Abstract
Objective: Platelets have an important role in atherosclerosis and arterial thrombosis. Cardiovascular complication prevalence of type 2 diabetes mellitus (type 2 DM) may be associated with glycosylated hemoglobin (HbA1c) and mean platelet volume (MPV). The aim of the study was to investigate if platelets were activated in diabetes and its associated vascular complications by measuring the MPV in the diabetics compared to the non-diabetics, and to determine the correlation of MPV with fasting serum glucose (FSG), HbA1c and duration of diabetes in the diabetic patients, respectively. Materials and Methods: The study carried out in 65 patients with type 2 DM and 40 non-diabetic subjects. In addition to non-diabetic patients, all diabetic patients were divided into two groups according to their HbA1c levels: group A consisted of patients with HbA1c levels ≤7% and group B consisted of patients with HbA1c levels >7%. Results: MPV was significantly higher in Group B as compared to both non-diabetics and Group A. MPV had a high positive correlation with HbA1c and FSG, as with diabetes duration. It is found that MPV was increased in type 2 DM. Conclusion: Our findings suggested an association between MPV and HbA1c. Therefore, MPV would be a beneficial prognostic marker of cardio-vascular complications in patients with type 2 DM.
Keywords: MPV, atherosclerosis, glucose regulation, diabetes duration
Introduction
Type 2 DM is both metabolic disorder and major worldwide health problem because of its high prevalence and morbidity [1]. Type 2 DM is a part of metabolic syndrome which comprises dyslipidemia, hypertension, impaired fibrinolysis, and increased procoagulation factors [2,3]. Vascular disorders such as coronary arterial disease enhance the morbidity and mortality of type 2 DM [4-6]. Type 2 DM induces atherosclerosis, circulation dysfunction, and dysregulation of coagulation [7,8]. It is reported that cardiovascular mortality risk is correlated with blood glucose concentration in cases with type 2 DM [9]. Hyperglycemia is thought to have a harmful effect on the blood vessels [10].
Platelets are involved in homeostatic process and have an important role in atherosclerosis and arterial thrombosis [11,12]. When vascular injury occurred, platelets adhere to damaged endothelium to form platelet plug [13]. Platelet volume is a marker of platelet function and activation. It can be quantified as mean platelet volume (MPV) by clinical hematology analyzers [14]. It has been reported that platelets from diabetic patients synthesize more thromboxane than normal platelets [2]. It is found that hyperglycemia causes larger platelets. Larger platelets also release more prothrombotic factors such as thromboxane A2 [15]. It is also suggested that the increased platelet activity enhances vascular complications in these patients [16,17]. In addition, it is revealed that increased MPV plays a role in myocardial infarction, thromboembolism and stroke [18]. Cardiovascular complication prevalence of type 2 DM may be associated with HbA1c [19] and MPV [20,21].
The aim of the study was to determine whether platelets were activated in diabetes and its associated vascular complications by measuring the MPV in the diabetics compared to the non-diabetics, and to evaluate the correlation of MPV with blood glucose regulation and duration of diabetes in the type 2 diabetic patients. We also compared the MPV of diabetics (regulated) having HbA1c≤7% to that of diabetics (non-regulated) having HbA1c>7%.
Materials and methods
This is a prospective study carried out in 65 patients with type 2 DM and 40 control subjects. All the diabetic and healthy subjects had a clinical examination in terms of macro or microvascular complications and history of drug usage. MPV was analyzed by an automatic blood counter (Abbott Cell Dyn 3200 Hematology Analyzer). Venous blood samples were collected in hemogram tubes with dipotassium EDTA and biochemistry tubes, and tested within 1 hour of collection to minimize variations due to sample aging. Samples were maintained at room temperature. HbA1c was measured by automated ion-exchange high performance liquid chromatography (Bio-Rad Variant II, Hercules, CA), serum glucose by hexokinase enzymatic method (Abbott Architect c8000 Chemistry Analyzer).
Male patients with hemoglobin below 12 mg/dl and female patients below 11 mg/dl were excluded from the study due to any possible nutritional anemias causing for reactive thrombocytosis and hence, increased MPV levels. Diabetics on antiplatelet drugs such as aspirin and clopidogrel were excluded. Subjects with any diagnosed pregnancy or malignancy were also not included. All diabetic patients were divided into two groups according to their HbA1c levels: group A HbA1c levels ≤7% and group B with HbA1c levels >7% [22,23].
Statistical evaluation was performed by SPSS statistics package program version 15 (for Windows) using One-Way ANOVA and Pearson correlation test (r value as the coefficient). Data were expressed as mean ± standard deviation. P value <0.05 was considered statistically significant.
Results
Among 86 diabetic subjects, 21 were excluded due to anemia or using antiplatelet drugs such as aspirin. Similarly, among the 44 non-diabetic individuals, 4 were also excluded due to anemia or having history of coronary artery disease. There were 21 non-diabetic males and 19 non-diabetic females whereas there were 33 male and 32 female diabetics in the study.
The diabetic group was divided based on the HbA1c levels into Group A (HbA1c≤7%) and Group B (HbA1c>7%). The mean age of the population according to subgroups was 51.8±12.2 (non-diabetic), 56.6±11.1 (Group A), and 56.7±10.9 (Group B) years. As seen in Table 1, there was no significant difference among the subgroups for demographic characteristics of study participants, including age, body mass index (BMI) and diabetes duration.
Table 1.
Demographic characteristics of study participants according to subgroups
| Factors | Non-Diabetics (n:40) | Diabetics | |
|---|---|---|---|
|
| |||
| HbA1c≤7 (n:33) | HbA1c>7 (n:32) | ||
| Age (year) | 51.8±12.2 | 56.6±11.1 | 56.7±10.9 |
| Gender | 21 (m)/19 (f) | 16 (m)/17 (f) | 17 (m)/15 (f) |
| BMI (kg/m2) | 28.8±3.2 | 31.58±5.32 | 30.9±5.1 |
| DM duration (year) | - | 7.7±8.5 | 5.6±4.7 |
Data are shown as the mean ± standard deviation. BMI, body mass index; m, male; f, Female.
The mean fasting serum glucose (FSG) level in the non-diabetic population was 84.5±6.0 mg/dL, while that of the diabetic groups were 207.2±82.4 mg/dL (Group A) and 246.5±90.3 (Group B). As seen in Figure 1, there was a significant difference between the diabetic subgroups in terms of FSG (p=0.047), as well as between diabetics and non-diabetics (p< 0.001). The mean HbA1c levels were as 5.8±0.7, 6.3±0.3 and 9.6±2.3 in the non-diabetic, Group A and B, respectively.
Figure 1.
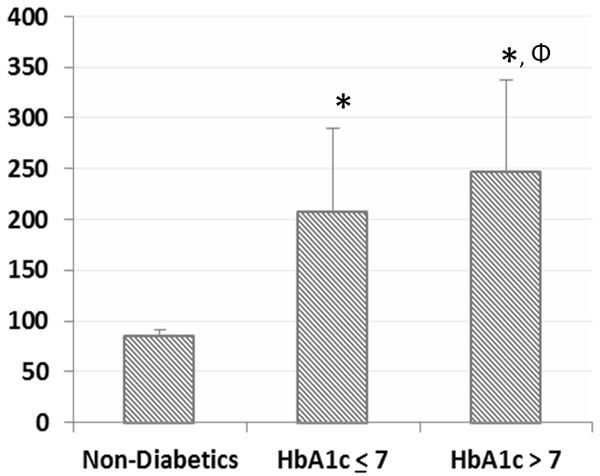
Mean values of fasting serum glucose (mg/dl) according to the subgroups, including non-diabetics, HbA1c≤7 and HbA1c>7. Data are shown as the mean ± standard deviation. P values were calculated using the One-Way ANOVA and posthoc Tukey HSD. (*) p<0.001 when compared to non-diabetics. (Φ) p<0.05 when compared to HbA1c≤7 group.
As seen in Figure 2, MPV was significantly higher (8.3±1.3 fl) in Group B as compared to both non-diabetics (7.1±1.0 fl; p<0.001) and Group A (7.5±1.1 fl; p=0.039). MPV had a high positive Pearson Correlation with HbA1c (r=0.393; p<0.001) and FSG (r=0.41; p<0.001), as with diabetes duration in a significance of p=0.02 (r=0.222) (Table 2). Scotter-Plot graphic was given in terms of correlations of MPV, HbA1c and FSG in Figure 3.
Figure 2.
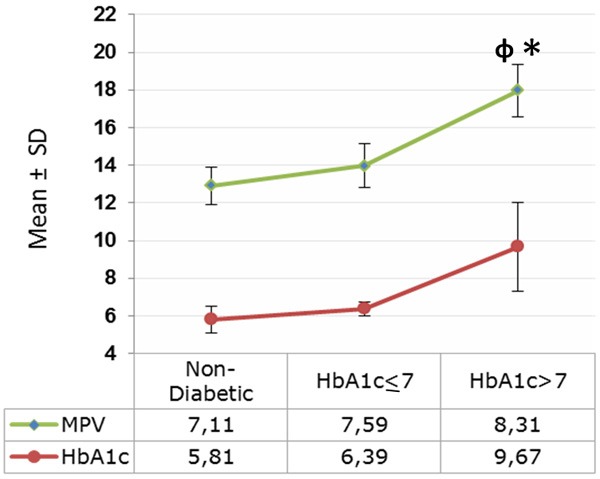
Mean values of MPV (mean platelet volume) and HbA1c according to the subgroups, including non-diabetics, HbA1c≤7 and HbA1c>7. Graphic data are shown as the mean ± standard deviation. P values were calculated using the One-Way ANOVA and posthoc Tukey HSD. (φ) p<0.001 when compared to non-diabetic group. (*) p=0.039 when compared to HbA1c≤7 group.
Table 2.
Correlations between mean platelet volume and various parameters
| Factors | r | p |
|---|---|---|
| Age (year) | 0.010 | 0.91 |
| BMI (kg/m2) | 0.181 | 0.06 |
| DM duration (year) | 0.222 | 0.02 |
| FSG (mg/dl) | 0.410 | <0.001 |
| HbA1c (%) | 0.393 | <0.001 |
Coefficients (r) and P-values are calculated using the Pearson’s correlation model. FSG, Fasting Serum Glucose; BMI, Body Mass Index.
Figure 3.
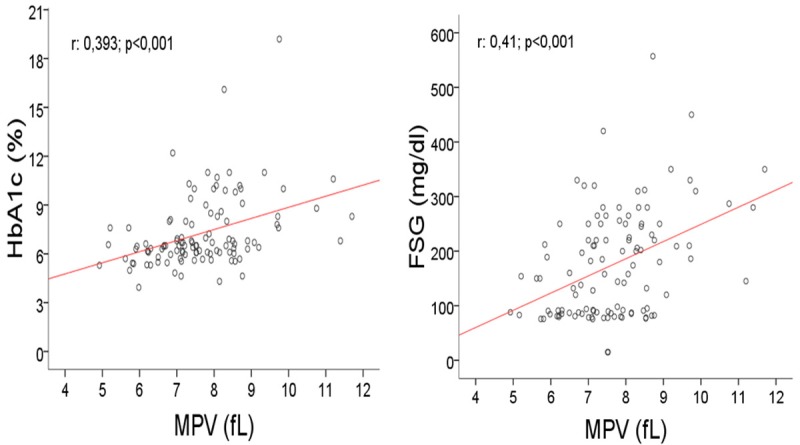
Mean Platelet Volume levels scatter-plotted against plasma HbA1c (r=0.393; p<0.001) and FSG (r=0.41; p<0.001). FSG, Fasting Serum Glucose; MPV, Mean Platelet Volume.
Discussion
Type 2 DM is a complex metabolic syndrome characterized by chronic hyperglycemia resulting in several complications regarding micro and macrovascular structures such as retinopathy, nephropathy and coronary artery disease. Insulin resistance and impaired insulin secretion are very important for type 2 DM pathogenesis. Demirtunc R et al. suggested that increased HbA1c level was associated with raised MPV. They also proposed that ameliorated glycemic control decreases MPV and avoid the possible role of platelets in cardiovascular events in type 2 DM [24].
Kodiatte et al. suggested a relation between MPV and retinopathy bu not with diabetes duration [25], in our study, it is found that mean volume (MPV) is increased in type 2 DM and we found that elevated HbA1c concentration and diabetes duration was directly correlated with increased MPV. In another study, it is shown that HbA1c and diabetes duration have individually induces cardiovascular adverse effects in adolescents with type 2 diabetes mellitus [26].
However, Hekimsoy Z et al. did not find any correlation between MPV and FSG in patients with type 2 diabetes mellitus [14], we found a correlation between MPV and FSG. Shimodaira et al also confirm a relationship between MPV and FSG in prediabetic subjects. Our results are consisted with their study [27]. Kodiatte et al reported that increased platelet activity have an important role in the development of vascular complications in type 2 DM [25]. It can be suggested that increased platelet volume may be an important factor in the enhanced risk of vascular complications in these cases. In this respect, MPV can be used as a favorable test in the monitoring of type 2 DM in terms of atherosclerosis development.
Kakouros N et al suggested that hyperglycemia causes to generate of larger platelets [15]. Abnormal platelet-endothelial interactions have been identified as an essential pathogenic mechanism in the development of atherosclerosis [13,28]. It is suggested that the pathogenesis of cardiovascular diseases can be influenced endothelial cell damage. Endothelial cell damage can be induced by hyperglycemia, increased free fatty acids, altered lipoproteins, hypertension and type 2 DM [29]. It is proposed that hyperglycemia can increase platelet reactivity by inducing some mechanisms includes the osmotic effect of glucose, nonenzymatic protein glycation of the platelet and protein kinase C activation [15,17,30]. Schneider DJ et al. also suggested that hyperglycemia may intensify the platelet activity by increasing megakaryocytic glycoprotein production [30].
However, it is proposed that FSG is not directly associated with increased cardiovascular events in patients with type 2 DM [31]. In our study we have found increased MPV levels, and it is correlated with elevated FSG and HbA1c levels. Eckel RH et al determined that there is a relationship between hyperglycemia and endothelial cell damage [29]. Therefore it may be suggested that hyperglycemia induces atherosclerosis via abnormal platelet-endothelial interactions. Our finding may have shown that elevated MPV levels may be associated with increased coronary arterial disease.
It has been proposed that diabetic patients have larger platelets and synthesize more thromboxane. Therefore, the larger platelets include denser granules, release more β-thromboglobulin, serotonin, and produce more thromboxane A2 [2,14]. The patients with type 2 DM have larger platelets that are more reactive and aggregable and it is thought that platelets may have an important role in the development of atherosclerosis in diabetes [25,32-34]. It is confirmed that the diabetic cases have increased levels in plasma procoagulant and decreased concentrations of functionally diminished antithrombotic factors [35]. Kaplan ZS and Jackson SP pointed that platelets have an important role in the initiation and propagation of atherosclerosis. It is also reported that the enhanced platelet activity intensify the vascular complications associated with type 2 DM [17].
Kakouros et al suggested that platelet hyperreactivity and increased baseline activation in patients with diabetes is multifactorial and connected to biochemical factors such as hyperglycemia, insulin resistance and hyperlipidemia [15]. Platelets have insulin binding site and it is assumed that insulin reduces platelet responses against aggregant factors including thrombin, ADP and platelet activating factor. Thus it is found that insulin resistance results in platelet dysfunction [17].
MPV is an indicator of the average size and activity of platelets and it is suggested that increased MPV plays an important role in stroke, myocardial infarction and thromboembolism [18]. It is also reported that high MPV is an independent risk factor for coronary atherosclerosis and myocardial infarction [20]. In addition, it was assumed that MPV increases in patients with some conditions including type 2 DM and metabolic syndrome. Han JY et al. also found that platelet activity have predictive value for ischemic stroke or coronary arterial diseases in patients with type 2 DM [20].
It is confirmed that there is a relationship between cardiovascular complications prevalence due to type 2 DM and MPV [20,21]. Growing evidence showed that MPV is an important risk factor for the vascular complications regarding type 2 DM [18]. And it is believed that type 2 DM is a prothrombotic state due to intensified platelet activity [36]. Therefore, increased MPV can generate a pro-coagulant effect and cause thrombotic vascular complications. It can be suggested that there is link between MPV and diabetic vascular complications associated with thrombogenesis [14,37]. Sewell R et al. reported that platelet size changed as a result of myocardial infarction. At the same time, MPV can also be elevated due to some coronary arterial risk factors including type 2 DM, smoking, hypertension and hypercholesterolemia [24,38].
In our study, the diabetic group had significantly higher MPV than the non-diabetic subjects. This result is consisted with the other previous studies [14,18,21,25,36,39]. However, Hekimsoy Z et al did not find any correlation between MPV and HbA1c levels [14]. In the current study, MPV was statistically increased in diabetics with HbA1c levels ≥7% than in diabetics with HbA1c levels <7%. There was also a significant correlation between HbA1c and MPV. These results are also similar to the study of Demirtunc et al. [39]. Andersson C. et al. reported a relationship between in high baseline HbA1c concentration in patient with type 2 DM and high cardiovascular risk [19]. In this article, it was concluded that glycemic control reduces the platelet activity and it may prevent or delay vascular complications in patient with type 2 DM.
Conclusions
In the current study, our findings showed a relationship between MPV and HbA1c. It may be suggested that platelets of diabetic patients become more aggregable and reactive due to increased MPV. Increased risk of atherosclerosis in regard with type 2 DM may be a result of high MPV. Therefore, MPV might be a useful prognostic marker of cardio-vascular complications in patients with type 2 DM. Further studies with the larger samples are needed to clarify these relations in terms of the pathogenesis.
Disclosure of conflict of interest
The authors of this paper have no conflicts of interest, including specific financial interests, relationships, and/or affiliations, relevant to the subject matter or materials included.
References
- 1.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 2.Colwell JA, Nesto RW. The platelet in diabetes: focus on prevention of ischemic events. Diabetes Care. 2003;26:2181–2188. doi: 10.2337/diacare.26.7.2181. [DOI] [PubMed] [Google Scholar]
- 3.Gundogan K, Bayram F, Capak M, Tanriverdi F, Karaman A, Ozturk A, Altunbas H, Gokce C, Kalkan A, Yazici C. Prevalence of metabolic syndrome in the Mediterranean region of Turkey: evaluation of hypertension, diabetes mellitus, obesity, and dyslipidemia. Metab Syndr Relat Disord. 2009;7:427–434. doi: 10.1089/met.2008.0068. [DOI] [PubMed] [Google Scholar]
- 4.Diabetes mellitus: a major risk factor for cardiovascular disease A joint editorial statement by the American Diabetes Association; The National Heart, Lung, and Blood Institute; The Juvenile Diabetes Foundation International; The National Institute of Diabetes and Digestive and Kidney Diseases; and The American Heart Association. Circulation. 1999;100:1132–1133. doi: 10.1161/01.cir.100.10.1132. [DOI] [PubMed] [Google Scholar]
- 5.Carson JL, Scholz PM, Chen AY, Peterson ED, Gold J, Schneider SH. Diabetes mellitus increases short-term mortality and morbidity in patients undergoing coronary artery bypass graft surgery. J Am Coll Cardiol. 2002;40:418–423. doi: 10.1016/s0735-1097(02)01969-1. [DOI] [PubMed] [Google Scholar]
- 6.Saglam H, Seyfeli E, Gul I, Duru M, Gokce C. Index of myocardial performance in patients with type 2 diabetes without hypertension and its relationship with clinical and echocardiographic parameters. J Diabetes. 2009;1:50–56. doi: 10.1111/j.1753-0407.2008.00005.x. [DOI] [PubMed] [Google Scholar]
- 7.Vericel E, Januel C, Carreras M, Moulin P, Lagarde M. Diabetic patients without vascular complications display enhanced basal platelet activation and decreased antioxidant status. Diabetes. 2004;53:1046–1051. doi: 10.2337/diabetes.53.4.1046. [DOI] [PubMed] [Google Scholar]
- 8.Chiha M, Njeim M, Chedrawy EG. Diabetes and coronary heart disease: a risk factor for the global epidemic. Int J Hypertens. 2012;2012:697240. doi: 10.1155/2012/697240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danaei G, Lawes CM, Vander Hoorn S, Murray CJ, Ezzati M. Global and regional mortality from ischaemic heart disease and stroke attributable to higher-than-optimum blood glucose concentration: comparative risk assessment. Lancet. 2006;368:1651–1659. doi: 10.1016/S0140-6736(06)69700-6. [DOI] [PubMed] [Google Scholar]
- 10.Temelkova-Kurktschiev TS, Koehler C, Henkel E, Leonhardt W, Fuecker K, Hanefeld M. Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care. 2000;23:1830–1834. doi: 10.2337/diacare.23.12.1830. [DOI] [PubMed] [Google Scholar]
- 11.van der Loo B, Martin JF. Megakaryocytes and platelets in vascular disease. Baillieres Clin Haematol. 1997;10:109–123. doi: 10.1016/s0950-3536(97)80053-4. [DOI] [PubMed] [Google Scholar]
- 12.Tufano A, Cimino E, Di Minno MN, Ierano P, Marrone E, Strazzullo A, Di Minno G, Cerbone AM. Diabetes mellitus and cardiovascular prevention: the role and the limitations of currently available antiplatelet drugs. Int J Vasc Med. 2011;2011:250518. doi: 10.1155/2011/250518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan ZS, Jackson SP. The role of platelets in atherothrombosis. Hematology Am Soc Hematol Educ Program. 2011;2011:51–61. doi: 10.1182/asheducation-2011.1.51. [DOI] [PubMed] [Google Scholar]
- 14.Hekimsoy Z, Payzin B, Ornek T, Kandogan G. Mean platelet volume in Type 2 diabetic patients. J Diabetes Complications. 2004;18:173–176. doi: 10.1016/S1056-8727(02)00282-9. [DOI] [PubMed] [Google Scholar]
- 15.Kakouros N, Rade JJ, Kourliouros A, Resar JR. Platelet function in patients with diabetes mellitus: from a theoretical to a practical perspective. Int J Endocrinol. 2011;2011:742719. doi: 10.1155/2011/742719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torun AN, Eren MA, Ulaş T, Demir M, Arslan I, Sabuncu T. Mean Platelet Volume in Various Degrees of Disturbed Carbohydrate Metabolism. Turkish Journal of Endocrinology and Metabolism. 2012;16:6–9. [Google Scholar]
- 17.Vinik AI, Erbas T, Park TS, Nolan R, Pittenger GL. Platelet dysfunction in type 2 diabetes. Diabetes Care. 2001;24:1476–1485. doi: 10.2337/diacare.24.8.1476. [DOI] [PubMed] [Google Scholar]
- 18.Zuberi BF, Akhtar N, Afsar S. Comparison of mean platelet volume in patients with diabetes mellitus, impaired fasting glucose and non-diabetic subjects. Singapore Med J. 2008;49:114–116. [PubMed] [Google Scholar]
- 19.Andersson C, van Gaal L, Caterson ID, Weeke P, James WP, Coutinho W, Finer N, Sharma AM, Maggioni AP, Torp-Pedersen C. Relationship between HbA1c levels and risk of cardiovascular adverse outcomes and all-cause mortality in overweight and obese cardiovascular high-risk women and men with type 2 diabetes. Diabetologia. 2012;55:2348–2355. doi: 10.1007/s00125-012-2584-3. [DOI] [PubMed] [Google Scholar]
- 20.Han JY, Choi DH, Choi SW, Kim BB, Ki YJ, Chung JW, Koh YY, Chang KS, Hong SP. Stroke or coronary artery disease prediction from mean platelet volume in patients with type 2 diabetes mellitus. Platelets. 2013;24:401–6. doi: 10.3109/09537104.2012.710858. [DOI] [PubMed] [Google Scholar]
- 21.Papanas N, Symeonidis G, Maltezos E, Mavridis G, Karavageli E, Vosnakidis T, Lakasas G. Mean platelet volume in patients with type 2 diabetes mellitus. Platelets. 2004;15:475–478. doi: 10.1080/0953710042000267707. [DOI] [PubMed] [Google Scholar]
- 22.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81–90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 24.Boos CJ, Lip GY. Assessment of mean platelet volume in coronary artery disease - what does it mean? Thromb Res. 2007;120:11–13. doi: 10.1016/j.thromres.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Kodiatte TA, Manikyam UK, Rao SB, Jagadish TM, Reddy M, Lingaiah HK, Lakshmaiah V. Mean platelet volume in Type 2 diabetes mellitus. J Lab Physicians. 2012;4:5–9. doi: 10.4103/0974-2727.98662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah AS, Dolan LM, Kimball TR, Gao Z, Khoury PR, Daniels SR, Urbina EM. Influence of duration of diabetes, glycemic control, and traditional cardiovascular risk factors on early atherosclerotic vascular changes in adolescents and young adults with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2009;94:3740–3745. doi: 10.1210/jc.2008-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimodaira M, Niwa T, Nakajima K, Kobayashi M, Hanyu N, Nakayama T. Correlation between mean platelet volume and fasting plasma glucose levels in prediabetic and normoglycemic individuals. Cardiovasc Diabetol. 2013;12:14. doi: 10.1186/1475-2840-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balasubramaniam K, Viswanathan GN, Marshall SM, Zaman AG. Increased atherothrombotic burden in patients with diabetes mellitus and acute coronary syndrome: a review of antiplatelet therapy. Cardiol Res Pract. 2012;2012:909154. doi: 10.1155/2012/909154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eckel RH, Wassef M, Chait A, Sobel B, Barrett E, King G, Lopes-Virella M, Reusch J, Ruderman N, Steiner G, Vlassara H. Prevention Conference VI: Diabetes and Cardiovascular Disease: Writing Group II: pathogenesis of atherosclerosis in diabetes. Circulation. 2002;105:e138–143. doi: 10.1161/01.cir.0000013954.65303.c5. [DOI] [PubMed] [Google Scholar]
- 30.Schneider DJ. Factors contributing to increased platelet reactivity in people with diabetes. Diabetes Care. 2009;32:525–527. doi: 10.2337/dc08-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeboah J, Bertoni AG, Herrington DM, Post WS, Burke GL. Impaired fasting glucose and the risk of incident diabetes mellitus and cardiovascular events in an adult population: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2011;58:140–146. doi: 10.1016/j.jacc.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Ramirez C, Sabate M, Jimenez-Quevedo P, Hernandez R, Moreno R, Escaned J, Alfonso F, Banuelos C, Costa MA, Bass TA, Macaya C. Platelet function profiles in patients with type 2 diabetes and coronary artery disease on combined aspirin and clopidogrel treatment. Diabetes. 2005;54:2430–2435. doi: 10.2337/diabetes.54.8.2430. [DOI] [PubMed] [Google Scholar]
- 33.Khandekar MM, Khurana AS, Deshmukh SD, Kakrani AL, Katdare AD, Inamdar AK. Platelet volume indices in patients with coronary artery disease and acute myocardial infarction: an Indian scenario. J Clin Pathol. 2006;59:146–149. doi: 10.1136/jcp.2004.025387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khode V, Sindhur J, Kanbur D, Ruikar K, Nallulwar S. Mean platelet volume and other platelet volume indices in patients with stable coronary artery disease and acute myocardial infarction: A case control study. J Cardiovasc Dis Res. 2012;3:272–275. doi: 10.4103/0975-3583.102694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider DJ, Nordt TK, Sobel BE. Attenuated fibrinolysis and accelerated atherogenesis in type II diabetic patients. Diabetes. 1993;42:1–7. doi: 10.2337/diab.42.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Jindal S, Gupta S, Gupta R, Kakkar A, Singh HV, Gupta K, Singh S. Platelet indices in diabetes mellitus: indicators of diabetic microvascular complications. Hematology. 2011;16:86–89. doi: 10.1179/102453311X12902908412110. [DOI] [PubMed] [Google Scholar]
- 37.Bae SH, Lee J, Roh KH, Kim J. Platelet activation in patients with diabetic retinopathy. Korean J Ophthalmol. 2003;17:140–144. doi: 10.3341/kjo.2003.17.2.140. [DOI] [PubMed] [Google Scholar]
- 38.Sewell R, Ibbotson RM, Phillips R, Carson P. High mean platelet volume after myocardial infarction: is it due to consumption of small platelets? Br Med J (Clin Res Ed) 1984;289:1576–1578. doi: 10.1136/bmj.289.6458.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demirtunc R, Duman D, Basar M, Bilgi M, Teomete M, Garip T. The relationship between glycemic control and platelet activity in type 2 diabetes mellitus. J Diabetes Complications. 2009;23:89–94. doi: 10.1016/j.jdiacomp.2008.01.006. [DOI] [PubMed] [Google Scholar]


