Abstract
Several lines of evidence demonstrate that isoflurane anesthesia would be a great risk factor for the patients undergoing surgeries to suffer from postoperative cognitive dysfunction (POCD). Additionally, diabetes is also an important pathogenic factor for the emergence of cognitive dysfunction. If patient is suffering from diabetes, the incidence of cognitive dysfunction greatly increased. We therefore aimed to investigate the effects of isoflurane anesthesia on cognitive dysfunction in a diabetic rat model induced by a single injection of streptozotocin (STZ). Wistar rats received 2 h of 2% isoflurane or oxygen exposure 1 month after a single intraperitoneal injection of 60 mg/kg of STZ or the vehicle. The results showed that isoflurane anesthesia significantly aggravates STZ-induced an increase of the latency to the platform and a decrease of the proportion of time spent in the target quadrant of rats in Morris water maze test. In addition to the expression of amyloid-β (Aβ), superoxide dismutase (SOD), malonyldialdehyde (MDA), tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), isoflurane anesthesia significantly increased as compared with a single injection of STZ. However, isoflurane anesthesia had no effect on the blood glucose and leptin. In conclusion, our results suggested that isoflurane anesthesia aggravating cognitive impairment induced by STZ is probably related to the activation of oxidative stress and inflammatory response in rat hippocampus.
Keywords: Isoflurane, cognitive impairment, streptozotocin, inflammatory response, oxidative stress
Introduction
Postoperative cognitive dysfunction (POCD) is a severe neurological dysfunction with a morbidity of approximately 25%, which is characterized by hypomnesia, insanity and an impaired ability to study [1,2]. Additionally, it also would be a negative effect on patients’ quality of life, and contributes greatly to healthcare costs [3]. Increasing studies have shown that inhaled anesthesia may be an important risk factor for the emergence of POCD [4,5]. Although the present clinical data cannot provide a causal connection between anesthesia and cognitive impairment, a great number of studies have proven that volatile anesthetics exposure causes cognitive impairment for days or even weeks in animal models [5-8].
Inhaled anesthetics such as isoflurane have been widely used in clinical practices. However, it has been reported that isoflurane anesthesia would be an inducing factor to impair cognitive function [9]. Although the pathogenesis of the isoflurane-induced cognitive impairment has not been fully elucidated, increasing studies have supported the view that the excessive expression of proinflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, are involved in cognitive impairment after surgeries and general anesthesia [10,11]. Lin et al. [12] has also demonstrated that increased expression of inflammatory cytokines and reduced neuronal density in hippocampus is probably involved in the isoflurane-induced cognitive impairment. Besides of activated inflammatory response implicated in the pathogenesis of POCD, oxidative stress-elicited neuron cell death may be, at least partially, involved in it [13]. Anesthesia and surgical trauma are considered major oxidative stressors which results in the development of POCD [14]. A previous study performed by Keller et al. [15] has displayed that oxidative damage may occur in the brain of subjects with cognitive impairment.
In routine clinical anesthesia practice, we found that after anesthesia and surgeries, the individuals with diabetes showed a significant higher incidence of POCD than those without diabetes. Thourani and colleagues [16] have reported that perioperative neurologic complications were more frequent among patients with diabetes than among patients without diabetes. It also have been reported that diabetes is a key pathogenic factor associated with the emergence of postoperative cognitive dysfunction for patients undergoing coronary artery bypass grafting [17].
However, there is little literature reporting the effects of inhaled anesthetic isoflurane on POCD in diabetic subjects. Thus, the present study planed to observe whether isoflurane anesthesia can induce cognitive impairment in a diabetic rat model which induced by a single injection of streptozotocin (STZ), and moreover, try to explain its possible pathogenic mechanisms.
Materials and methods
Animals
Forty-eight male Wistar rats weighing 220-300 g were purchased from Shanghai Animal Center, Shanghai, China. Six rats were housed per cage with food and water available ad libitum and maintained on a 12-h light/dark cycle (lights on at 07:00 AM). Rats were randomly divided into 4 groups (n = 12 each). Rats were intraperitoneally pretreated with either a single injection of saline or STZ (Sigma, St. Louis, MO, USA) at the dose of 60 mg/kg. One month later, rats in the control + isoflurane and STZ + isoflurane groups exposure to the isoflurane anesthesia in a chamber prefilled with 2% isoflurane in 100% O2. Subsequently, the rats were continuously exposed to 2% isoflurane in 100% O2 of 2 L/min for 2 h. After anesthesia, the mice were placed back into the home cage for recovery with 100% O2 of 2 L/min supply, whereas the rats in the respective control groups exposure to 100% O2 of 2 L/min for 2 h. Animal care was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and was approved by the Animal Use and Protection Committee of Soochow University.
Morris water maze test
Cognitive function was performed between 09:00 h and 15:00 h by using Morris water maze test system. According to a previous study [18], the maze, 80 cm deep and 100 cm in diameter, was divided into four quadrants of equal size on the monitor screen of a computer, filled to a depth of 30 cm with water. The water temperature was maintained at 23-24°C. The swimming paths of the rats were recorded by a video camera and analyzed by Videomot software (Huaibei Zhenghua Biologic Apparatus Facilities Co., Ltd., Huaibei, China). The trials were conducted for 4 consecutive days to observe escape latency and time spent in the quadrant of rats in the Morris water maze. Rats were placed in the maze from four random points of the tank and were allowed to search for the platform for 120 sec. However, if this was not achieved, the rat was gently placed on the platform and left for 20 sec. The escape latency and the proportion of time spent in the target quadrant were recorded.
Aβ1-42 measurement
Animals were sacrificed immediately by decapitation. Hippocampus tissues were harvested and homogenized in RIPA buffer (Beyotime P0013C, Haimen, Jiangsu, China) plus protease inhibitors. Protein concentrations were determined by using BCA method assay kit (Beyotime P0012S, Haimen, Jiangsu, China). After that, samples were centrifuged at 3000G at 4°C for 30 min to obtain the supernatants. Protein were separated by SDS-PAGE. The proteins were then transferred onto polyvinylidene difluoride membrane. After blocking with 5% nonfat milk, membranes were incubated with the primary antibodies: rabbit anti-Aβ (1:400, Cell Signaling Technology, Inc. Danvers, MA, USA). Subsequently, membranes were incubated for 1 hour at room temperature with secondary antibody of anti-rabbit HRP-conjugated IgG (1:20000, CWBIO, Beijing, China). Labeled protein was detected using chemiluminescence reagents (ECL; Amersham Bio-sciences, Little Chalfont, Buckinghamshire, UK) and the band intensity was analyzed (Image J software).
TNF-α and IL-1β measurement
TNF-α and IL-1β levels were determined by using ELISA kits. The ELISA was performed by adding 100 μl of each sample to wells in a 96-well plate of a commercially available rat ELISA kit (Wuhan Huamei Bioengineering Company, Wuhan, China). The samples were tested in duplicate. The ELISA was performed according to the manufacturer’s instructions. Microtiter plates (96-well flat-bottom) were coated for 24 h with the samples diluted 1:2 in sample diluent. The plates were then washed three times with sample diluent and a monoclonal anti-TNF-α and IL-1β antibodies diluted 1:1000 in sample diluent was added to each well and incubated for 3 h at room temperature. After washing, a peroxidase conjugated anti-rabbit antibody (diluted 1:1000) was added to each well and incubated at room temperature for 1 h. After addition of streptavidin-enzyme, substrate and stop solution, the amount of TNF-α and IL-1β were determined by absorbance in 450 nm respectively. The standard curve demonstrates a direct relationship between Optical Density (OD) and test concentrations. Total protein was measured by Lowry’s method using bovine serum albumin as a standard.
Superoxide dismutase (SOD) and malondialdehyde (MDA) measurement
SOD activity is measured by testing the capacity of pyrogallol to autoxidize. The inhibition of autoxidation of this compound occurs when SOD is present, and the enzymatic activity can be assayed indirectly using a temperature-controlled double-beam spectrophotometer at an absorbance of 420 nm. A 50% inhibition of pyrogallol autoxidation was defined as 1 U SOD. For testing MDA levels, the samples were mixed with 1 mL 10% trichloroacetic acid and 1 mL 0.67% thiobarbituric acid, and then were heated in a boiling water bath for 30 minutes. Malondialdehyde equivalents were determined in both tissue and submitochondrial particles of the rat brain using a spectrophotometer at an absorbance of 532 nm.
Blood glucose and leptin measurement
The blood samples from the rats were collected from the carotid artery by anaesthetizing with 10% chloral hydrate (0.4 ml/100 g, i.p.). Blood glucose levels were determined with a blood glucometer (One Touch UltraEasy, Johnson). Plasma levels of leptin were measured by Linco Research Rat leptin Radioimmunoassay (Linco Research, St Charles, MO, USA).
Statistical analysis
Data are expressed as mean ± SD. Statistical analyses were performed by one-way analysis of variance and post hoc analyses were performed using Fisher›s least significant difference tests. Statistical analyses were conducted using Statistical Product for Social Sciences (SPSS), version17.0 (SPSS, Inc., Chicago, IL, USA). Percentages of time spent in quadrant of rats in Morris water maze were evaluated by using X 2 tests. P < 0.05 was considered to indicate a statistically significant difference.
Results
Effects of isoflurane exposure on escape latency and percent of time spent in quadrant of rats in Morris water maze test and hippocampal Aβ levels
A single injection of STZ resulted in cognitive impairment verified by the performance of rats in the Morris water maze test. The results demonstrated that rats undergoing STZ administration significantly increased the escape latency as compared to the control group (Figure 1A), and decreased the percentage of time spent in the 4th quadrant (Figure 1B). For Aβ expression, a single injection of STZ significantly increased its expression in hippocampus (Figure 1C). Additionally, our results also showed that rats undergoing isoflurane anesthesia could further increased the escape latency and decreased the percentage of time spent in the 4th quadrant and increased hippocampal Aβ levels than those received a single intraperitoneal injection of STZ (Figure 1A-C).
Figure 1.
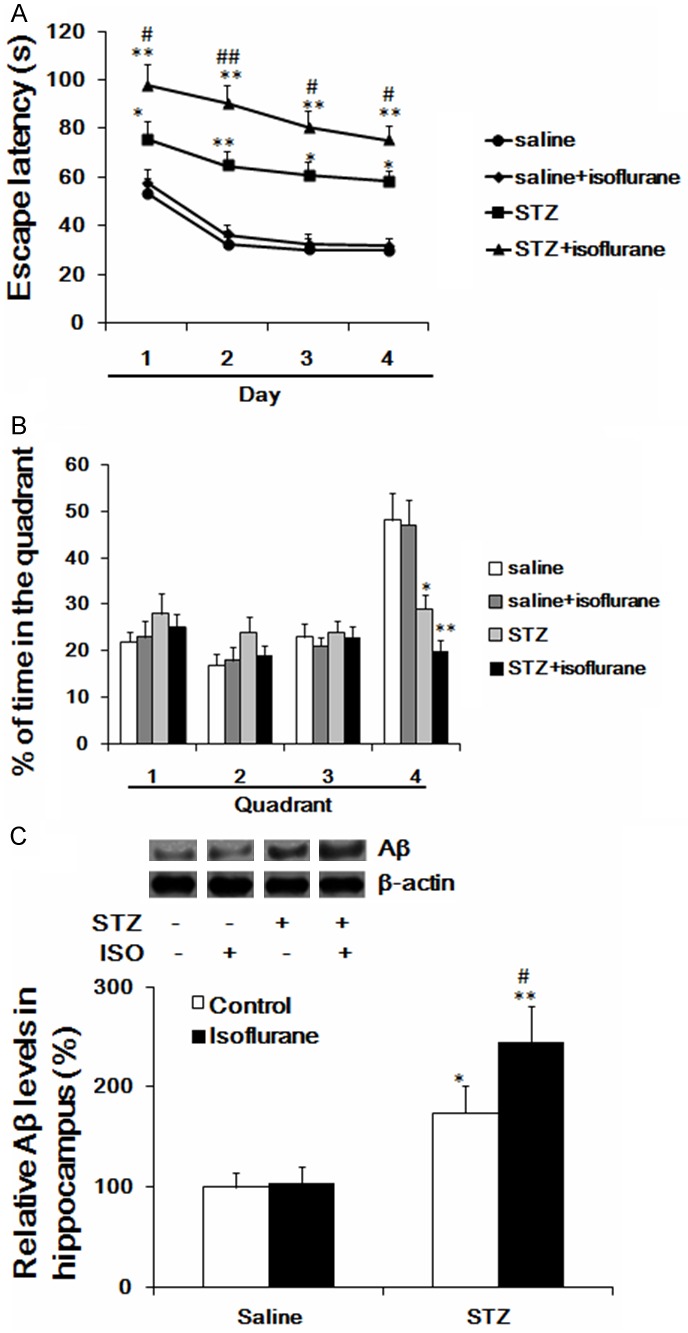
Effects of isoflurane anesthesia on escape latency and percent of time spent in quadrant of diabetic rats in Morris water maze test (A, B); (C) Effect of isoflurane anesthesia on the expression of Aβ in STZ-induced diabetic rat hippocampus. *p < 0.05 vs saline, **p < 0.01 vs saline, #p < 0.05 vs STZ, #p < 0.01 vs STZ. Aβ, amyloid-β; STZ, streptozotocin.
Isoflurane anesthesia increased the TNF-α and IL-1β levels in STZ-induced diabetic rat hippocampus
STZ administration could induce a significant increase of TNF-α and IL-1β levels in rat hippocampus. And more importantly, our results showed that isoflurane anesthesia significantly increased the TNF-α and IL-1β levels in STZ-induced diabetic rat hippocampus (Figure 2A and 2B).
Figure 2.

Effects of isoflurane anesthesia on the expression of the TNF-α (A) and IL-1β (B) levels in STZ-induced diabetic rat hippocampus. ***p < 0.001 vs saline, #p < 0.05 vs STZ. TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; STZ, streptozotocin.
Effects of isoflurane anesthesia on oxidative stress in STZ-induced diabetic rat hippocampus
A single intraperitoneal injection of STZ significantly increased SOD and MDA expression in rat hippocampus. Furthermore, isoflurane anesthesia showed a significant increase of TNF-α and IL-1β levels in STZ-induced diabetic rat hippocampus (Figure 3A and 3B).
Figure 3.

Effects of isoflurane anesthesia on SOD (A) and MDA (B) in STZ-induced diabetic rat hippocampus. **p < 0.01 vs saline, #p < 0.05 vs STZ. SOD, superoxide dismutase; MDA, malonyldialdehyde; STZ, streptozotocin.
Isoflurane anesthesia did not affect the blood glucose and leptin levels
Rats intraperitoneally administered with STZ significantly increased the blood glucose and leptin levels. However, isoflurane anesthesia has no effect on the blood glucose and leptin levels as compared to a single injection of STZ group (Figure 4A and 4B).
Figure 4.

Effects of isoflurane anesthesia on the blood glucose (A) and leptin (B) levels. ***p < 0.001 vs saline, #p < 0.05 vs STZ. STZ, streptozotocin.
Discussion
In the present study, the results showed that isoflurane anesthesia aggravated cognitive impairment in diabetic rat induced by a single intraperitoneal injection of STZ. Moreover, isoflurane anethesia further increased Aβ, TNF-α, IL-1β, SOD and MDA levels in hippocampus after STZ administration.
A large number of clinical studies indicating that isoflurane anesthesia can cause cognitive dysfunction [7]. However, in preclinical studies, observing the effect of isoflurane anesthesia on cognitive function is often using the elderly animal models. As we known, POCD often occurs in the elderly who are older than 65 years. Besides, our clinical experience told us the incidence of cognitive dysfunction in patients with diabetes is significantly higher than those without diabetes. Several studies validated our view that once a patient with diabetes-associated factors, the incidence of postoperative cognitive dysfunction is higher than the control [19,20]. Currently, researches about the effects of anesthetics on cognitive dysfunction in diabetes animal models have not been reported. In this study, we successfully constructed STZ-induced diabetic rat model, and observed that isoflurane anesthesia may further aggravate cognitive dysfunction. Although numerous studies have evidenced that cognitive impairment is caused primarily by intracerebroventricular injection of STZ [21-23]. We did not use this protocol since the intracerebroventricular injection approach may cause cognitive impairment. Additionally, intracerebroventricular injection of STZ is mainly used to construct Alzheimer’s disease model, but our study requires diabetic animal models. Different STZ injection approach may cause the emergence of cognitive impairment, but for investigating diabetes-related diseases, intraperitoneal injection of STZ is an appropriate method for administration.
At present, isoflurane has been reported it can cause a significant higher incidence of POCD [8]. Lin et al. [12] have showed that isoflurane exposure can cause cognitive dysfunction in rats, and also indicated that the pathogenesis is likely related to isoflurane-induced cell damage and excessive expression of inflammatory mediators in hippocampus. In addition, an in-vitro study by Xie et al [24] has concluded that isoflurane causing cognitive dysfunction is attributed to the activation of the apoptosis and overexpression of Aβ in the cultured human neuroglioma cells. Our data also showed the overexpressed Aβ and activated TNF-α and IL-1β in rat hippocampus after isoflurane anesthesia. However, Cibelli et al. [25] have displayed an inconsistent result, which showed that isoflurane could not induce the increase of IL-1β in hippocampus. We considered that the inconsistency may be related to different animal model since we chose a diabetic-associated cognitive impairment model.
Oxidative stress is defined as a cytological consequence, which caused by an imbalance between the production of free radicals and the ability to clean them [26]. Pratico et al [27] have demonstrated that increased brain oxidative stress may be used to predict the onset of cognitive impairment. Additionally, another preclinical study has suggested that oxidative damage to the rat synapse in the cerebral cortex and hippocampus may contribute to cognitive impairment [28]. On the other hand, increasing studies have evidenced that patients with cognitive impairment after treatment, their oxidative stress in peripheral blood showed a down-regulated trend [29]. Moreover, Sharma and colleagues [30] reported that intracerebroventricular injection of STZ could cause a significant increase of MDA expression in rat brain. Similarly, a subsequent study from this group has demonstrated that preventing STZ-induced oxidative stress is helpful to improve cognitive impairment [31]. In the present study, we observed that isoflurane anesthesia can attenuate cognitive impairment as well as over-activate hippocampal oxidative stress. Currently, studies regarding the pathogenesis of cognitive dysfunction caused by isoflurane is whether associated with oxidative stress have not been reported. Thus, our study reported for the first and implied that oxidative stress activation is likely related to the aggravation of cognitive impairment after isoflurane anesthesia in diabetic animal model.
It has been reported that TNF-α and IL-1β are two important pro-inflammatory cytokines, which may be involved in the pathogenesis of cognitive impairment [32]. Our previous study also has showed that chronic infection of LPS could induce cognitive impairment and associated with up-regulation of IL-1β in rat hippocampus [33]. In the present study, our results showed that isoflurane anesthesia increased hippocampal TNF-α and IL-1 levels in STZ-induced diabetic rat model. Moreover, Mina et al. [34] have demonstrated that IL-1β receptor antagonist could restore sepsis-induced cognitive impairment. Medeiros and colleagues [35] also have validated that TNF-α is probably implicated in the onset of cognitive impairment which induced by Aβ. Collectively, these results showed that significantly increased proinflammatory cytokines such as TNF-α and IL-1 may play critical roles in cognitive impairment.
As previously mentioned, intraperitoneal injection of STZ has been widely used to build the diabetic animal model by destroying pancreatic β cells. More attention has been attracted in the role of leptin in diabetes-related cognitive impairment. It is well documented that leptin is a potential cognitive enhancer in hippocampus [36]. In the present study, we observed that STZ elicit a significant decrease of leptin levels in blood, while isoflurane anesthesia did not further decrease the leptin levels. The similar phenomenon also appears in the result of blood glucose. However, Kain et al [37] found that leptin levels were decreased in patient peripheral blood after balanced anesthesia. Although we did not observe such changes in the expression of leptin after isoflurane as we expected, we considered that this study just used a single anesthetic isoflurane which could not fully simulate the clinical impact of anesthesia and surgical stress on the leptin expression. Similarly, our unpublished clinical data showed a significant reduction of peripheral serum leptin levels in individuals with cognitive dysfunction as compared with the control group. Future studies are required to ascertain the role of leptin in cognitive impairment.
In conclusion, the present study reported that isoflurane anesthesia can aggravate cognitive impairment in STZ-induced diabetic animal model, which is probably related to the activation of inflammatory response and oxidative stress in rat hippocampus.
Acknowledgements
We acknowledge Dr. Ri-Yue Jiang for her suggestive comments.
Disclosure of conflict of interest
None.
References
- 1.Bryson GL, Wyand A. Evidence-based clinical update: general anesthesia and the risk of delirium and postoperative cognitive dysfunction. Can J Anaesth. 2006;53:669–677. doi: 10.1007/BF03021625. [DOI] [PubMed] [Google Scholar]
- 2.Jungwirth B, Zieglgansberger W, Kochs E, Rammes G. Anesthesia and postoperative cognitive dysfunction (POCD) [J] . Mini Rev Med Chem. 2009;9:1568–1579. doi: 10.2174/138955709791012229. [DOI] [PubMed] [Google Scholar]
- 3.Dodds C, Allison J. Postoperative cognitive deficit in the elderly surgical patient. Br J Anaesth. 1998;81:449–462. doi: 10.1093/bja/81.3.449. [DOI] [PubMed] [Google Scholar]
- 4.Mandal PK, Schifilliti D, Mafrica F, Fodale V. Inhaled anesthesia and cognitive performance. Drugs Today (Barc) 2009;45:47. doi: 10.1358/dot.2009.45.1.1315075. [DOI] [PubMed] [Google Scholar]
- 5.Culley DJ, Xie Z, Crosby G. General anesthetic-induced neurotoxicity: an emerging problem for the young and old? Curr Opin Anaesthesiol. 2007;20:408–413. doi: 10.1097/ACO.0b013e3282efd18b. [DOI] [PubMed] [Google Scholar]
- 6.Wei H, Xie Z. Anesthesia, calcium homeostasis and Alzheimer’s disease. Curr Alzheimer Res. 2009;6:30. doi: 10.2174/156720509787313934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hudson AE, Hemmings HC. Are anaesthetics toxic to the brain? Br J Anaesth. 2011;107:30–37. doi: 10.1093/bja/aer122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perouansky M, Hemmings HC Jr. Neurotoxicity of general anesthetics: cause for concern? Anesthesiology. 2009;111:1365. doi: 10.1097/ALN.0b013e3181bf1d61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su D, Zhao Y, Wang B, Xu H, Li W, Chen J, Wang X. Isoflurane-induced spatial memory impairment in mice is prevented by the acetylcholinesterase inhibitor donepezil. PLoS One. 2011;6:e27632. doi: 10.1371/journal.pone.0027632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miki C, Ohmori Y, Yoshiyama S, Toiyama Y, Araki T, Uchida K, Kusunoki M. Factors predicting postoperative infectious complications and early induction of inflammatory mediators in ulcerative colitis patients. World J Surg. 2007;31:522–529. doi: 10.1007/s00268-006-0131-4. [DOI] [PubMed] [Google Scholar]
- 11.Peng L, Xu L, Ouyang W. Role of Peripheral Inflammatory Markers in Postoperative Cognitive Dysfunction (POCD): A Meta-Analysis. PLoS One. 2013;8:e79624. doi: 10.1371/journal.pone.0079624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin D, Zuo Z. Isoflurane induces hippocampal cell injury and cognitive impairments in adult rats. Neuropharmacology. 2011;61:1354–1359. doi: 10.1016/j.neuropharm.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.An LN, Yue Y, Guo WZ, Miao YL, Mi WD, Zhang H, Lei ZL, Han SJ, Dong L. Surgical trauma induces iron accumulation and oxidative stress in a rodent model of postoperative cognitive dysfunction. Biol Trace Elem Res. 2013;151:277–283. doi: 10.1007/s12011-012-9564-9. [DOI] [PubMed] [Google Scholar]
- 14.Wan Y, Xu J, Ma D, Zeng Y, Cibelli M, Maze M. Postoperative impairment of cognitive function in rats: a possible role for cytokine-mediated inflammation in the hippocampus. Anesthesiology. 2007;106:436–443. doi: 10.1097/00000542-200703000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, Butterfield DA, Markesbery WR. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–1156. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- 16.Thourani VH, Weintraub WS, Stein B, Gebhart SS, Craver JM, Jones EL, Guyton RA. Influence of diabetes mellitus on early and late outcome after coronary artery bypass grafting. Ann Thorac Surg. 1999;67:1045–1052. doi: 10.1016/s0003-4975(99)00143-5. [DOI] [PubMed] [Google Scholar]
- 17.Deaton C, Thourani V. Patients with type 2 diabetes undergoing coronary artery bypass graft surgery: predictors of outcomes. Eur J Cardiovasc Nurs. 2009;8:48–56. doi: 10.1016/j.ejcnurse.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Okada M, Amamoto T, Tomonaga M, Kawachi A, Yazawa K, Mine K, Fujiwara M. The chronic administration of docosahexaenoic acid reduces the spatial cognitive deficit following transient forebrain ischemia in rats. Neuroscience. 1996;71:17–25. doi: 10.1016/0306-4522(95)00427-0. [DOI] [PubMed] [Google Scholar]
- 19.Kadoi Y, Saito S, Fujita N, Goto F. Risk factors for cognitive dysfunction after coronary artery bypass graft surgery in patients with type 2 diabetes. J Thorac Cardiovasc Surg. 2005;129:576–583. doi: 10.1016/j.jtcvs.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 20.Gregg EW, Yaffe K, Cauley JA, Rolka DB, Blackwell TL, Narayan KM, Cummings SR. Is diabetes associated with cognitive impairment and cognitive decline among older women? Arch Intern Med. 2000;160:174. doi: 10.1001/archinte.160.2.174. [DOI] [PubMed] [Google Scholar]
- 21.Tiwari V, Kuhad A, Bishnoi M, Chopra K. Chronic treatment with tocotrienol, an isoform of vitamin E, prevents intracerebroventricular streptozotocin-induced cognitive impairment and oxidative-nitrosative stress in rats. Pharmacol Biochem Behav. 2009;93:183–189. doi: 10.1016/j.pbb.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Sharma M, Gupta YK. Chronic treatment with trans resveratrol prevents intracerebroventricular streptozotocin induced cognitive impairment and oxidative stress in rats. Life Sci. 2002;71:2489–2498. doi: 10.1016/s0024-3205(02)02083-0. [DOI] [PubMed] [Google Scholar]
- 23.Sharma M, Gupta YK. Effect of alpha lipoic acid on intracerebroventricular streptozotocin model of cognitive impairment in rats. Eur Neuropsychopharmacol. 2003;13:241–247. doi: 10.1016/s0924-977x(03)00008-7. [DOI] [PubMed] [Google Scholar]
- 24.Xie Z, Dong Y, Maeda U, Zhang G, Zhang B, Moir RD, Frosch MP, Crosby G, Tanzi RE. The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid β protein levels. Anesthesiology. 2006;104:988–994. doi: 10.1097/00000542-200605000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, Feldmann M, Takata M, Lever IJ, Nanchahal J, Fanselow MS, Maze M. Role of interleukin-1β in postoperative cognitive dysfunction. Ann Neurol. 2010;68:360–368. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith JV, Luo Y. Elevation of oxidative free radicals in Alzheimer’s disease models can be attenuated by Ginkgo biloba extract EGb 761. J Alzheimers Dis. 2003;5:287–300. doi: 10.3233/jad-2003-5404. [DOI] [PubMed] [Google Scholar]
- 27.Pratico D, Clark CM, Liun F, Rokach J, Lee VY, Trojanowski JQ. Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer disease. Arch Neurol. 2002;59:972. doi: 10.1001/archneur.59.6.972. [DOI] [PubMed] [Google Scholar]
- 28.Fukui K, Omoi NO, Hayasaka T, Shinnkai T, Suzuki S, Abe K, Urano S. Cognitive impairment of rats caused by oxidative stress and aging, and its prevention by vitamin E. Ann N Y Acad Sci. 2002;959:275–284. doi: 10.1111/j.1749-6632.2002.tb02099.x. [DOI] [PubMed] [Google Scholar]
- 29.Padurariu M, Ciobica A, Hritcu L, Stoica B, Bild W, Stefanescu C. Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and Alzheimer’s disease. Neurosci Lett. 2010;469:6–10. doi: 10.1016/j.neulet.2009.11.033. [DOI] [PubMed] [Google Scholar]
- 30.Sharma M, Gupta YK. Intracerebroventricular injection of streptozotocin in rats produces both oxidative stress in the brain and cognitive impairment. Life Sci. 2001;68:1021–1029. doi: 10.1016/s0024-3205(00)01005-5. [DOI] [PubMed] [Google Scholar]
- 31.Sharma M, Gupta YK. Chronic treatment with trans resveratrol prevents intracerebroventricular streptozotocin induced cognitive impairment and oxidative stress in rats. Life Sci. 2002;71:2489–2498. doi: 10.1016/s0024-3205(02)02083-0. [DOI] [PubMed] [Google Scholar]
- 32.Paganelli R, Di Iorio A, Patricelli L, Ripani F, Sparvieri E, Faricelli R, Iarlori C, Porreca E, Di Gioacchino M, Abate G. Proinflammatory cytokines in sera of elderly patients with dementia: levels in vascular injury are higher than those of mild-moderate Alzheimer’s disease patients. Exp Gerontol. 2002;37:257–263. doi: 10.1016/s0531-5565(01)00191-7. [DOI] [PubMed] [Google Scholar]
- 33.Zhu B, Wang ZG, Ding J, Liu N, Wang DM, Ding LC, Yang C. Chronic lipopolysaccharide exposure induces cognitive dysfunction without affecting BDNF expression in the rat hippocampus. Exp Ther Med. 2014;7:750–754. doi: 10.3892/etm.2014.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mina F, Comim CM, Dominguini D, Cassol OJ Jr, Dall Igna DM, Ferreira GK, Silva MC, Galant LS, Streck EL, Quevedo J, Dal-Pizzol F. Il1-β Involvement in Cognitive Impairment after Sepsis. Mol Neurobiol. 2014;49:1069–76. doi: 10.1007/s12035-013-8581-9. [DOI] [PubMed] [Google Scholar]
- 35.Medeiros R, Figueiredo CP, Pandolfo P, Duarte FS, Prediger RD, Passos GF, Calixto JB. The role of TNF-α signaling pathway on COX-2 upregulation and cognitive decline induced by β-amyloid peptide. Behav Brain Res. 2010;209:165–173. doi: 10.1016/j.bbr.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 36.Harvey J, Shanley LJ, O’Malley D, Irving AJ. Leptin: a potential cognitive enhancer? Biochem Soc Trans. 2005;33:1029–1032. doi: 10.1042/BST20051029. [DOI] [PubMed] [Google Scholar]
- 37.Kain ZN, Zimolo Z, Heninger G. Leptin and the perioperative neuroendocrinological stress response. J Clin Endocrinol Metab. 1999;84:2438–2442. doi: 10.1210/jcem.84.7.5850. [DOI] [PubMed] [Google Scholar]


