Abstract
Matrine, one of the Chinese herbal medicine, has anti-tumor activity in a variety of tumor cells. However, its anti-tumor activity in human medulloblastoma remains unclear. The aim of this study was to investigate the presence and mechanism of matrine-induced proliferation and apoptosis in human medulloblastoma D341 cells. D341 cells were divided into experimental groups in which matrine were added at different concentrations and a control group under the same conditions without matrine applied. D341 cell proliferation was analyzed using a cell counting kit-8 assay, apoptosis was detected by annexin-V FITC/PI double-staining, and the expression of Bax, Bcl-2, caspase-3 and caspase-9 was detected by Western blotting. Results showed that matrine significantly inhibited the proliferation of D341 cells. The cells displayed more and larger cytoplasmic vacuoles, and formed apoptotic bodies after matrine treatment. Western blotting analysis showed that expressions of Bax, caspase-3 and caspase-9 increased, while that of Bcl-2 decreased as the drug concentration gradually increased. The study suggests that matrine could induce human medulloblastoma D341 cells apoptosis and inhibit the cells proliferation in vitro by activating Bax, caspase-3 and caspase-9 and reducing Bcl-2 expression.
Keywords: Apoptosis, Bax, Bcl-2, caspase-3, caspase-9, human medulloblastoma, matrine
Introduction
In 1925, Bailey and Cushing first proposed the diagnosis of medulloblastoma. Since then, people have widely studied on the tissue origin, structure and biological characteristics of the tumor. Medulloblastoma is a highly aggressive tumor and its clinical features are rapid growth, short clinical course, and rapid progression. The tumor cells can transfer along the cerebrospinal fluid channel. The tumor cells are not sensitive to conventional radiotherapy and chemotherapy. It has the tendency to recurrence and the prognosis is very poor. Although the surgical technique of neurosurgeon and the use of sophisticated operation support system have made continuous progress, the treatment effect is still poor and the prognosis is bad in a portion of patients [1]. There is still a lack of effective treatment means and reliable prognostic indicators on medulloblastoma at present. Therefore, it is extremely important to find an effective anti-tumor drug for medulloblastoma [2].
Matrine, one of the main alkaloid components extracted from Sophora, bitter beans, broad beans, and other leguminous Sophora root plants, belongs to tetracyclic quinolizidine alkaloids. Recent evidence indicates that matrine plays an important role in the treatment of tumors, induces the apoptosis of cells and inhibits the cell proliferation [3]. However, few studies on the effect of matrine on medulloblastoma have been reported, and the molecular mechanism underlying the antitumor function of matrine remains unclear. Therefore, we studied the proliferation and apoptosis effects of matrine on D341 medulloblastoma cells and analyzed the expression of Bax, Bcl-2, caspase-3, caspase-9 after matrine treatment, which may provide the important biological basis for the pathogenesis as well as the drug treatment of human medulloblastoma.
Materials and methods
Materials
Matrine was provided by Ningbo Team Pharm Co., Ltd., Ningbo, China. D341 cells were provided by the Peking Union Medical College Hospital Cell Library, Beijing, China. Apoptosis detection kits, Bax, Bcl-2, caspase-3, caspase-9 antibodies were provided by Shanghai BlueGene Biotech Co., Ltd., Shanghai, China. Transmission electron microscope (TEM; JEM-1011; JEOL, Tokyo, Japan) was used in this study.
Cell culture
The D341 cells were cultured in RPMI-1640 medium supplemented with 1% penicillin-streptomycin and 10% fetal bovine serum at 37°C with 5% CO2.
Cell ultrastructure observation
D341 cells cultured in vitro were divided into experimental and control groups. Cells in each experimental group were treated with matrine 2.0 mg/mL, while those in the control group were not treated. Cells were harvested by trypsinization with matrine for 24, 48 or 72 h. The cells were then fixed with 2.5% glutaraldehyde for 48 h, post-fixed in 1% osmium tetraoxide for 90 min, dehydrated by gradient ethanol-acetone, and embedded in paraffin. The blocks were cut into ultra-thin sections with a microtome and then stained with saturated uranyl acetate and lead citrate. The ultrastructure of the cells was then examined by TEM.
In vitro proliferation assay by a cell counting kit-8 (CCK-8)
D341 cells were trypsinized to a single cell suspension with 0.25% trypsin adjusting the cell density to 5 × 104/mL. A cell suspension aliquot (100 μL) was added to each well of a 96-well cell culture plate and cultured 5% CO2, at 37°C for 24 h. Matrine was diluted to the desired concentration (0.5 mg/mL, 1.0 mg/mL, 1.5 mg/mL, and 2.0 mg/mL) with culture medium and 200 μL was added to each well. Cell culture was continued for 24, 48, and 72 h at 37°C, 5% CO2 and assessed using the CCK-8. The absorbance value of each well was determined at 450 nm using a microplate reader and inhibition rates were calculated. The experiment was repeated for 3 times.
Cells apoptosis analysis by annexin-V FITC/PI double-staining
D341 cells at the logarithmic growth phase were seeded into six-well plates. Matrine was diluted to the desired concentration (0.5 mg/mL, 1.0 mg/mL, 1.5 mg/mL, and 2.0 mg/mL) with culture medium and added to each well. The cells were cultured at 37°C, 5% CO2 for 24, 48, or 72 h. The cells (5 × 105) were collected, centrifuged at 2,000 rpm for 5 min, and re-suspended in 500 μL of binding buffer. Annexin V-FITC (5 μL) was added and mixed. After the addition of 5 μL of propidium iodide (PI) staining solution, the cells were incubated for 5-15 min in the dark at room temperature and analyzed using a flow cytometer to detect cell apoptosis.
Western blot analysis of Bax, Bcl-2, caspase-3 and caspase-9 expression
D341 cells at the logarithmic growth phase were seeded at a density of 2 × 105/mL in 24-well plates and divided into four groups after cell attachment. In the matrine groups, matrine was added at concentrations of 0.5 mg/mL, 1.0 mg/mL, 1.5 mg/mL, and 2.0 mg/mL, while cells in the control group were cultured under the same conditions with no matrine. Cell culture was continued for 24, 48, or 72 h at 37°C, 5% CO2. Cells were lysed on ice with 100 mL of pre-cooled cell lysis buffer for 30 min and the total protein was isolated. The protein concentration was determined by the bicinchonininc acid method. Proteins were separated by electrophoresis with sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred onto polyvinylidene difluoride membranes.
After blocking with 5% non-fat dry milk or bovine serum albumin in TBST (20 mM Tris-HCl, 150 mM NaCl, and 0.05% Tween-20) for 1 h at room temperature, the membranes were incubated with primary antibodies [Bax (1:100), Bcl-2 (1:100), caspase-3 (1:1,000), caspase-9 (1:500), β-actin (1:1,000)] overnight at 4°C, followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit secondary antibodies (1:5,000) for 2 h at room temperature. The membranes were washed 3 times with TBST for 10 min each. The specific protein bands were developed using a chemiluminescent substrate and imaged using a gel scanner. Protein levels were normalized to β-actin as a reference.
Statistical analysis
All results were analyzed with SPSS 17.0 software. (SPSS Inc. 233 South Wacker Drive, 11th Floor, Chicago, IL 60606-6412) One-way analysis of variance (ANOVA) was used for the statistical analysis between the experimental and control groups.
Results
D341 cell ultrastructure observation with TEM after matrine treatment
The ultrastructural morphology of the D341 cells was observed by TEM after exposure to matrine (2.0 mg/mL) for 24, 48, and 72 h. Control cells without matrine exposure exhibited normal ultrastructural morphology. In contrast, after incubation with matrine, cells underwent apoptosis, characterized by cell shrinkage, plasma membra ne blebbing, chromatin condensation with margination of chromatin to the nuclear membrane, and the formation of apoptotic bodies (Figure 1).
Figure 1.
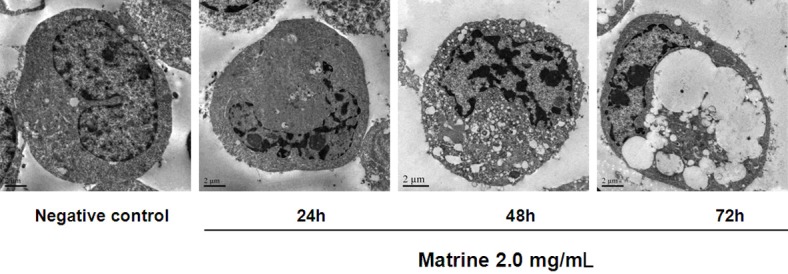
Cell ultrastructure observation with TEM.
Control cells exhibited normal ultrastructural morphology with membrane integrity and even chromatin distribution. Early apoptotic cells with acoustic cavitation bubble structure, chromatin condensation, and marginalization were observed after 24 h of drug incubation. After 48 h treatment with matrine, nuclear chromatin condensation and more vacuoles in the cytoplasm were observed. After 72 h treatment with matrine, cells exhibited apoptotic characteristics with obvious karyopyknosis, significantly increased and larger cytoplasmic vacuoles, and apoptotic bodies.
Inhibitory effects of matrine on D341 cell proliferation
Cell proliferation was assessed by the CCK-8 assay using WST-8 (2-[2-methoxy-4-nitrophenyl]-3-[4-nitrophenyl]-5-[2,4-disulfophenyl]-2H-tetrazolium, monosodium salt). WST-8 is bio-reduced by dehydrogenase in cells to give a yellow-colored product (formazan), which is water-soluble in the tissue culture medium. The amount of the formazan dye generated by dehydrogenase activity in cells is directly proportional to the number of living cells. The absorbance at wavelength 450 nm was measured with a spectrophotometer. The CCK-8 method showed the inhibitory effects of matrine on D341 cell proliferation.
Under the condition with same treatment period, different concentrations of matrine have different degrees of inhibitory effects on cell proliferation. The proliferation inhibition rate increased with increasing drug concentrations under the same time. The proliferation rate gradually increased in a dose-dependent manner along with increasing drug concentrations. One-way ANOVA analysis showed a significant difference between the 24 h to 72 h groups (P<0.05), and no significant difference between the 48 h to 72 h or 24 h to 48 h groups (P>0.05). The inhibitory effect of matrine on cell proliferation increased with incubation time under the condition with same drug concentration. The inhibitory effects were more obvious at matrine concentrations of 1.5 mg/mL and 2.0 mg/mL (Table 1).
Table 1.
Proliferation of D341 cells by Cell Counting Kit-8 assay after matrine treatment
| Group | Inhibitory rate % (X̅ ± S, n = 3) | ||
|---|---|---|---|
|
| |||
| 24 h | 48 h | 72 h | |
| Negative control | __ | __ | __ |
| Matrine 0.5 mg/mL | 10.496 ± 0.11 | 11.598 ± 0.062 | 12.698 ± 0.114 |
| Matrine 1.0 mg/mL | 21.297 ± 0.109 | 27.596 ± 0.24 | 28.296 ± 0.15 |
| Matrine 1.5 mg/mL | 31.597 ± 0.364 | 36.995 ± 0.239 | 47.496 ± 0.047 |
| Matrine 2.0 mg/mL | 37.998 ± 0.26 | 47.198 ± 0.011 | 59.3 ± 0.206 |
Apoptosis induction effects of matrine on D341 cells
To further confirm the apoptosis induction effects of matrine on D341 cells, Annexin V and PI double-staining was performed and detected using flow cytometry following matrine treatment for 24, 48, or 72 h (Table 2 and Figure 2). Annexin V and PI double-staining can distinguish among live, apoptotic, and necrotic cells. The lower left (LL) quadrants showed the live cells (FITC−/PI−), the lower right (LR) quadrants represent the early apoptotic cells (FITC+/PI−), and the upper right (UR) quadrants represent the terminal apoptotic cells (FITC+/PI+). These results showed matrine induced apoptosis in D341 cells and that the apoptotic rate increased with increasing matrine concentrations. The apoptotic rate significantly increased in the presence of matrine at different concentration compared with control group (P<0.01).
Table 2.
Cell apoptosis analysis by Annexin-V FITC/PI double-staining
| Group | Apoptosis (%) | ||
|---|---|---|---|
|
| |||
| 24 h | 48 h | 72 h | |
| Negative control | 3.31 | 5.09 | 5.74 |
| 0.5 mg/mL | 23.03 | 24.20 | 25.33 |
| 1.0 mg/mL | 27.38 | 31.24 | 38.33 |
| 1.5 mg/mL | 31.91 | 38.22 | 48.30 |
| 2.0 mg/mL | 36.88 | 44.90 | 56.10 |
Figure 2.
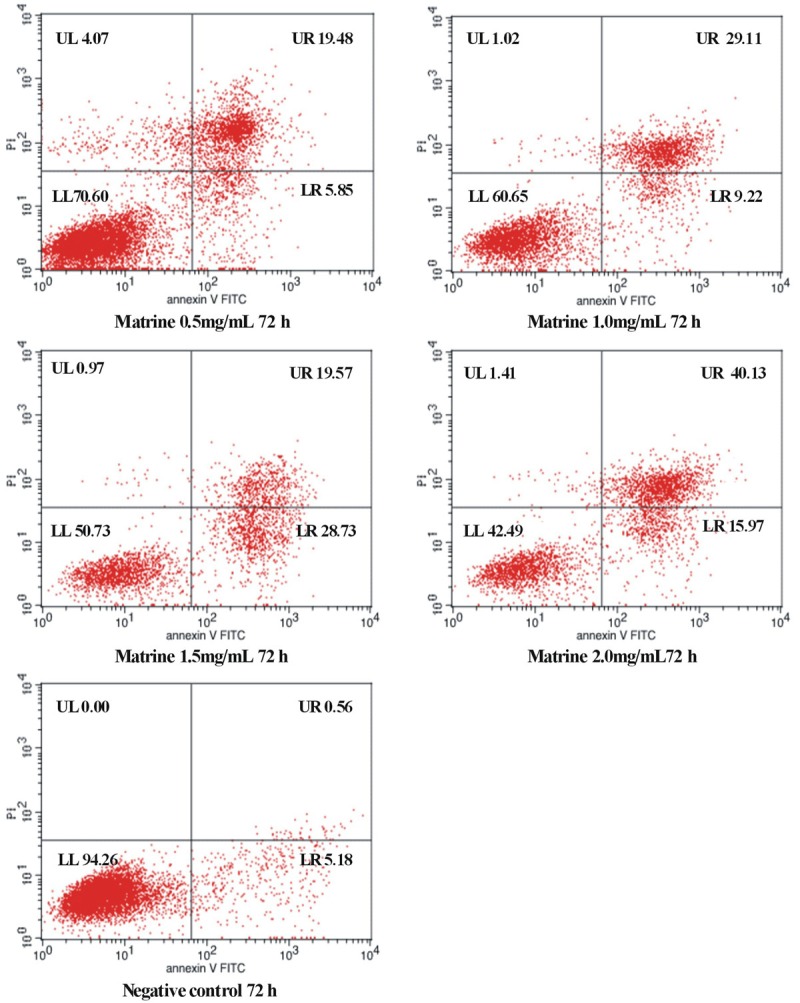
D341 cell apoptosis analysis by flow cytometry after treatment with matrine (72 h).
Expression of Bax, Bcl-2, caspase-3 and caspase-9
To further study the mechanism of matrine on D341 cells proliferation and apoptosis, Bax and Bcl-2 expressions were analyzed by western blotting after treatment with matrine at different concentrations for 24 h, 48 h, 72 h (Figure 3). Caspase-3 and Caspase-9 expressions were analyzed by western blotting after treatment with matrine at different concentrations for 48 h (Figure 4). The results showed that the expression of Bax, Caspase-3 and Caspase-9 gradually increased while that of Bcl-2 gradually decreased as the drug concentration increased (P<0.05). The expression of Bcl-2 gradually decreased as the time prolonged (P<0.01).
Figure 3.
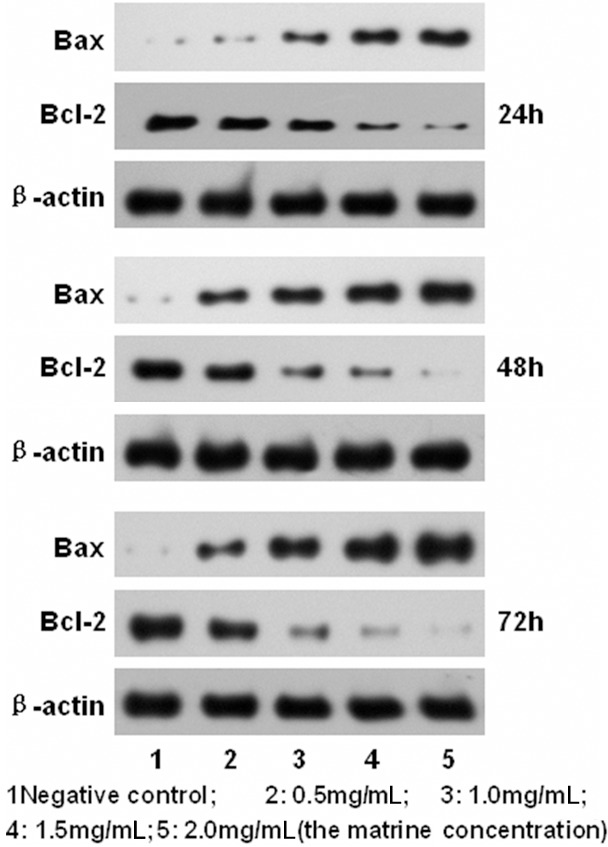
Western blot analysis of Bax, Bcl-2, β-actin expression.
Figure 4.
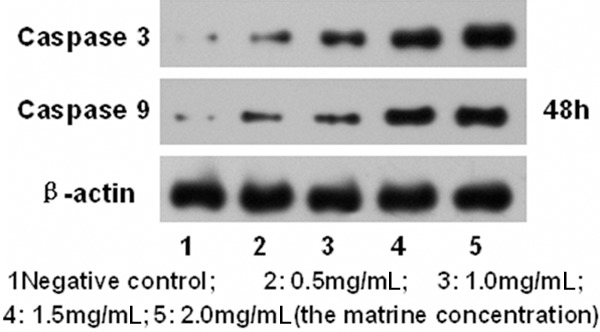
Western blot analysis of Caspase 3, Caspase 9 expression.
Discussion
The apoptosis is a normal and programmed cell death and is a clearing means of abnormal cells or senescent cells. Inducing the apoptosis of tumor cells is one of the most important strategy to discover anti-tumour drugs based on apoptosis mechanism. The apoptosis is a very important way of the Chinese herbal medicine to inhibit the proliferation of tumor cells.
Matrine, one of the main alkaloid components extracted from Sophora, bitter beans, broad beans, and other leguminous Sophora root plants, belongs to tetracyclic quinolizidine alkaloids. It was noted by Shen Nong’s Herbal Classic that Sophora, which tastes bitter, is mainly used for treatment of heartburn, abdominal masses, and jaundice. It has been shown to possess various anti-inflammatory, antiviral, hepatoprotective, and antiarrhythmic properties [4,5]. Recent evidence indicates that matrine plays an important role in the treatment of tumors without obvious toxicity and side effects. The anti-tumor activity of matrine is mainly reflected in the inducing the differentiation of tumor cells, promoting the apoptosis of tumor cells, inhibiting the proliferation and adhesion and metastasis of tumor cells, and modulating the host immune function [6,7].
Interest has been generated in the apoptosis induction effect of matrine. It has been reported [8] that matrine inhibited proliferation and induced apoptosis of human small cell lung cancer cells in a dose-dependent manner. Matrine-induced apoptosis and inhibition of proliferation may be an attractive strategy for enhancing the antitumor potential of matrine in the human small cell lung cancer cells. And it may be by activating Bax and reducing Bcl-2 expression. Matrine also inhibited proliferation and induced apoptosis in human rhabdomyosarcoma cells [9]. And it related to the cyclin D1 and Survivin genes. In addition, matrine significantly inhibited cell proliferation and promoted apoptosis in human glioma cells via inducing the upregulation of caspase-3 in the death receptor and mitochondrial pathways [10]. It has been reported that matrine inhibited the gastric cancer cells and inducing the apoptosis of tumor cells may be play an important role [11].
Matrine inhibiting proliferation and promoting apoptosis of medulloblastoma cells has rarely been reported. In this study, we studied the apoptotic effects of matrine on D341 medulloblastoma cells and analyzed the expressions of Bax, Bcl-2, caspase-3 and caspase-9 after matrine treatment. We found that matrine inhibited proliferation and induced apoptosis of D341 cells in vitro. The inhibition rate of D341 cell proliferation gradually increased with the increase of drug concentration and showed a dose-dependent manner under the same exposure time to matrine.
We further studied the changes of the apoptosis related genes such as Bax, caspase-3 and caspase-9 in the process of apoptosis. We found that the expressions of Bax, caspase-3 and caspase-9 gradually increased while that of Bcl-2 gradually decreased along with increasing matrine concentrations. The Bcl-2 family plays an important role in the mitochondrial signal transduction pathway of the apoptosis [12]. The Bcl-2 protein usually is the high expression and plays an important role in the inhibition of cell apoptosis in tumor cells. The vitro studies found that the high expression of Bcl-2 was the main reason for chemotherapy failure and inhibited the apoptosis of tumor cells induced by chemotherapy or radiotherapy [13]. It had been found that controlling the expression of Bcl-2 protein would be reduced, Bax protein expression increased, Bcl-2/Bax decreased, Bcl-2 and Bax of two heterologous dimers reduced, and Bcl-2 and Bax of homodimer with two increased in the process of cell apoptosis.
It is currently considered that the mechanism of Bcl-2 inhibiting cell apoptosis would be related to counteract the apoptosis gene of Bax, control the cytochrome C to release from mitochondria to cytoplasm, stop cytochrome C to activate the Caspaseprotease [14]. Bcl-2 can promote the glutathione to enter the nucleus, change the oxidation reduction reaction in the nucleus, inhibit the Caspase protease activity and cell apoptosis. We through qualitative and quantitative methods in this study to conclude that matrine could increase the expression of Bax and decrease the expression of Bcl-2, matrine could be induced the apoptosis of human medulloblastoma D341 cells and inhibited the proliferation of tumor cells by regulating the expression of Bcl-2 and Bax.
Caspases are crucial mediators of programmed cell death (apoptosis). It had been found that caspase-3 is a frequently activated death protease among them that catalyzes the specific cleavage of many key cellular proteins [15,16]. It was reported that matrine increased the expression of caspase-3 in gastric cancer cells [17], and induced apoptosis of rat osteosarcoma MG-63 cells activated by caspase-3 and caspase-9 [18]. Consistent with these observations, our studies also showed that matrine induced apoptosis in D341 cells via activation of crucial caspases such as caspase-3 and caspase-9. These results indicated that caspase-3 activation is responsible for matrine-induced D341 cell apoptosis.
It was observed by transmission electron microscope that after incubation with matrine, cells underwent apoptosis, characterized by cell shrinkage, plasma membrane blebbing and cytoplasmic vacuolar degeneration, chromatin condensation with margination of chromatin to the nuclear membrane, and the formation of apoptotic bodies. Some of these vacuoles are expansion of the smooth endoplasmic reticulum or lipid droplets. These endoplasmic reticulum are a response to drugs. The lipid droplets are due to the interference caused by drugs or destruction of fat metabolism. Therefore, matrine can inhibit apoptosis and promote proliferation of human medulloblastoma cells obviously, and it may be a potential anticancer drug. With the further experiments in vivo including drug blood barrier test and intratumoral therapy, we hope eventually to provide experimental evidence in the clinical trial of the drug.
Acknowledgements
This study was supported by the Project of Traditional Chinese Medicine Science and Technology Plan of Zhejiang Province (No2013ZA133).
Disclosure of conflict of interest
None.
References
- 1.De Braganca KC, Packer RJ. Treatment Options for Medulloblastoma and CNS Primitive Neuroectodermal Tumor (PNET) Curr Treat Options Neurol. 2013;15:593–606. doi: 10.1007/s11940-013-0255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kostaras X, Easaw JC. Management of recurrent medulloblastoma in adult patients: a systematic review and recommendations. J Neurooncol. 2013;115:1–8. doi: 10.1007/s11060-013-1206-3. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, Zhang J, Wang Y, Xing R, Yi C, Zhu H, Chen X, Guo J, Guo W, Li W, Wu L, Lu Y, Liu S. Matrine, a novel autophagy inhibitor, blocks trafficking and the proteolytic activation of lysosomal proteases. Carcinogenesis. 2013;34:128–138. doi: 10.1093/carcin/bgs295. [DOI] [PubMed] [Google Scholar]
- 4.Huang WC, Chan CC, Wu SJ, Chen LC, Shen JJ, Kuo ML, Chen MC, Liou CJ. Matrine attenuates allergic airway inflammation and eosinophil infiltration by suppressing eotaxin and Th2 cytokine production in asthmatic mice. J Ethnopharmacol. 2014;151:470–477. doi: 10.1016/j.jep.2013.10.065. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Chu W, Liu J, Xue X, Lu Y, Shan H, Yang B. Antiarrhythmic properties of long-term treatment with matrine in arrhythmic rat induced by coronary ligation. Biol Pharm Bull. 2009;32:1521–1526. doi: 10.1248/bpb.32.1521. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Li Y, Chen X, Liu T, Chen Y, He W, Zhang Q, Liu S. Autophagy is involved in anticancer effects of matrine on SGC-7901 human gastric cancer cells. Oncol Rep. 2011;26:115–124. doi: 10.3892/or.2011.1277. [DOI] [PubMed] [Google Scholar]
- 7.Yang ZY, Wang L, Hou YX, Wang XB. Effects of matrine on oval cellmediated liver regeneration and expression of RBPJκ and HES1. Mol Med Rep. 2013;7:1533–1538. doi: 10.3892/mmr.2013.1398. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Zhang H, Yu P, Liu Q, Liu K, Duan H, Luan G, Yagasaki K, Zhang G. Effects of matrine against the growth of human lung cancer and hepatoma cells as well as lung cancer cell migration. Cytotechnology. 2009;59:191–200. doi: 10.1007/s10616-009-9211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo L, Xue TY, Xu W. Effects of matrine on the proliferation and apoptosis of human rhabdomyosarcoma RD cells. Zhongguo Dang Dai Er Ke Za Zhi. 2012;14:780–784. [PubMed] [Google Scholar]
- 10.Zhang S, Qi J, Sun L, Cheng B, Pan S, Zhou M, Sun X. Matrine induces programmed cell death and regulates expression of relevant genes based on PCR array analysis in C6 glioma cells. Mol Biol Rep. 2009;36:791–799. doi: 10.1007/s11033-008-9247-y. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Zhang J, Ma H, Chen X, Liu T, Jiao Z, He W, Wang F, Liu X, Zeng X. Protective role of autophagy in matrineinduced gastric cancer cell death. Int J Oncol. 2013;42:1417–1426. doi: 10.3892/ijo.2013.1817. [DOI] [PubMed] [Google Scholar]
- 12.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2013;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 13.Keshavarz M, Emamghoreishi M, Nekooeian AA, J Warsh J, Zare HR. Increased bcl-2 Protein Levels in Rat Primary Astrocyte Culture Following Chronic Lithium Treatment. Iran J Med Sci. 2013;38:255–262. [PMC free article] [PubMed] [Google Scholar]
- 14.Morales-Cano D, Calviño E, Rubio V, Herráez A, Sancho P, Tejedor MC, Diez JC. Apoptosis induced by paclitaxel via Bcl-2, Bax and caspases 3 and 9 activation in NB4 human leukaemia cells is not modulated by ERK inhibition. Exp Toxicol Pathol. 2013;65:1101–1108. doi: 10.1016/j.etp.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Fiandalo MV, Kyprianou N. Caspase control: protagonists of cancer cell apoptosis. Exp Oncol. 2012;34:165–175. [PMC free article] [PubMed] [Google Scholar]
- 16.Rodrigue-Gervais IG, Saleh M. Caspases and immunity in a deadly grip. Trends Immunol. 2013;34:41–49. doi: 10.1016/j.it.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Dai ZJ, Gao J, Ji ZZ, Wang XJ, Ren HT, Liu XX, Wu WY, Kang HF, Guan HT. Matrine induces apoptosis in gastric carcinoma cells via alteration of Fas/FasL and activation of caspase-3. J Ethnopharmacol. 2009;123:91–96. doi: 10.1016/j.jep.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Liang CZ, Zhang JK, Shi Z, Liu B, Shen CQ, Tao HM. Matrine induces caspase-dependent apoptosis in human osteosarcoma cells in vitro and in vivo through the upregulation of Bax and Fas/FasL and downregulation of Bcl-2. Cancer Chemother Pharmacol. 2012;69:317–331. doi: 10.1007/s00280-011-1699-4. [DOI] [PubMed] [Google Scholar]


