.
The result of the TOS levels were in correlation with TAR levels; significant increase in CPF group, significant decrease in CAPE and IL groups was observed
| Dependent | Variable | (I) GROUPS | (J) GROUPS | Mean Difference (I-J) | Std. Error | Sig. |
|---|---|---|---|---|---|---|
| TOS | Tukey | C | CPF | -5.19857* | 1.10545 | 0 |
| HSD | IL | -0.05857 | 1.10545 | 1 | ||
| CAPE | 0.804 | 1.15058 | 0.981 | |||
| CPF+CAPE | -3.70574* | 1.01917 | 0.009 | |||
| CPF+CAPE+IL | -0.74956 | 1.04222 | 0.978 | |||
| CPF | C | 5.19857* | 1.10545 | 0 | ||
| IL | 5.14000* | 1.10545 | 0 | |||
| CAPE | 6.00257* | 1.15058 | 0 | |||
| CPF+CAPE | 1.49283 | 1.01917 | 0.688 | |||
| CPF+CAPE+IL | 4.44902* | 1.04222 | 0.002 | |||
| IL | C | 0.05857 | 1.10545 | 1 | ||
| CPF | -5.14000* | 1.10545 | 0 | |||
| CAPE | 0.86257 | 1.15058 | 0.974 | |||
| CPF+CAPE | -3.64717* | 1.01917 | 0.011 | |||
| CPF+CAPE+IL | -0.69098 | 1.04222 | 0.985 | |||
| CAPE | C | -0.804 | 1.15058 | 0.981 | ||
| CPF | -6.00257* | 1.15058 | 0 | |||
| IL | -0.86257 | 1.15058 | 0.974 | |||
| CPF+CAPE | -4.50974* | 1.06796 | 0.002 | |||
| CPF+CAPE+IL | -1.55356 | 1.08998 | 0.712 | |||
| CPF+CAPE | C | 3.70574* | 1.01917 | 0.009 | ||
| CPF | -1.49283 | 1.01917 | 0.688 | |||
| IL | 3.64717* | 1.01917 | 0.011 | |||
| CAPE | 4.50974* | 1.06796 | 0.002 | |||
| CPF+CAPE+IL | 2.95618* | 0.95023 | 0.037 | |||
| CPF+CAPE+IL | C | 0.74956 | 1.04222 | 0.978 | ||
| CPF | -4.44902* | 1.04222 | 0.002 | |||
| IL | 0.69098 | 1.04222 | 0.985 | |||
| CAPE | 1.55356 | 1.08998 | 0.712 | |||
| CPF+CAPE | -2.95618* | 0.95023 | 0.037 |
The mean difference is significant at the 0.05 level.
Hematoxylin and eosin
The brain and cerebellum specimens examined under light microscope (×200 and ×400) for inflammation, oedema, congestion, degeneration, necrosis and necrobiosis findings.
Scoring
Semiquantative grading was performed as: Oedema and congestion: None: 0, Minimal: 1 Moderate: 2 Severe: 3; Inflammation: Negative: 0, Positive: 1; Degeneration: None: 0, Minimal: 1, Moderate: 2 Severe: 3; Necrosis and necrobiosis: None: 0, Minimal: 1, Moderate: 2, Severe: 3; The score results were summarized at Table 4.
Table 4.
Score results of degeneration findings
| GROUPS | TOTAL SCORE/70 |
|---|---|
| 1. Control group | 7/70 |
| 2. CPF | 37/70 |
| 3. CAPE | 9/70 |
| 4. IL | 11/70 |
| 5. CPF+CAPE | 17/70 |
| 6. CPF+IL | 32/70 |
| 7. CPF+CAPE+IL | 21/70 |
Neurons were normal in control group (Figures 1 and 2). In CPF group there was significant neuronal degeneration with the findings of dark picnotic nucleus, vacuolation and shrunken cytoplasm (Figure 3), significant oedema, congestion and inflammation (Figure 4), and necrosis (Figure 5). CPF+IL group evaluation showed that neuronal degeneration was fewer than CPF group but there was no statistical significance. In CAPE group, all of the degeneration findings were significantly fewer than CPF group (Figure 6).
Figure 1.
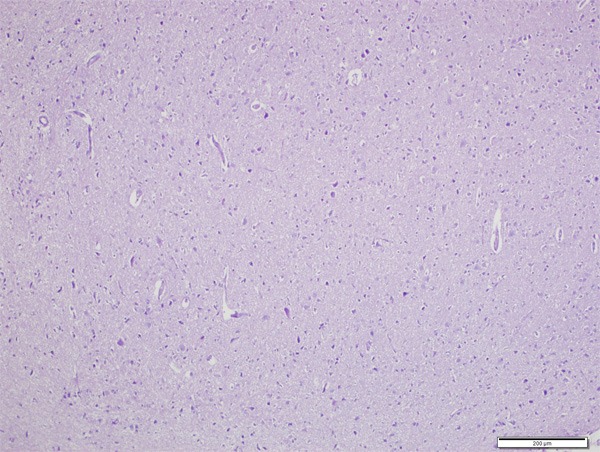
Normal histologic view of rat brain cortex (H&E, ×200).
Figure 2.
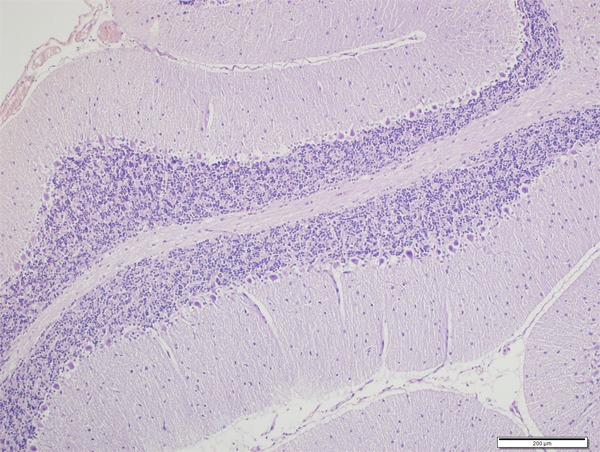
Normal histologic view of rat cerebellum (H&E, ×200).
Figure 3.
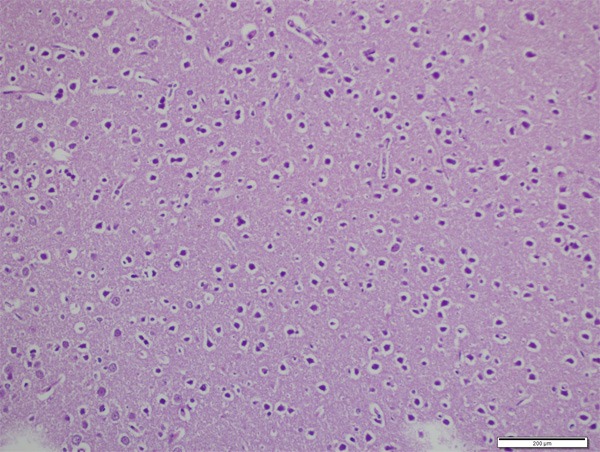
Vacuolar degeneration, dark pyknotic nucleus and shrunken cytoplasm (H&E, ×400).
Figure 4.
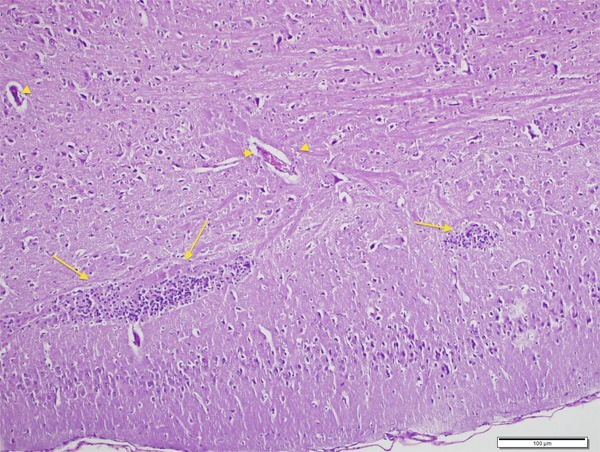
Besides degenerative findings, inflammation (arrow) and congestion (arrow head) can be seen (H&E, ×200).
Figure 5.
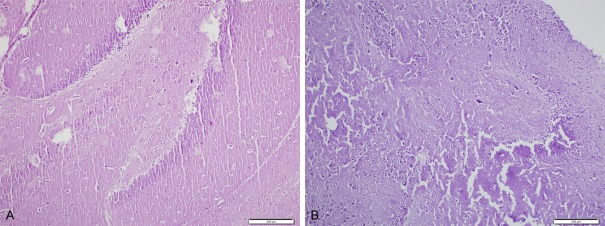
A: Cerebellar necrosis (H&E, ×400), B: Cortical necrosis (H&E, ×400) in CPF group.
Figure 6.
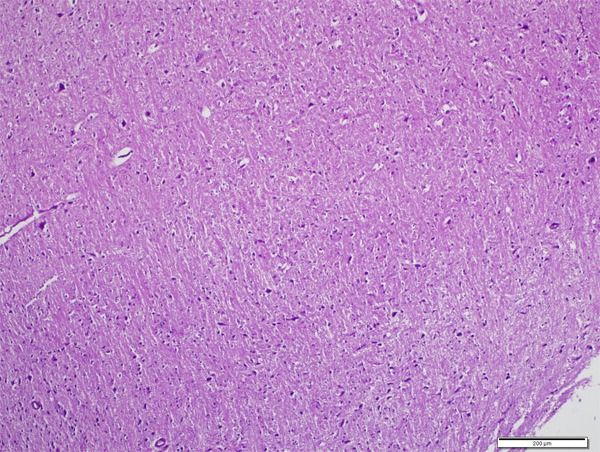
Histologic examination of CPF+CAPE group. Degeneration findings are significantly fewer than CPF group (H&E, ×100).
Immunohistochemical findings
Seven rats were evaluated in each group. Caspase-3, bcl-2 and bax antibodies were used for apoptotic cell counting. Randomized ten microscope fields (×400) were counted (Table 5).
Table 5.
Amount of apoptotic cells. Randomized ten microscope fields (×400) were counted
| GROUPS | TOTAL APOPTOTIC CELLS |
|---|---|
| 1. CONTROL | 25 |
| 2. CPF | 147 |
| 3. CAPE | 31 |
| 4. IL | 38 |
| 5. CPF+CAPE | 61 |
| 6. CPF+IL | 108 |
| 7. CPF+CAPE+IL | 78 |
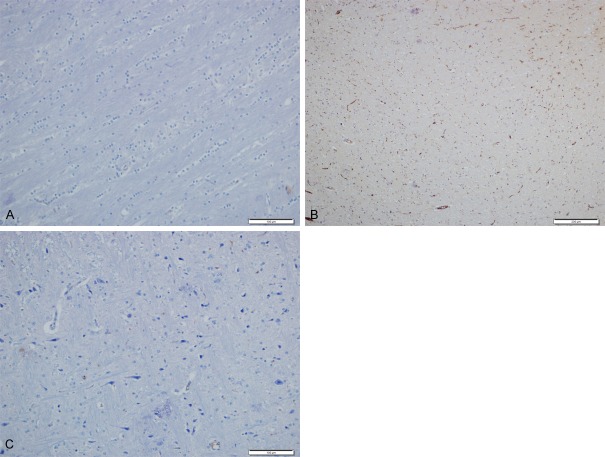
CAPE and IL group had similar amount of apoptotic cells with control group. In CPF group there was significant increase of dead cells. CPF+ CAPE group showed that CAPE protects the apoptotic process caused by CPF (Figure 7).
Figure 7.
Immunohistochemical examination with caspase-3, bcl-2 and bax antibodies. A: normal brain tissue (IHC ×200). B: CPF group had significant apoptotic cells (IHC ×100), C: CPF+CAPE group had fewer amount of apoptotic cells than CPF group (IHC ×200).
Discussion
Chlorpyriphos is a broad-spectrum OP insecticide that is increasingly used both in agriculture and in the home because it is generally safer than parathion and related compounds [12]. OP insecticides produce toxicity by inhibiting cholinesterase enzymes in both vertebrate and invertebrate organisms. These enzymes are responsible for the removal of the neurotransmitter ACh from the synaptic cleft through hydrolysis [13,14]. In vertebrates, ACh acts as an excitatory transmitter for voluntary muscle in the somatic nervous system. ACh also serves as both a preganglionic and a postganglionic transmitter in the parasympathetic nervous system and as a preganglionic transmitter in the sympathetic nervous system. In critical regions of the central nervous system, ACh serves as an excitatory transmitter. When cholinesterases are inactivated by the binding of OPs, an accumulation of ACh occurs at the nerve synapse, interfering with the normal nervous system function. This produces rapid twitching of voluntary muscles followed by paralysis. Once bound, organophosphorus compounds are considered irreversible inhibitors, as recovery usually depends on new enzyme synthesis [14].
In this study, biochemical analysis showed that chlorpyriphos inhibited AChE as mentioned in the literature. The effect of CAPE on AChE is controversial. In some studies it is reported that CAPE does not alter the AChE serum levels [14,15], but some authors indicated that CAPE inhibits AChE [16]. Our results revealed that CAPE significantly inhibits AChE, therefore it is not suitable for managing OP toxicity.
The effect of IL on AChE has not been studied before. In this study, comparison the results of IL group with CAPE+CPF group, and CPF+IL group with CPF+CAPE group showed significant difference (p<0.05). The result indicates that IL has a protective effect on inhibition of AChE due to chlorpyriphos and CAPE.
Vidyasagar et al [17] reported that the effects of organophosphates on fish revealed that besides AChE inhibition, there were changes characteristic of oxidative stress. In humans, OP was shown to reduce the total cholesterol and phospholipid level of red blood cell membrane following phosphamidon and malathion, and increase lipid peroxides level following malathion. They have shown that there was a considerable increase in lipid peroxide levels indicating an enormous oxidative stress in OP poisoned patients. In this study, we found that CAPE and IL increases total antioxidant response and decreases total oxidant status generated by chlorpyriphos.
It has been reported that OP poisoning induces apoptosis through caspase-3 activation [18]. A previous study suggests that caspase-3 plays an important role in mediating paraoxon-induced apoptosis in EL4 cells [19]. Various cellular antioxidants, like Vitamins C, E and N-acetylcysteine, are able to prevent apoptosis induced by several different agents, other than oxidants. This also supports the argument for an important role of oxidative stress in apoptosis [20]. It has been shown that intracellular ROS generation or a depletion of cellular antioxidants can result in apoptosis and the supplement with antioxidant compounds can block apoptosis [21,22].
Güney et al [23] reported that there was a significant difference in immunolabelling of caspase-3 and -9 in endometrial epithelium and stroma between samples obtained from control and Methyl parathion (MPT) group. Activated caspase-3 and -9 is responsible for the breakdown of several cellular components related to DNA repair and regulation during apoptosis. Administration of Vitamins E and C along with MPT significantly reduced the extent of apoptosis.
In this study, apoptosis was evaluated by caspase-3, bcl-2 and bax antibodies. There was no sign of apoptosis induction in control, CAPE and IL groups. In chlorpyriphos group, there was a significant increase of apoptosis. The most important finding is the decrease of apoptosis caused by chlorpyriphos in CPF+IL and CPF+ CAPE group (p<0.001).
Conclusion
The results of this study gave us three key points which can be used in the future studies: (1) AChE activity is important for diagnosis of organophosphorus and organocarbamate pesticide intoxication and clinical signs of muscarinic-nicotinic receptors, but it has no value for determining the neuro-degeneration. (2) CAPE inhibits AChE activity and may increase the muscarinic-nicotinic hyperactivation. Therefore it should not be used for treatment of OP and OC intoxication. (3) IL decreases the severity of neurodegeneration and symptoms of OP and OC intoxication, therefore it can be used as a supportive agent for managing OP and OC poisoning.
Acknowledgements
This study with 1206M0122 ID number was supported by Mustafa Kemal University Scientific Research Project Coordination.
Disclosure of conflict of interest
None.
References
- 1.Mansour SA, Mossa ATH. Lipid peroxidation and oxidative stress in rat erythrocytes induced by chlorpyrifos and the protective effect of zinc. Pesticide Biochemistry and Physiology. 2009;93:34–39. [Google Scholar]
- 2.Yurumez Y, Durukan P, Yavuz Y, Ikizceli I, Avsarogullari L, Ozkan S, Akdur O, Ozdemir C. Acute organophosphate poisoning in university hospital emergency room patients. Intern Med. 2007;46:965–969. doi: 10.2169/internalmedicine.46.6304. [DOI] [PubMed] [Google Scholar]
- 3.Bicker W, Lammerhofer M, Genser D, Kiss H, Lindner W. A case study of acute human chlorpyrifos poisoning: Novel aspects on metabolism and toxicokinetics derived from liquid chromatography-tandem mass spectrometry analysis of urine samples. Toxicol Lett. 2005;159:235–251. doi: 10.1016/j.toxlet.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37:112–119. doi: 10.1016/j.clinbiochem.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Lukaszewicz-Hussain A. Role of oxidative stress in organophosphate insecticide toxicity - Short review. Pesticide Biochemistry and Physiology. 2010;98:145–150. [Google Scholar]
- 7.Alp H, Aytekin I, Esen H, Alp A, Büyükbaş S, Basarali K, Hatipoğlu NK, Kul S. Protective effects of caffeic acid phenethyl ester, ellagic acid, sulforaphan and curcuma on malathion induced damage in lungs, liver and kidneys in an acute toxicity rat model. Revue Méd Vét. 2011;162:333–340. [Google Scholar]
- 8.Attia AA, El-Mazoudy RH, El-Shenawy NS. Antioxidant role of propolis extract against oxidative damage of testicular tissue induced by insecticide chlorpyrifos in rats. Pesticide Biochemistry and Physiology. 2012;103:87–93. [Google Scholar]
- 9.Zhou Y. Intravenous lipid emulsions combine extracorporeal blood purification: a novel therapeutic strategy for severe organophosphate poisoning. Med Hypotheses. 2010;74:309–311. doi: 10.1016/j.mehy.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Weinberg GL, VadeBoncouer T, Ramaraju GA. Pretreatment or resuscitation with a lipid infusion shifts the dose-response to bupivacaine induced asystole in rats. Anesthesiology. 1998;88:1071–1075. doi: 10.1097/00000542-199804000-00028. [DOI] [PubMed] [Google Scholar]
- 11.Sirianni AJ, Osterhoudt KC, Calello DP. Use of lipid emulsion in the resuscitation of a patient with prolonged cardiovascular collapse after overdose of bupropion and lamotrigine. Ann Emerg Med. 2008;51:412–415. doi: 10.1016/j.annemergmed.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Song X, Violin JD, Seidler FJ, Slotkin TA. Modeling the Developmental Neurotoxicity of Chlorpyrifos in Vitro: Macromolecule Synthesis in PC12 Cells. Toxicol Appl Pharmacol. 1998;151:182–191. doi: 10.1006/taap.1998.8424. [DOI] [PubMed] [Google Scholar]
- 13.Habig C, Di Giulio RD. Biochemical characteristics of cholinesterases in aquatic organisms. In: Mineau P, editor. Cholinesterase inhibiting insecticides: Their impact on wildlife and the environment. Vol 2. New York: Elsevier; 1991. pp. 19–34. [Google Scholar]
- 14.Alp H, Sak ME, Evsen MS, Firat U, Evliyaoğlu O, Penbegül N, Sancaktutar AA, Söylemez H, Tuzcu M. Effects of Malathion in fetal kidney tissues in pregnant rats: Teratogenic effects induced by different doses. Kafkas Univ Vet Fak Derg. 2012;18:221–226. [Google Scholar]
- 15.Rezg R, Mornagui B, El-Fazaa S, Gharbi N. Caffeic acid attenuates malathion induced metabolic disruption in rat liver, involvement of acetylcholinesterase activity. Toxicology. 2008;250:27–31. doi: 10.1016/j.tox.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Anwar J, Spanevello RM, Thomé G, Stefanello N, Schmatz R, Gutierres J, Vieira J, Baldissarelli J, Carvalho FB, da Rosa MM, Rubin MA, Fiorenza A, Morsch VM, Schetinger MR. Effects of caffeic acid on behavioral parameters and on the activity of acetylcholinesterase in different tissues from adult rats. Pharmacol Biochem Behav. 2012;103:386–394. doi: 10.1016/j.pbb.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Vidyasagar J, Karunakar N, Reddy MS, Rajnarayana K, Surender T, Krishna DR. Oxidative stress and antioxidant status in acute organophosphorous insecticide poisoning. Indian J Pharmacol. 2004;36:76–79. [Google Scholar]
- 18.Masoud L, Vijayasarathy C, Fernandez-Cabezudo M, Petroianu G, Saleh AM. Effect of malathion on apoptosis of murine L929 fibroblasts: a possible mechanism for toxicity in low dose exposure. Toxicology. 2003;185:89–102. doi: 10.1016/s0300-483x(02)00596-6. [DOI] [PubMed] [Google Scholar]
- 19.Saleh AM, Vijayasarathy C, Masoud L, Kumar L, Shahin A, Kambal A. Paraoxon induces apoptosis in EL4 cells via activation of mitochondrial pathways. Toxicol Appl Pharmacol. 2003;190:47–57. doi: 10.1016/s0041-008x(03)00126-1. [DOI] [PubMed] [Google Scholar]
- 20.Llopisa SP, Ferrando MD, Peña JB. Fish tolerance to organophosphate-induced oxidative stress is dependent on the glutathione metabolism and enhanced by N-acetylcysteine. Aquat Toxicol. 2003;65:337–360. doi: 10.1016/s0166-445x(03)00148-6. [DOI] [PubMed] [Google Scholar]
- 21.Serbecic N, Beutelspacher SC. Anti-oxidative vitamins prevent lipid-peroxidation and apoptosis in corneal endothelial cells. Cell Tissue Res. 2005;320:465–475. doi: 10.1007/s00441-004-1030-3. [DOI] [PubMed] [Google Scholar]
- 22.Ramanathan B, Jan KY, Chen CH. Resistance to Paclitaxel is proportional to cellular total antioxidant capacity. Cancer Res. 2005;65:8455–8460. doi: 10.1158/0008-5472.CAN-05-1162. [DOI] [PubMed] [Google Scholar]
- 23.Güney M, Oral B, Demirin H, Özgüner M, Take G, Mungan T, Altuntas I. Evaluation of caspase-dependent apoptosis during methyl parathion-induced endometrial damage in rats: Ameliorating effect of Vitamins E and C. Environ Toxicol Pharmacol. 2007;23:221–227. doi: 10.1016/j.etap.2006.10.002. [DOI] [PubMed] [Google Scholar]