Abstract
To explore effects of natural crude extract of C. elegans on treatment of asthma. Method: Obtain crude extract of C. elegans from synchronically incubated C. elegans via centrifugation, washing and ultrasonic emulsification, etc.; measure C. elegans’s protein molecular weight via SDS-polyacrylamide gel electrophoresis (SDS-PAG electrophoresis); construct animal models of asthma with 6-8-week-old BALB/c female mice sensitized by chicken ovalbumin (OVA); conduct immunotherapy on animals with asthma with different doses of mixture of C. elegans and OVA (COM) respectively; take PBS buffer group and OVA group as control groups; conduct inspection of cell factors and differential count of cells in serum IgE, IgG1 and IgG2a antibodies and bronchoalveolar lavage fluid (BALF) via enzyme linked immunosorbent assay (ELISA); and incise lung tissue for pathology observation. Result: C. elegans’s protein molecular weight is about 50 kd. In bronchoalveolar lavage fluid (BALF) of OVA group, cell factors IL-5 and IL-13 are more than those in PBS buffer group, but IL-2 and IFN-γ are less than those in PBS buffer group; these differences are of statistical significance (P<0.05). Total cellular score and number of eosinophile granulocyte in BALF of OVA group are more than those in PBS buffer group (P<0.05), and the difference in serum IgE, IgG1 and IgG2a between these two groups is of statistical significance (P<0.05). For groups treatment by different doses of COM, cell factors IL-5 and IL-13 in bronchoalveolar lavage fluid (BALF) are less than those in OVA group, but IL-2 and IFN-γ are more than those in OVA group; these differences are of statistical significance (P<0.05). Total cellular score and number of eosinophile granulocyte in BALF of COM treatment groups are less than those in OVA group (P<0.05); serum IgE and IgG1 less than those in OVA group, but IgG2a is more than that in OVA group; these differences are of statistical significance (P<0.05). Conclusion: The natural crude extract of C. elegans has immunoregulation to animals with asthma.
Keywords: Caenorhabditis elegans, crude extract, asthma, immunoregulation
Introduction
According to the statistics there are more than 300 million people suffering from asthma, and the incidence has been on the rise for recent 10 years. It is expected that the incidence rate will be up to 400 million [1] by 2025, so research on prevention and treatment of asthma will be an important research subject in the future. Foreign experts predicted that the market of drugs for relieving asthma would be one of the ten most promising drug markets in the next 20 years. Market Letter reported that the average annual growth rate of drugs for relieving asthma in the world was 7%-8%. The 2012-2016 Strategic Research Report on Current Market Situation and Development Tendency of Industry of Drugs for Relieving Cough and Asthma shows China’s market of drugs for relieving cough and asthma has a great development space. Drugs for relieving asthma in the current market are mainly divided into two categories: anti-inflammatory drugs mainly controlling inflammation such as glucocorticoid and bronchodilators mainly controlling airway stenosis such as β2-adrenoreceptor agonist. Most of research and development on drugs for relieving asthma is at the stage I and II. In recent years, compounds of inhaled glucocorticoid and bronchodilators, desensitization therapy for determining sensitinogen of patients, etc. [2,3] also have gained a rapid development, but due to influences from drug adverse reactions, prices, difficulty in determining allergen, and other factors, it is necessary to strengthen research and development of drugs for relieving asthma. The study explored immunoregulation of natural crude extract of C. elegans to asthma and provided references for research and development of new drugs for relieving asthma.
Materials and methods
Materials
Caenorhabditis elegans: the experimental article is N2 wild-type C. elegans (C. elegans, the Bristol strain N2), and it is incubated on NGM culture medium containing uracil leaky mutant Escherichia coli OP50. Original C. elegans and Escherichia coli OP50 are provided by Caenorhabditis Genetics Center (CGC). Experimental animals are 6-8-week-old SPF-class female BALB/c mice provided by the Comparative Medicine Center of Yangzhou University.
Reagents and instruments
NGM culture medium: prepare 1000 ml solution with 3 g NaCl, 2.5 g peptone, 17 g agar and 25 ml of 1 mol/L K2HPO4-KH2PO4 buffer (pH=6.0); autoclave the solution at 121°C for 30 minutes; add 1 ml cholesterol solution (5 mg/ml ethanol), 1 ml 1 mol/L MgSO4 and 1 ml 1 mol/L CaCl2, respectively sterilized via suction filtration; and regulate PH with HCL or NaOH to neutrality. M9 buffer: prepare 1 L solution with 15.12 g Na2HPO4·12H2O, 3 g KH2PO4, 5 g NaCl and 0.25 g MgSO4·7H2O; and autoclave the solution at 121°C for 30 minutes. Ovalbumin (OVA) and aluminium hydroxide: being bought from Sigma Company. OVA sensitization liquid (each ml has PBS solution containing 3 mg AL (OH)3 and 50 μg OVA). OVA atomized liquid (containing 1% OVA solution). Mice IgE, IgG1 and IgG2a ELISA Kits were bought from Shanghai Genemy BioTech Co., Ltd. and mice IL-2, IL-5, IL-13 and IFN-γ ELISA Kits were bought from R&D Systems Company. Instruments: BioTek microplate reader ELx800, 402AI ultrasonic atomizer, ultrasonic cell disruptor, electrophoresis apparatus, automatic electric-heated vertical pressure steam sterilizer (HHT4-LX-B100L), table-top high-speed centrifuge (L3660D), thermostatic oscillation incubator (ZHWY-103B), clean bench (BCM biological clean bench), water-jacket thermostatic incubator (GHX-9270B), artificial climate incubator (RQH-750), OLYMPΜS microscope (SZX7), etc.
Preparation and molecular weight measurement of extract of C. elegans
Obtain Escherichia coli OP50 solution by shaking the test tube with incubated Escherichia coli OP50, making it stay overnight, centrifuging, removing upper clear liquid, diluting, etc.; inoculate the Escherichia coli OP50 on the NGM culture medium; obtain NGM culture medium containing Escherichia coli OP50 after staying for 24 hours at 37°C; transfer the synchronically incubated C. elegans to the NGM culture medium; incubate them in artificial climate incubator (temperature: 20°C; humidity: 75%); wash C. elegans away from the culture dish with M9 buffer (PH=7); centrifuge at 1500 r/min in 1.5 ml EP tube at 4°C for 5 minutes; remove upper clear liquid; obtain C. elegans after washing precipitants three times; put C. elegans in the ultrasonic cell disruptor (ultrasonic power: 200 W, ultrasonic time: 5 min); conduct sterilization via suction filtration to obtain crude extracts of C. elegans (C. elegans); prepare 50 μg/ml C. elegans solution with PBS solution; conduct 12.5% SDS-PAGE electrophoresis after addition of 0.2 ml sample; dye the solution with Coomassie brilliant blue dye liquor; and prepare mixed treatment solution COM of C. elegans and OVA (prepare COMA solution containing 50 μg C. elegans and COMB solution containing 75 μg C. elegans with PBS buffer containing 50 μg OVA and PBS buffer containing aluminum hydroxide adjuvant respectively).
Constructing animal models of asthma
Sensitize mice via intraperitoneal injection of 0.2 ml OVA sensitization liquid on the 1st, 7th, 14th day of the experiment while sensitizing mice in control groups with the same dose of PBS buffer at the same locations; put mice in an enclosed atomization inhalation case on 21st day of the experiment; conduct atomization inhalation provocation with portable atomizer and OVA sensitization liquid, 30 min each time, once a day for successive 3 days; and record positive reactions of mice every day to construct mouse models of asthma.
Immunotherapy of crude extract of C. elegans (C. elegans)
Randomly divide BALB/c mice into 4 groups: control group (PBS Group), asthma model group (OVA Group), C. elegans treatment mixture A group and B group (namely COMA group and COMB group), 10 mice each group; and start injecting treatment solution on the 24th day of the experiment - treating mice in PBS group and OVA group with PBS solution and 50 μg/ml OVA respectively, at an interval of 1 week, once a week for successive 4 weeks, 1 ml per injection.
Collection and inspection of specimens
(1) Inspection of Serum IgE, IgG1, and IgG2a antibodies: Mice for modeling and laboratory mice were sterilely killed by dislocation on the 24 day of the experiment and 24 hours after the last treatment respectively; draw blood from eyeball of mice and left serum; and conduct ELISA to inspect contents of specific serum IgE, IgG1 and IgG2a antibodies in strict accordance with the kit instruction.
(2) Inspection of cell factors in bronchoalveolar lavage fluid (BALF): Anaesthetize mice with 0.25 ml of 10% chloral hydrate; separate and cut open trachea of mice under aseptic conditions; insert a tube into the trachea; repeatedly conduct bronchoalveolar lavage by pouring sterilely precooled PBS solution (1 ml each time); collect bronchoalveolar lavage fluid (BALF) (recovery rate >85%) after lavaging three times; centrifuge BALF at a speed of 2000 r/min at 4°C for 5 minutes; collect upper clear liquid; conduct ELISA to inspect contents of cell factors IL-2, IL-5, IL-13 and IFN-γ in BALF in strict accordance with the kit instruction.
(3) Cell count in bronchoalveolar lavage fluid (BALF): Centrifuge the collected bronchoalveolar lavage fluid (BALF) at a speed of 2000 r/min at 4°C for 10 minutes; centrifuge dregs and conduct weight suspension with 1 ml PBS solution; fetch 2 μl and put on the blood cell counting plate to count cells under microscope; dye smear with Wright-Giemsa’s compound stain; and conduct cell classification and count under microscope.
(4) Pulmonary histopathologic observation: Wash unlavaged lateral lung tissue with normal saline, fix tissue with 10% formaldehyde solution overnight, conduct regular section, dye with HE, and observe infiltration of lymphocytes and eosinophils as well as damages of bronchial epithelial cells.
Statistical analysis
Found database for data with EXCELL, analyze with SPSS16.0 statistical package, and express measurement data with (x̅ ± s). Apply F inspection for comparison among several groups while applying t or t’ inspection for comparison between two groups.
Results
Protein molecular weight of crude extract of C. elegans (C. elegans)
Prepare 50 μg/ml C. elegans solution with PBS solution; conduct 12.5% SDS-PAGE electrophoresis after addition of 0.2 ml sample; and dye the solution with Coomassie brilliant blue dye liquor. Standard MARK 50 μg/ml OVA solution was taken as control solution, and the measured protein molecular weight of C. elegans was about 50 kd. See Figure 1.
Figure 1.
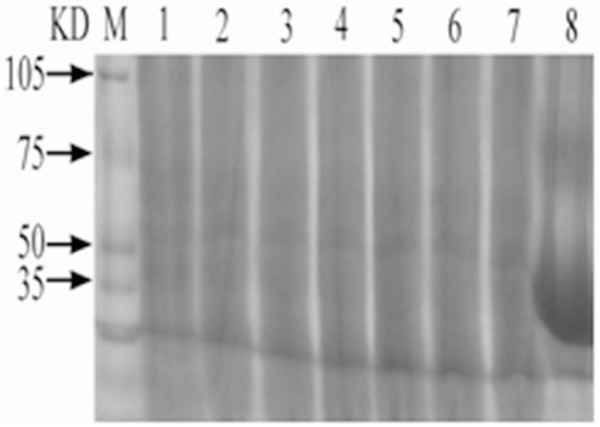
SDS-PAGE Analysis of C. elegans Proteins. M: Protein marker 1-7: Extract of C. elegans 8: OVA.
Status of animal models of asthma
During OVA sensitization and atomization provocation, tachypnea, nodding breathing, hair rising, hunchback and scratching face, shrinking and lifting of fore limbs, abdominal muscle convulsion, gatism, and other phenomena to varying extent occurred on mice. After stopping atomization every time symptoms for atomization provocation gradually occurred in advance, there’s still pant, but main symptoms had disappeared, and mice were quiet and made less movements at the later stage.
Content of serum IgE, IgG1, IgG2a antibodies
Contents of IgG1 and IgE in OVA group were higher than those in PBS group, and this difference was of statistical significance (P<0.05), but difference in content of IgG2a was of no statistical significance (P>0.05). In comparison with OVA group, contents of IgG1 and IgE in COMA and COMB groups were lower, but content of IgG2a antibody was higher; and these difference were of statistical significance (P< 0.05). See Figure 2.
Figure 2.
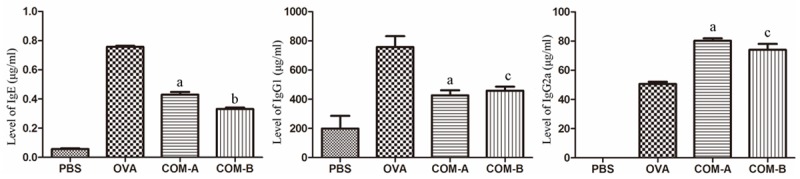
Comparison among contents of serum IgE, IgG1, IgG2a antibodies. a: In comparison with PBS and OVA groups, the difference was of statistical significance (P<0.001); b: in comparison with COM-A group, the difference is of statistical significance (P<0.01); c: in comparison with COM-A group, the difference was of no statistical significance (P>0.05).
Contents of cell factors in bronchoalveolar lavage fluid (BALF)
During comparison among cell factors IL-2, IL-5, IL-13 and IFN-γ, difference in each group was of statistical significance (P<0.001). In comparison with PBS group, contents of cell factors IL-5 and IL-13 in OVA group were higher while those of cell factors IL-2 and IFN-γ were lower, and these differences are of statistical significance (P<0.05). In comparison with 0VA group, contents of cell factors IL-5 and IL-13 in COMA and COMB groups were lower while those of cell factors IL-2 and IFN-γ in these two groups were higher, and these differences were of statistical significance (P<0.05). In comparison with COMA group, content of cell factor IFN-γ in COMB group was higher, and this difference was of statistical significance (P<0.05). The results is showed in Table 1.
Table 1.
Comparison among contents of cell factors in bronchoalveolar lavage fluid (BALF) of mice
| Group | Number of cases | IL-2 | IL-5 | IL-13 | IFN-γ |
|---|---|---|---|---|---|
| PBS | 10 | 21.6±2.22 | 16.5±1.27 | 206.6±6.24 | 392.5±4.91 |
| OVA | 10 | 14.1±0.99 | 67.6±4.69 | 510.0±13.85 | 225.10±11.92 |
| COMA | 10 | 20.2±1.23 | 27.9±1.97 | 359.70±17.42 | 1726.5±133.66 |
| COMB | 10 | 21.0±2.06 | 24.1±4.82 | 339.8±23.46 | 1982.1±90.36 |
| F | 41.19 | 412.64 | 569.10 | 1252.0 |
Cell classification and count in bronchoalveolar lavage fluid (BALF)
Both total cellular score and quantity of eosinophils in OVA group were higher than those in PBS group, and this difference was of statistical significance (P<0.05). In comparison with 0VA group, both total cellular score and quantity of eosinophils in COMA and COMB groups were lower while quantity of macrophage and lymphocyte in these two groups were higher, and these differences were of statistical significance (P<0.05) (Figure 3).
Figure 3.
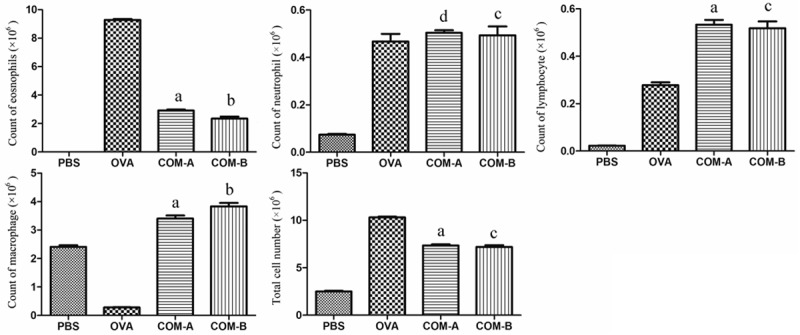
Cell classification and count in bronchoalveolar lavage fluid (BALF). a: In comparison with PBS and OVA groups, the difference was of statistical significance (P<0.001); b: in comparison with COM-A group, the difference was of statistical significance (P<0.01); c: in comparison with COM-A group, the difference was of no statistical significance (P>0.05); d: in comparison with OVA group, the difference was of no statistical significance (P>0.05).
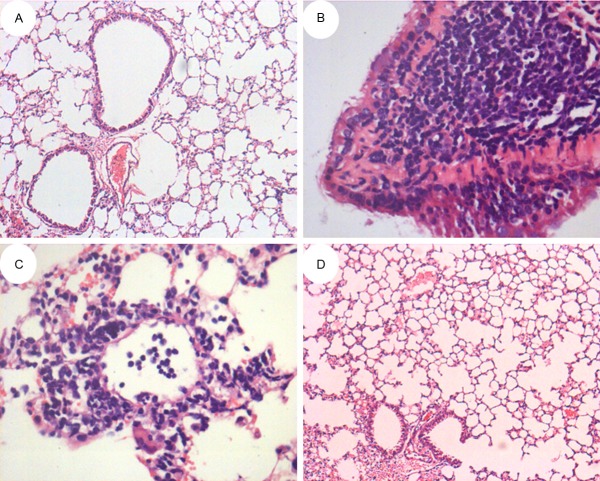
Pulmonary histopathologic changes
Visual observations showed that mice lung tissue in OVA model group was larger in volume than that in PBS control group and had swelling, congestion and irregularities on its surface as well as white foamy exudate on the section. After H-E staining, it was observed under microscope that there was obvious inflammatory cell infiltration (mainly eosinophils and lymphocytes) on mice bronchi and surrounding lung tissue as well as under vascular mucous membranes and that bronchial epithelial cells had several ruptures and defluvium (see Figure 4B). No change was observed in PBS group (see Figure 4A). After treatment with COM, phenomena on mice lung tissue mentioned above changed: obvious decrease of inflammatory cells, decrease of exudate from lung tissue, and no obvious increase in volume (see Figure 4C, 4D).
Figure 4.
Pulmonary histopathologic changes of mice with asthma in different groups. A: HE×100; B: HE×400; C: HE×400; D: HE×100.
Discussion
Epidemiological investigations show that allergies and autoimmune diseases in countries with prevalence of worms have occurred less than in countries without prevalence of worms. Animal model studies indicate that invermination can regulate immunity and that severity of damages caused by allergies and autoimmune diseases shall be reduced while establishing long-term chronic parasitic infection. For example, nematization (trichina, filaria, American hookworm, Angiostrongylus cantonensis, etc.) can inhibit incidence of asthma and arthritis [4-7]. A number of studies have also confirmed that secretion from live bugs may function as a modulating agent, and can intervene parasitifer immunological effect [8]. For example, total protein extract of earthworm plays a role in relieving asthma, with rapid action, less adverse reactions, etc [9]. C. elegans is a simple multicellular organism with extensive attention from scientists due to its application by molecular genetics expert Sydney Brenner to explore genetic regulatory mechanism of neurodevelopment in the 1960s. It is a hermaphroditic nematode freely living in soil, with a very small but transparent body. Male C. elegans is rare. This nematode is able to conduct self-fertilization and sense changes in such external environment as flavor, light and temperature. Because scientists have already drawn its cell lineage and completed gene sequencing, and the nematode is taken as a model organism and extensively applied in such fields as drug screening, water quality monitoring, heavy metal toxicity assessment, and study on mechanism of ageing. The latest studies at abroad indicate that extract of the nematode may have an anti-asthma role [10]. In the study, crude extract of C. elegans (C. elegans) was obtained from synchronically incubated C. elegans via centrifugation, washing and ultrasonic emulsification, etc. and then was prepared into treatment solution. The study showed that C. elegans had therapeutic effects on mouse models of asthma.
Asthma is an allergy disease caused by immune deficiency. TH1/TH2 cells unbalance in proportion of number, activation and function, and over-activation of Th2 and resulted cell factors play a main role in asthma and chronic airway inflammation [11]. TH2 cell mainly secrets IL-4, IL-5, IL-10, IL-13 and other Type II anti-inflammatory cell factors and assists in humoral immunity. TH1 cell primarily produces IL-2, IFN-γ and other Type I proinflammatory cell factors and assists in cellular immunity. Meanwhile, reciprocal inhibition may occur between TH1 and TH2 cell subsets. For example, cell factor IFN-γ is able to activate TH1 cell, promote release of proinflammatory cell factors, and inhibit TH2 cell and production of anti-inflammatory cell factors. Studies show incidence of asthmatoid diseases: after allergenic substance enters the human body at first, plasma cell in the body produces lgE; when the body contacts sensitinogen again, the sensitinogen combines with IgE, resulting in cell degranulation, release of various mediators of inflammation and then strengthened vasopermeability and airway smooth muscle contraction, thus causing airway stenosis and then asthma. This disease is related to sensitinogen- and IgE-mediated Type 1 immunoreaction or Type III immunoreaction resulted from complement activation by immune complex of IgG and antigen. Pathology of asthma is characterized by chronic airway inflammation involved by eosinophils, lymphocytes, mast cells and other inflammatory cells.
In the study, OVA were taken as sensitinogen to induce mice, resulting in typical symptoms of bronchial asthma. In comparison with PBS group, EOS% in BLAF of OVA group increased obviously, contents of specific IgG1, IgE and IgG2a antibodies in BLAF of OVA group were higher than PBS group, and contents of specific cell factors IL-5 and IL-13 were higher while those of IL-2 and IFN-γ were lower. Such phenomena as infiltration of a large amount of inflammatory cells were observed on the lung tissue slice. Mixture of C. elegans and OVA was used to prepare different concentrations of treatment solution. The study showed that all different concentrations of treatment solution had immunoregulation to mouse models of asthma. In comparison with OVA group, contents of IgG1 and IgE in treatment group were lower; those of IL-2 and IFN-γ in treatment group were higher while those of IL-5 and IL-13 were lower. BALF cell count showed increase of total cellular score, decrease of eosinophils, and increase of macrophage and lymphocyte in treatment group. After treatment, significant decrease of inflammatory cells in mice lung tissue, decrease of exudate from lung tissue, and basic recovery in volume was observed. It could be seen that C. elegans immunoregulated asthma by regulating Th1/Th2 balance and there’s difference in IFN-γ produced by treatment with different concentrations of C. elegans. This showed a certain dose-effect relationship between C. elegans and content of IFN-γ as well as possible relation between function of C. ELEGANS and immunoregulation of cell factor IFN-γ.
Invermination can regulate immunity. Different worms are different in migration path, parasitic site and effect of immune response in their parasitifers. It is important significance and clinical application value to explore immunoregulation mechanism of worms. “Worm therapy” is expected to be developed into a new therapy and thought for such autoimmune diseases as inflammatory or anaphylactic diseases. C. elegans in the study are characterized by simple, convenient and rapid oviposition and hatching, high yield, applicability to large-scale cultivation as pharmaceutical raw material, etc. providing a new view for research and development of drugs for relieving asthma.
Acknowledgements
The research work was supported by National Natural Science Foundation of China (No. 81270091) and the Provincial Natural Science Foundation of Colleges and Universities in Anhui Province under Grant (No. KJ2013B306).
Disclosure of conflict of interest
None.
References
- 1.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 2.Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2007;119:780–791. doi: 10.1016/j.jaci.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 3.Larche M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–771. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 4.Park HK, Cho MK, Choi SH, Kim YS, Yu HS. Trichinella spiralis: infection reduces airway allergic inflammation in mice. Exp Parasitol. 2011;127:539–544. doi: 10.1016/j.exppara.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Feary JR, Venn AJ, Mortimer K, Brown AP, Hooi D, Falcone FH, Pritchard DI, Britton JR. Experimental hookworm infection: a randomized placebo-controlled trial in asthma. Clin Exp Allergy. 2010;40:299–306. doi: 10.1111/j.1365-2222.2009.03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen QR, Guan F, Yan DJ, Lei DS, Fu L, Xia HS, Zhu YH, Chen ZW, Niu AO. The dynamic expression of allograft inflammatory factor-1 in hepatic tissues and splenic cells of BALB/c mice with Schistosoma japonicum infection. Tissue Antigens. 2012;79:33–41. doi: 10.1111/j.1399-0039.2011.01809.x. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez ML, Herbst M, Lay JC, Alexis NE, Brickey WJ, Ting JP, Zhou H, Peden DB. Atopic asthmatic patients have reduced airway inflammatory cell recruitment after inhaled endotoxin challenge compared with healthy volunteers. J Allergy Clin Immunol. 2012;130:869–876. e2. doi: 10.1016/j.jaci.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hewitson JP, Grainger JR, Maizels RM. Helminth immunoregulation: the role of parasite secreted proteins in modulating host immunity. Mol Biochem Parasitol. 2009;167:1–11. doi: 10.1016/j.molbiopara.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H. TCM treatment of asthma in children with yin hua wu mei tang--Professor Liu Bichen’s experience in treating asthma. J Tradit Chin Med. 2000;20:180–184. [PubMed] [Google Scholar]
- 10.Kim SE, Kim JH, Min BH, Bae YM, Hong ST, Choi MH. Crude extracts of C. elegans suppress airway inflammation in a murine model of allergic asthma. PLoS One. 2012;7:e35447. doi: 10.1371/journal.pone.0035447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mochizuki H, Shigeta M, Morikawa A. Development of bronchial hyperresponsiveness during childhood. J Asthma. 2001;38:1–21. doi: 10.1081/jas-100000018. [DOI] [PubMed] [Google Scholar]