Abstract
Objective: To investigate the therapeutic effects of a novel fluid resuscitation protocol (early fluid resuscitation plus 2% hydrogen inhalation) on acute kidney injury during septic shock induced by lipopolysaccharide in rats. Methods: Sixty male Wistar rats were randomly divided into four groups (n = 15 per group): control group (C), septic shock group (S), septic shock with early fluid resuscitation group (R), and septic shock with early fluid resuscitation plus 2% hydrogen inhalation group (R+R+H2). The rats were ventilated, and a 2% hydrogen mixture was used in Group R+H2. Lipopolysaccharide (10 mg/kg) was administered to establish the septic shock model in rats and fluid resuscitation was performed in Groups R and R+R+H2. Results: Fluid resuscitation with 2% hydrogen inhalation decreased serum creatinine, blood urea nitrogen, and neutrophil gelatinase-associated lipocalin. It also reduced oxidative stress injury and decreased renal tumor necrosis factor-α and interleukin-6 levels compared with fluid resuscitation alone. Conclusion: Early fluid resuscitation plus 2% hydrogen inhalation provided more protection against AKI during septic shock.
Keywords: Septic shock, acute kidney injury, fluid resuscitation, hydrogen inhalation, oxidative stress, inflammation
Introduction
Despite substantial advances in therapy for septic shock and understanding its pathogenesis, it is still associated with high morbidity and mortality and often accompanied by multiple organ dysfunctions [1,2]. Early fluid resuscitation is an important and necessary means of reversing the reduction of circulating blood volume and reducing the incidence of multiple organ dysfunction syndrome (MODS). Numerous clinical trials have shown that early fluid resuscitation can significantly improve the prognosis of septic shock patients [3-5]. However, fluid resuscitation itself may have untoward effects, including the possible risk of liquid overload, which can lead to interstitial edema and organ dysfunction [6,7]. Managing fluid resuscitation is important to improve the prognosis of patients with septic shock.
The incidence of acute kidney injury (AKI) is nearly 65% in critically ill patients [8,9] and can aggravate the condition of patients with septic shock, resulting in higher mortality [10-12]. Therefore, protecting renal function in patients with septic shock is important. So far, early treatment for AKI induced by septic shock is still liquid resuscitation. But during this protocol, there is the risk of liquid overload and aggravation of kidney injury. So a novel liquid resuscitation protocol is in urgent need.
The hydrogen molecule is a selective antioxidant and has good therapeutic effects in animal models of various diseases [13]. The therapeutic mechanisms of action of hydrogen mainly include antioxidant, inhibition of apoptosis, and inhibition of an excessive inflammatory response. We assumed that early fluid resuscitation plus hydrogen inhalation will provide more protection on AKI during septic shock induced by LPS in rats.
Materials and methods
Animals
Male Wistar rats, weighing 180-200 g, were provided by the Experimental Animal Center of China Medical University. The rats were housed with free access to a normal rat diet and tap water and maintained in a temperature-controlled room with a 12 h/12 h light/dark cycle. All of the experimental procedures were approved by the Institutional Animal Care and Use Committee of China Medical University.
Drugs and materials
An HX300 animal ventilator was obtained from Tai Meng Technology Company (Chengdu, China). An HP portal ECG and blood pressure monitor was obtained from Bao Lai Te (Guangdong, China). An AVLOMNI blood gas analyzer was obtained from AVL Company (Switzerland). LPS was obtained from Sigma (St. Louis, MO, USA). Myeloperoxidase (MPO), superoxide dismutase (SOD), and malonaldehyde (MDA) assay kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Interleukin-6 (IL-6), Interleukin-10 (IL-10), tumor necrosis factor-α (TNF-α), and neutrophil gelatinase-associated lipocalin (NGAL) enzyme-linked immunosorbent assay (ELISA) kits were purchased from Wuhan Boster Bio-engineering Limited Company (Wuhan, China). 2% hydrogen mixed with 21% oxygen and 77% nitrogen was prepared by a medical gas station at the First Affiliated Hospital of China Medical University.
Experimental design
Sixty male Wistar rats were randomly divided into four groups (n = 15 per group): control group (Group C), septic shock group (Group S), septic shock with early fluid resuscitation group (Group R), and septic shock with early fluid resuscitation plus 2% hydrogen inhalation group (Group R+H2). The rats were anesthetized with 10% chloral hydrate (300 mg/kg, i.p.) and ventilated through tracheotomy with a respiratory frequency of 100 breaths/min and a tidal volume of 10 ml/kg. A 2% hydrogen mixture was used for Group R+H2, and air alone was used for the other three groups. The left carotid artery was cannulated to mean arterial pressure (MAP) through an HP portal ECG and blood pressure monitor. The femoral vein was cannulated for LPS administration and fluid resuscitation. Thirty minutes after the rats reached stable status, LPS (10 mg/kg, 10 mg/ml) was administered to establish the septic shock rat model, with the exception of Group C. Saline (0.2 ml) was then used to ensure that LPS that remained in the syringe was completely injected. Rats in Group C received intravenous injections of the same volume of saline. Fluid resuscitation (10 ml/kg saline per 15 min plus 0.5-6 μg/kg norepinephrine per minute) was performed when MAP decreased to 80% of baseline values and was maintained at normal levels [14]. MAP, saline and norepinephrine usage were recorded in each group. The rats were sacrificed by carotid artery bleeding 4 h after the experiment. Blood samples were centrifuged at 3000 × g for 10 min, and serum samples were collected to analyze creatinine (Cr), blood urea nitrogen (BUN), and NGAL. The left kidney was dissected and perfused with phosphate-buffered saline (PBS) to remove all blood. It was then cut into four pieces along the renal pedicle. One piece was fixed in 4% paraformaldehyde at 4°C for light microscopy. One piece was fixed in 2.5% glutaraldehyde for transmission electron microscopy. The other two pieces were stored at -80°C to analyze oxidation indicators and inflammatory mediators.
Analysis of renal function
Serum BUN and Cr were used to evaluate renal function. The samples were analyzed on a COBAS Mira chemical analyzer (Roche, Basel, Switzerland) using commercial kits from Sigma (St. Louis, MO, USA).
Measurement of NGAL
The concentration of NGAL was determined using sandwich assay by a commercially available ELISA kit according to the manufacturer’s instructions.
Histologic observation
Renal samples were fixed with 4% paraformaldehyde at 4°C for 24 h and embedded in paraffin, and 4 μm sections were stained with hematoxylin and eosin for light microscopy. Another renal sample was fixed with 2.5% glutaraldehyde, dehydrated, embedded in epoxy resin, and made into ultrathin sections to analyze changes in organelles using transmission electron microscopy (JEM-2000EX, JEOL, Japan).
Measurement of MDA, MPO, and SOD in kidney tissues
Renal MDA, MPO, and SOD activity was determined using the chemical method described in the manufacturer’s instructions (Nanjing Jian cheng Biochemistry Co., Nanjing, China). Kidney tissues were homogenized and then centrifuged at 12,000 × g for 20 min. The activity of MDA, MPO, and SOD in the supernatant was measured using the corresponding kits.
Determination of TNF-α, IL-6 and IL-10 levels in kidney tissues
Kidney tissues were thawed on ice and homogenized. The homogenates were centrifuged at 3000 × g at 4°C for 15 min. The levels of TNF-α, IL-6 and IL-10 were measured using a commercial ELISA kit according to the manufacturer’s instructions (Wuhan Boster Bio-engineering Limited Company, Wuhan, China). Absorbance was read on a microplate reader, and the concentrations were calculated according to the standard curve. Protein content in the sample was determined using the Coomassie blue assay, and the results were corrected per microgram of protein.
Statistical analysis
The results are expressed as mean ± SD. The statistical analyses were performed using SPSS 13.0 software. One-way analysis of variance was used to establish whether the differences among the four groups were statistically significant. Values of p < 0.05 were considered statistically significant.
Results
MAP, PO2, fluid volume and norepinephrine usage
In the current study, mean artery pressure (MAP) decreased to 80% of the baseline values 20 min after LPS injection. There was no significant difference of MAP in R group and R+H2 group during the experiment (Table 1). The fluid volume and norepinephrine usage in R+H2 group were significantly less than in R group (p < 0.05; Table 1). There was an obviously reduction of oxygen partial pressure (PO2) in septic shock group compared with control group. The two resuscitation methods all made increase of oxygen partial pressure, but a remarkable increase was seen in R+H2 group compared with R group (p < 0.05; Table 1).
Table 1.
Changes of MAP, PO2, fluid volume and norepinephrine usage of different groups
| Groups | MAP at baseline (mmHg) | MAP at 20 min after LPS administration (mmHg) | MAP at 4 h of the experiment (mmHg) | Fluid volumes (ml) | Norepinephrine (μg/kg per minute) | PO2 (mmHg) |
|---|---|---|---|---|---|---|
| C | 102.5 ± 9.1 | 100.4 ± 8.3 | 99.5 ± 8.1 | 0 | 0 | 90.4 ± 5.1 |
| S | 101.4 ± 8.3 | 82.7 ± 7.6 | 35.6 ± 7.6﹟ | 0 | 0 | 45.3 ± 6.2 |
| R | 100.7 ± 10.5 | 80.3 ± 9.1 | 97.6 ± 9.3Δ | 15.6 ± 5.4Δ | 4.5 ± 1.5Δ | 62.2 ± 6.1 |
| R+H2 | 103.4 ± 10.4 | 81.8 ± 8.6 | 99.8 ± 7.5Δ | 7.4 ± 3.1† | 1.2 ± 0.6† | 80.4 ± 4.3 |
Data are expressed as mean ± SD, n = 15.
p < 0.05 compared with Group C;
p < 0.05 compared with Group S;
p < 0.05 compared with Group R.
Serum BUN, Cr, and NGAL levels
Serum BUN, Cr, and NGAL levels were significantly higher in Group S than in Group C at the end of the experiment (p < 0.05; Figure 1A-C). When compared with Group S, Groups R and R+H2 had significant reductions of serum BUN, Cr, and NGAL levels. The reduction in Group R+H2 was more significant than the reduction in Group R (p < 0.05; Figure 1A-C). Though the two resuscitation methods reduced the BUN and Cr levels, there was no significantly difference of BUN/Cr ratio in the four groups (0.18 ± 0.05 vs 0.19 ± 0.06 vs 0.17 ± 0.04 vs 0.17 ± 0.05).
Figure 1.
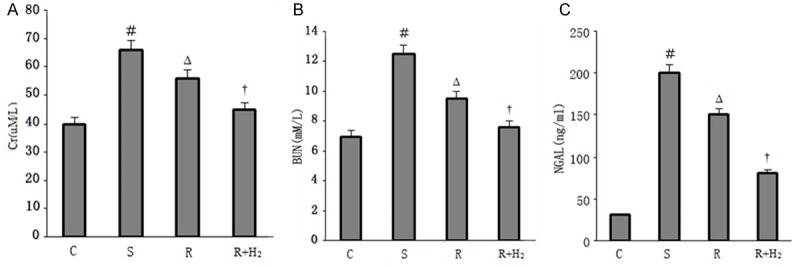
A. Serum Cr in different groups; B. Serum BUN in different groups; C. NGAL levels in different groups. Data are expressed as mean ± SD, n = 15. ﹟p < 0.05 versus Group C; Δp < 0.05 versus Group S; †p < 0.05 versus Group R.
Hematoxylin and eosin staining of kidney tissues
The kidneys in Group C presented normal glomeruli and tubules. In contrast, in Group S, renal tubular epithelial cells exhibited significant edema, brush border damage, and interstitial edema with hemorrhage. Tubular epithelial cell damage was ameliorated in Groups R and R+H2, but the improvements were more significant in Group R+H2 (Figure 2A).
Figure 2.
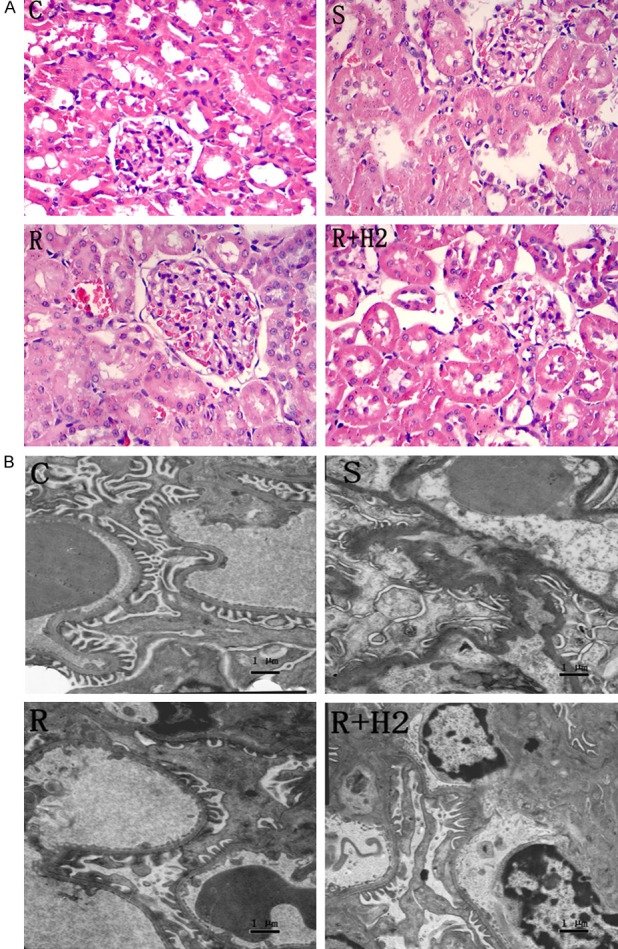
A. Photomicrographs of kidney sections of different groups. Routine hematoxylin and eosin stained (200×). B. Transmission electron microscopies of glomerular filtration membrane of different groups, (TEM 5000×, Bar scale = 1 μm).
Transmission electron microscopic analysis of glomerular filtration membrane
The glomerular filtration membrane comprises a glomerular basement membrane, podocytes, and endothelial cells. In Group C, the glomerular basement membrane was continuous with the same thickness, with neatly arranged podocytes and clear endothelial cells. In Group S, the glomerular basement membrane was circuitous with an irregular thickness and mostly fused podocytes and endothelial cells, indicating severe damage of the glomerular filtration membrane. Glomerular filtration membrane damage was ameliorated in Groups R and R+H2, especially in Group R+H2 (Figure 2B).
Levels of MDA, MPO and SOD in kidney tissues
We observed a significant increase in MDA and MPO levels and decrease in SOD levels in Group S compared with Group C. After resuscitation, the levels of MDA and MPO decreased and SOD increased simultaneously. However, a marked decrease in MDA and MPO levels and increase in SOD levels were seen in Group R+H2 compared with Group R (p < 0.05; Figure 3A-C).
Figure 3.
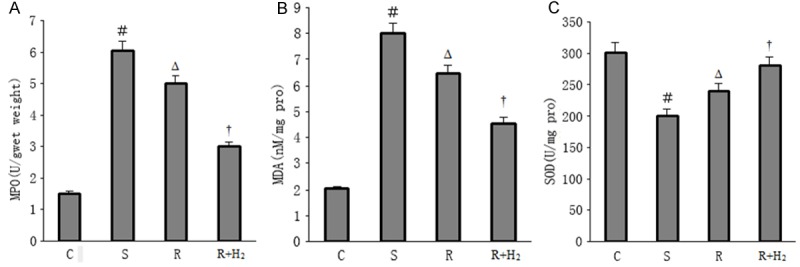
A. MPO expression of different groups; B. MDA expression of different groups; C. SOD expression of different groups. Data are expressed as mean ± SD, n = 15. ﹟p < 0.05 versus Group C; Δp < 0.05 versus Group S; †p < 0.05 versus Group R.
Proinflammatory cytokine levels in kidney tissues
The ELISA indicated that the levels of TNF-α and IL-6 were markedly increased in Group S compared with Group C. Fluid resuscitation reduced the elevation of TNF-α and IL-6 levels compared with the septic shock group. Additionally, the expression of TNF-α and IL-6 was even lower in Group R+H2 than in Group R, and the difference was significant (p < 0.05; Figure 4A, 4B). There was no significantly difference of IL-10 levels in Group S, Group R and Group R+H2 (Figure 4C). But there was significantly decrease of IL-6/IL-10 ratio in Group R and Group R+H2 compared with Group S, especially in Group R+H2 (p < 0.05; Figure 4D).
Figure 4.
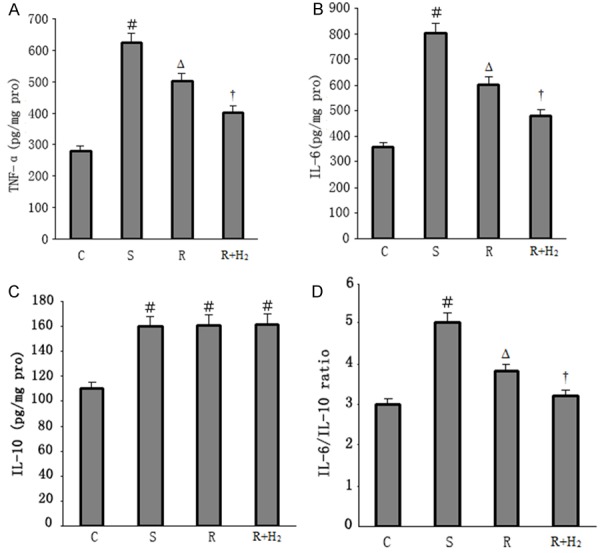
A. TNF-α levels of different groups; B. IL-6 levels of different groups; C. IL-10 levels of different groups; D. Ratio of IL-6/IL-10 of different groups. Data are expressed as mean ± SD, n = 15. ﹟p < 0.05 versus Group C; Δp < 0.05 versus Group S; †p < 0.05 versus Group R.
Discussion
The results of current study indicated that when combined with hydrogen inhalation, less fluid and norepinephrine were needed to maintain the MAP at normal level. As we know that overload liquid can lead to the aggravation of organ edema. So hydrogen inhalation appeared to enhance the beneficial effects of fluid resuscitation and reduce the side effects (e.g., liquid overloading) at the early stage of the septic shock.
The present study showed significant kidney injury at the end of the experiment in septic shock group. Though the two resuscitation methods reduced the BUN and Cr levels, there was no significantly difference of BUN/Cr ratio in the four groups. As we know, BUN/Cr ratio is used to analyze whether it is a pre-renal azotemia or tubular ischemia in AKI. During septic shock, there is an obvious reduction of renal blood flow, which can cause a pre-renal injury. But at the same time, LPS and ischemia can release excessive proinflammatory cytokines, resulting in renal parenchyma injury. So our data suggested that AKI during septic shock induced by LPS is not just hemodynamic in nature but also associated with alterations in kidney structure (Figure 2B).
Neutrophil gelatinase-associated lipocalin, a member of the apolipoprotein superfamily, has low expression levels in epithelial cells under normal conditions and can be highly expressed in renal tubular injury. It is a highly predictive biomarker of the early stage of AKI [15,16]. In the present study, we observed an obvious increase in NGAL in the septic shock group, and the two resuscitation protocols decreased NGAL levels, especially in the hydrogen inhalation group. The results indicated that fluid resuscitation plus hydrogen inhalation had a more protective effect in renal tubular epithelial cells. The transmission electron microscopy results indicated that the glomerular filtration membrane was severely damaged during septic shock. Although both resuscitation methods attenuated glomerular filtration membrane injury, the more apparent amelioration was observed in the fluid resuscitation plus hydrogen inhalation group. Therefore, fluid resuscitation plus hydrogen inhalation exerted more protection in renal tubular epithelial cells and the glomerular filtration membrane in the course of septic shock compared with fluid resuscitation alone.
During sepsis, LPS can activate neutrophils, monocytes/macrophages and endothelial cells, inducing the release of cytokines and accumulation of reactive oxygen species (ROS), which can negatively affect the prognosis of patients in various ways [17,18]. At the same time, glomerular afferent arterioles shrink with the slowing of blood flow and neutrophils adhesion and aggregation. When hypotension continues, glomerular efferent arteries shrink, resulting in kidney hypoxia and high expression of renal vascular endothelial cell adhesion molecule, which accelerated neutrophil adhesion to vascular endothelium. Activated neutrophils can release amounts of inflammatory cytokines and oxygen radicals, aggravating the hypoxia and injury of kidney [19]. So the improvement of oxygenation and decrease of oxidative stress injury is important to protect kidney during septic shock. Myeloperoxidase (MPO) is a peroxidase enzyme that is released by neutrophils and may reflect neutrophil infiltration levels. Malonaldehyde (MDA), a major metabolite of lipid peroxidation, may reflect the degree of damage caused by oxidative stress. Superoxide dismutase (SOD), one of the most powerful free radical-scavenging enzymes, has a protective effect against renal injury. The results of this study indicated that resuscitation plus hydrogen inhalation was associated with greater improvement not only with oxygenation but also ROS-induced injury of AKI.
Although the pathogenesis of AKI during septic shock is not entirely clear, excessive inflammation plays an important role [20-23]. Tumor necrosis factor-α (TNF-α) is a key proinflammatory factor in AKI. During sepsis, TNF-α has been shown to be overexpressed in renal tubular epithelial cells, with invasion of a large number of inflammatory cells, resulting in renal injury [24]. Treatment with TNF-α receptor antagonist can significantly reduce AKI [25]. Additionally, Interleukin-6 (IL-6) is highly expressed during AKI and cause injury to both the kidneys and lungs [26]. Inhibition of the overexpression of TNF-α and IL-6 is important in AKI. Nevertheless, Interleukin-10 (IL-10) is an anti-inflammatory cytokine, which can inhibit the expression of proinflammatory cytokines such as TNF-α, IL-1β, IL-6 and IL-8. And it is representative of the initiation of the compensatory anti-inflammatory response (CARS). It has been shown that IL-10 is effective as a therapeutic when exogenously delivered to rodents undergoing AKI [27].
In the present study, TNF-α and IL-6 levels significantly increased in the septic shock group, indicating the involvement of excessive inflammation during AKI. The two resuscitation methods reduced the overexpression of TNF-α and IL-6, but the more evident reduction was seen in the hydrogen inhalation group. The CARS response is typically described as occurring “later” in the course of sepsis. However, our data suggested that IL-10 increased nearly simultaneously with TNF-α and IL-6 in the septic shock group at the end of the experiment. There was no significantly difference of IL-10 levels in Group S, Group R and Group R+H2. But there was significantly decrease of IL-6/IL-10 ratio in Group R and Group R+H2 compared with Group S, especially in Group R+H2. As reported in previous study, elevated ratio of IL-6/IL-10 was related with poor prognosis of sepsis [28]. The results demonstrated that resuscitation plus hydrogen inhalation reduced more expression of proinflammatory cytokines and maintained the balance of pro-inflammation and anti-inflammation system better than resuscitation alone, which could have more protection on AKI.
The hydrogen molecule is a selective antioxidant and has good therapeutic effects in animal models of various diseases. The therapeutic mechanisms of action of hydrogen mainly include antioxidant, inhibition of apoptosis, and inhibition of an excessive inflammatory response [29-31], which are also involved in the mechanisms of sepsis. With regard to septic shock, microcirculation dysfunction or capillary dysfunction is a key issue that can determine the effect of fluid resuscitation. Increased capillary permeability increases the permeation of liquid into the tissue space, resulting in organ edema and inadequate circulating blood volume, ultimately resulting in the aggravation of organ ischemia and hypoxia accompanied by sustained hypotension. Early fluid resuscitation clearly plays an important role in septic shock treatment, but the recovery liquid can lead to aggravation of organ edema, which can result in multi-organ failure if treated inappropriately. With good therapeutic antioxidant effects, including the inhibition of apoptosis and excessive inflammation, hydrogen molecules likely improve capillary permeability and reduce the volume that leaks into the interstitial space. Hydrogen inhalation appeared to maximize the beneficial effects of fluid resuscitation and minimize the side effects (e.g., liquid overloading). The results of the present study indicate that fluid resuscitation combined with hydrogen inhalation may provide more protection on AKI during septic shock. The present protocol may have broad applications for the clinical treatment of septic shock.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 81101415).
Disclosure of conflict of interest
None.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence outcome and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Rivers E, Nguyen B, Havstad S. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 4.Trzeciak S, McCoy JV, Phillip Dellinger R. Early increases in microcirculatory perfusion during protocol directed resuscitation are associated with reduced multi-organ failure at 24 h in patients with sepsis. Intensive Care Med. 2008;34:2210–2217. doi: 10.1007/s00134-008-1193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones AE, Focht A, Horton JM, Kline JA. Prospective external validation of the clinical effectiveness of an emergency department-based early goal-directed therapy protocol for severe sepsis and septic shock. Chest. 2007;132:425–432. doi: 10.1378/chest.07-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell JP, Schuller D, Calandrino FS. Improved outcome based on fluid management in critically ill patients requiring pulmonary artery catheterization. Am Rev Respir Dis. 1992;145:990–998. doi: 10.1164/ajrccm/145.5.990. [DOI] [PubMed] [Google Scholar]
- 7.Schrier RW. Fluid administration in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol. 2010;5:733–739. doi: 10.2215/CJN.00060110. [DOI] [PubMed] [Google Scholar]
- 8.Singbartl K, Kellum JA. AKI in the ICU: Definition, epidemiology, risk stratification, and outcomes. Kidney Int. 2012;81:819–825. doi: 10.1038/ki.2011.339. [DOI] [PubMed] [Google Scholar]
- 9.Mandelbaum T, Scott DJ, Lee J. Outcome of critically ill patients with acute kidney injury using the Acute Kidney Injury Network criteria. Crit Care Med. 2011;39:2659–2664. doi: 10.1097/CCM.0b013e3182281f1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bagshaw SM, George C, Bellomo R ANZICS Database Management Committe. A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23:1569–1574. doi: 10.1093/ndt/gfn009. [DOI] [PubMed] [Google Scholar]
- 11.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 12.Barrantes F, Feng Y, Ivanov O. Acute kidney injury predicts outcomes of non-critically ill patients. Mayo Clin Proc. 2009;84:410–416. doi: 10.1016/S0025-6196(11)60559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dixon BJ, Tang J, Zhang JH. The evolution of molecular hydrogen: a noteworthy potential therapy with clinical significance. Med Gas Res. 2013;3:10. doi: 10.1186/2045-9912-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sennoun N, Montemont C, Gibot S. Comparative effects of early versus delayed use of norepinephrine in resuscitated endotoxic shock. Crit Care Med. 2007;35:1736–1740. doi: 10.1097/01.CCM.0000269028.28521.08. [DOI] [PubMed] [Google Scholar]
- 15.Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–1024. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 16.Wheeler DS, Devarajan P, Ma Q, Harmon K, Monaco M, Cvijanovich N, Wong HR. Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Crit Care Med. 2008;36:1297–1303. doi: 10.1097/CCM.0b013e318169245a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trzeciak S, McCoy JV, Phillip Dellinger R, Arnold RC, Rizzuto M, Abate NL, Shapiro NI. Early increases in microcirculatory perfusion during protocol directed resuscitation are associated with reduced multi-organ failure at 24 h in patients with sepsis. Intensive Care Med. 2008;34:2210–2217. doi: 10.1007/s00134-008-1193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu DD, Kao SJ, Chen HI. N-acetylcysteine attenuates acute lung injury induced by fat embolism. Crit Care Med. 2008;36:565–571. doi: 10.1097/01.CCM.0000299737.24338.5C. [DOI] [PubMed] [Google Scholar]
- 19.Kurniati NF, van Meurs M, Vom HF, Jongman RM. Pleiotropic effects of angiopoietin-2 deficiency do not protect mice against endotoxin-induced acute kidney injury. Nephrol Dial Transplant. 2013;28:567–575. doi: 10.1093/ndt/gfs336. [DOI] [PubMed] [Google Scholar]
- 20.Ramesh G, Zhang B, Uematsu S, Akira S, Reeves WB. Endotoxin and cisplatin synergistically induce renal dysfunction and cytokine production in mice. Am J Physiol Renal Physiol. 2007;293:F325–332. doi: 10.1152/ajprenal.00158.2007. [DOI] [PubMed] [Google Scholar]
- 21.Zager RA, Johnson AC, Geballe A. Gentamicin suppresses endotoxin-driven TNF-alpha production in human and mouse proximal tubule cells. Am J Physiol Renal Physiol. 2007;293:F1373–1380. doi: 10.1152/ajprenal.00333.2007. [DOI] [PubMed] [Google Scholar]
- 22.Zager RA, Johnson AC, Lund S. Uremia impacts renal inflammatory cytokine gene expression in the setting of experimental acute kidney injury. Am J Physiol Renal Physiol. 2009;297:F961–970. doi: 10.1152/ajprenal.00381.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang W, Bansal S, Falk S, Ljubanovic D, Schrier R. Ghrelin protects mice against endotoxemia-induced acute kidney injury. Am J Physiol Renal Physiol. 2009;297:F1032–1037. doi: 10.1152/ajprenal.00044.2009. [DOI] [PubMed] [Google Scholar]
- 24.Cunningham PN, Dyanov HM, Park P, Wang J, Newell KA, Quigg RJ. Acute renal failure in endotoxemia is caused by TNF acting directly on TNF receptor-1 in kidney. J Immunol. 2002;168:5817–5823. doi: 10.4049/jimmunol.168.11.5817. [DOI] [PubMed] [Google Scholar]
- 25.Knotek M, Rogachev B, Wang W, Ecder T, Melnikov V, Gengaro PE, Esson M, Edelstein CL, Dinarello CA, Schrier RW. Endotoxemic renal failure in mice: role of tumor necrosis factor independent of inducible nitric oxide synthase. Kidney Int. 2001;59:2243–2249. doi: 10.1046/j.1523-1755.2001.00740.x. [DOI] [PubMed] [Google Scholar]
- 26.Grigoryev DN, Liu M, Hassoun HT, Cheadle C, Barnes KC, Rabb H. The local and systemic inflammatory transcriptome after acute kidney injury. J Am Soc Nephrol. 2008;19:547–558. doi: 10.1681/ASN.2007040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng J, Kohda Y, Chiao H, Wang Y, Hu X, Hewitt SM, Miyaji T, McLeroy P, Nibhanupudy B, Li S, Star RA. Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int. 2001;60:2118–2128. doi: 10.1046/j.1523-1755.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- 28.Taniguchi T, Koido Y, Aiboshi J, Yamashita T, Suzaki S, Kurokawa A. Change in the ratio of Interleukin-6 to interleukin-10 predicts a poor outcome in patients with systemic inflammatory response syndrome. Crit Care Med. 1999;27:1262–1264. doi: 10.1097/00003246-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 30.Xie K, Yu Y, Pei Y. Protective effects of hydrogen gas on murine polymicrobial sepsis via reducing oxidative stress and HMGB1 release. Shock. 2010;34:90–97. doi: 10.1097/SHK.0b013e3181cdc4ae. [DOI] [PubMed] [Google Scholar]
- 31.Fukuda K, Asoh S, Ishikawa M. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem Biophys Res Commun. 2007;361:670–674. doi: 10.1016/j.bbrc.2007.07.088. [DOI] [PubMed] [Google Scholar]


