Abstract
Objective: To study the efficacy of bone marrow mesenchymal stem cell (MSC) transplantation to treat pulmonary emphysema in rats. Methods: Sixty rats were randomly divided into control, model and transplantation groups. Each group contained 20 rats. Rat models of emphysema were established via intratracheal instillation of lipopolysaccharide and exposure of model and transplantation groups to smoke. Then, cultured bone marrow MSCs were injected into rats in the transplantation group via the tail vein. Pathological changes of the lung in rats were observed. Results: Emphysemic pathological changes were found in model and transplantation groups, but changes were significantly attenuated after transplantation, compared with that of the model group. The mean alveoli number (MAN) and pulmonary alveolar area (PAA) showed statistically significant differences among the three groups (P < 0.001). The MAN in the transplantation group was higher than that in the model group, but still lower than that in the control group. The PAA in the transplantation group was lower than that in the model group, but higher than that in the control group (P < 0.05). After transplantation, Brdu-positive cells were observed and CK was expressed in a small number of Brdu-positive cells. Brdu- and CK-positive cells were not found in control and model groups. Conclusion: Transplantation of bone marrow MSCs can significantly attenuate lung inflammation and pathological changes in emphysemic rats, which may be associated with MSC differentiation into alveolar epithelial cells in recipient lung tissues.
Keywords: Bone marrow, mesenchymal stem cells, transplantation, emphysema
Introduction
Chronic obstructive pulmonary disease (COPD) is a common chronic disease of the respiratory system, and persistent airway obstruction caused by emphysema and/or chronic bronchitis often leads to dyspnea and decline of activity tolerance because of lung function impairment [1]. Emphysema occurs as a result of the destruction of alveolar walls, and alveolar expansion, and it is one of the leading causes of airway obstruction in patients with COPD, thus greatly affecting patient health. Mesenchymal stem cells (MSCs) are present in bone marrow and other tissues, with multipotency, high plasticity and a chemotactic ability [2,3]. MSCs can migrate into tissues and differentiate into several mesoderm-derived cell types under appropriate conditions [4,5]. The advanced progress of stem cell research in the past 20 years has provided a novel insight into bone marrow MSC treatment of many diseases and achieved preliminary results. This study aimed to elucidate the effect of MSC transplantation on an animal model of emphysema.
Materials and methods
Reagents
Lipopolysaccharide (LPS); mouse anti-rat cytokeratin monoclonal antibody; 5-bromo-2-deoxy-uridine (Brdu); anti-Brdu monoclonal antibody; polymer double staining kit.
Subjects
Sixty male Wistar rats, weighing 160 ± 12 g, aged 8-10 weeks, were used in this study. Rats were randomly divided into three groups: transplantation, model and control, with 20 rats in each.
MSC isolation, culture and labeling
Rat bilateral femurs were removed under sterile conditions, and bone marrow was collected. MSCs were isolated from bone marrow using an adherent method and seeded into 25 cm2 culture flasks. MSCs were incubated in L-DMEM supplemented with 10% fetal bovine serum at 37°C with 5% CO2 for 24 hours. Culture medium was exchanged to remove non-adherent cells, and then medium was exchanged every 3-5 days. At 80% confluence, cells were digested with 2.5 g/L trypsin and subcultured. Cells were subcultured to passage seven and then labeled with 10 mg/L Brdu.
Experimental procedure
Model and transplantation groups of rats received intratracheal instillation of 1 g/L LPS (200 μL), while control rats received 200 μL normal saline on days 1 and 15. Model and transplantation groups were exposed to fumigation in custom-made smoke boxes for 1 hour per day, during days 2-14 and 16-35. Control rats were housed in a conventional environment and allowed free access to food and water. Rats in the transplantation group were transplanted with a 0.5 mL Brdu-labeled MSC suspension (5 × 106 cells) via the tail vein on day 36. Control and model groups were injected with 0.5 mL normal saline via the tail vein.
Pathological examination
All rats were sacrificed on day 30 after transplantation, and lung tissue was collected, rinsed with normal saline and fixed in paraformaldehyde. The middle and inferior lobe of the right lung was embedded in paraffin, sectioned and stained with hematoxylin-eosin (HE). Two randomly selected fields of vision from each section were observed with a micro-computer image processing system (× 200). Mean alveoli number (MAN) and pulmonary alveolar area (PAA) were calculated according to the following formula: MAN = number of alveoli within each field/area of the field, this value indicated the alveolar density. PAA indicated the alveolar size by measuring the gray value of the lung parenchyma in each field.
MSC detection
Lung tissue sections were stained with DAB in strict accordance with the instructions of the ready-to-use SP series kit. The mouse anti-rat Brdu monoclonal antibody dilution was 1:60.
CK detection
CK expression in Brdu-labeled cells within lung tissue sections was determined with immunohistochemical staining. According to the manufacturer’s instructions of the double staining kit, sections were stained with DAB and 3-amino-9-ethylcarbazole. Primary monoclonal antibodies were mouse anti-rat Brdu (1:60) and mouse anti-rat CK (1:100). The secondary antibody was biotinylated goat anti-mouse working fluid. The negative control was injected with PBS instead of the primary antibody. The Brdu-positive signal was red under a light microscope and the CK-positive signal was yellow-brown.
Statistical analysis
Data were analyzed with SPSS 13.0 software. The PAA and MAN among the three groups were compared using one-way analysis of variance, and pairwise comparisons were performed using the LSD-t test. The test level was defined as α = 0.05.
Results
Histopathological changes in lung tissues of rats
At 1 week after smoke exposure, rats showed a low appetite, poor diet, lusterless hair, listless performance, unsteady gait and decreased activity. Microscopic observations after HE staining showed that changes in the lung tissue of rats in model and transplantation groups were chronic bronchitis- and emphysemic-like. Moreover, inflammatory cell infiltration and pulmonary congestion were significantly attenuated in the transplantation group, compared with that of the model group (Figure 1). The PAA and MAN of the three groups are shown in Table 1.
Figure 1.
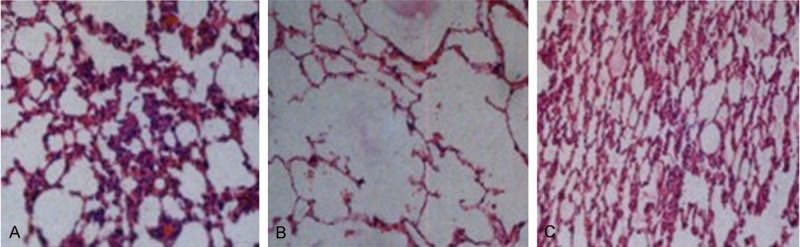
Histopathological changes in lung tissues of rats in the three groups (hematoxylin-eosin staining, × 200). (A) Transplantation, (B) model and (C) control groups.
Table 1.
Mean alveoli number (MAN) and pulmonary alveolar area (PAA) in rats of the three groups
| Group | N | MAN/(n/mm) | PAA/% |
|---|---|---|---|
| Control | 20 | 341.21 ± 17.12 | 55.37 ± 2.13 |
| Model | 20 | 183.34 ± 15.46 | 76.52 ± 4.02 |
| Transplant | 20 | 244.79 ± 15.71 | 62.35 ± 3.69 |
| F | 485.046 | 196.372 | |
| P | < 0.001 | < 0.001 |
P < 0.01, transplantation vs. control group; P < 0.01, transplantation vs. model group.
Identification of MSCs in lung tissue
After transplantation, cell nuclei in pulmonary alveoli and bronchial walls of rat lung tissue were stained brown, while no staining was found in lung tissue sections of rats without transplantation (Figure 2).
Figure 2.
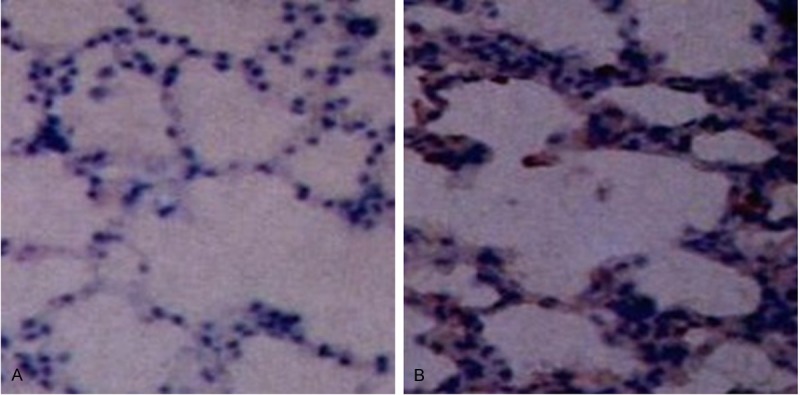
Brdu-labeled mesenchymal stem cells (SP, × 400). (A) Control and (B) transplantation groups.
CK detection results
Double-staining immunohistochemistry showed that the yellow-stained cytoplasm was also stained red, while double-staining was not observed in lung tissues of the other two groups (Figure 3).
Figure 3.
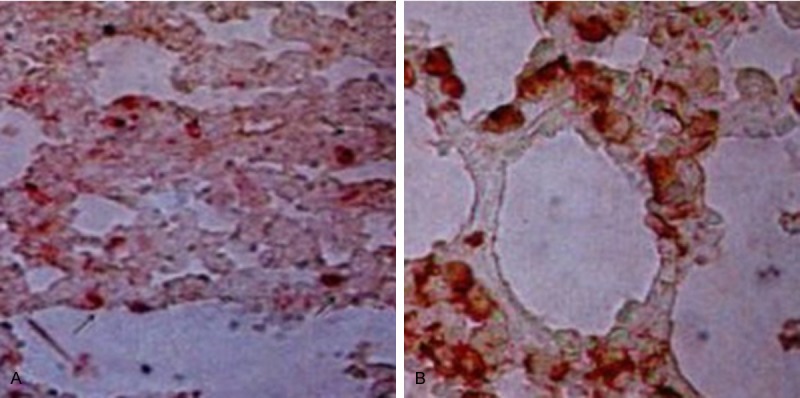
CK staining results (immunohistochemical double-staining, × 400). (A) Control and (B) transplantation groups.
Discussion
Gross et al [6] was the first to find that intratracheal instillation of papain can induce emphysema, and accordingly numerous researchers have designed a number of animal models for emphysema. Song et al [7] modeled emphysema via intratracheal instillation of LPS and fumigation. In this study, we established rat models of emphysema using this method. Rat models showed lusterless hair, decreased activity, increased oral secretions and wheezing at rest. Pathological changes of lung tissue sections included alveolar expansion and convergence, thickened alveolar septa, large alveolar diameter, inflammatory cell infiltration, capillary dilatation and congestion. The appearance and pathological changes are very similar to that of emphysema in humans. Lung inflammation in rats was significantly reduced after transplantation, compared with that of the model group, and pathological indicators were obviously improved. These results indicate that MSC transplantation has a positive effect on emphysemic rats.
MSCs are able to differentiate into alveolar cells. For example, alveolar type I and II cells as well as fibroblasts can be obtained using a whole bone marrow transplantation method [8-10]. Krause et al [11] injected labeled bone marrow stem cells from male mice into female mice after irradiation, and obtained a group of long-period aggravated updated cells, which homed to the bone marrow after 48 hours. These cells were harvested and diluted, then injected into another group of female recipient mice so that each female mouse had been injected with bone marrow cells from only a male mouse. Eleven months later, Y chromosome and epithelial cell-specific marker CK expression confirmed the differentiation of donor stem cells, including alveolar type II cells, which accounted for more than 20% of cells and expressed keratin and surface-active substance B, and tracheal epithelial cells accounted for less than 4%. These two kinds of cells were both positive for the Y chromosome. This study also demonstrated that Brdu-labeled MSCs were detectable in the lungs of rats after transplantation, and a small number of Brdu-positive cells expressed CK, indicating that the transplanted MSCs differentiated into epithelial cells in the lungs. In this study, MSC transplantation can ameliorate the inflammatory and pathological changes in the lung of rat models of emphysema, and this improvement may be related to transplanted MSC colonization and differentiation into alveolar cells in the lung.
Acknowledgements
Science and Technology Department of Henan Province, No. 112102310180.
Disclosure of conflict of interest
Gratitude is extended to the Science and Technology Department of Henan Province for its funding.
References
- 1.Tochino Y, Asai K, Hirata K. Bronchial asthma: progress in diagnosis and treatments. Topics: I. Basic knowledge; 4. Differentiation and coexistence with COPD. Nihon Naika Gakkai Zasshi. 2013;102:1352–8. doi: 10.2169/naika.102.1352. [DOI] [PubMed] [Google Scholar]
- 2.Zhou JB, Zhang J, Bai CX. Progress in the mechanisms of chronic obstructive pulmonary disease co-morbid lung cancer. Zhonghua Jie He He Hu Xi Za Zhi. 2012;35:288–90. [PubMed] [Google Scholar]
- 3.Dunt D, Doyle C. Signs of progress in the Australian post-2000 COPD experience, but some old problems remain. Int J Chron Obstruct Pulmon Dis. 2012;7:357–66. doi: 10.2147/COPD.S30003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawamoto K, Konno M, Nagano H, Nishikawa S, Tomimaru Y, Akita H, Hama N, Wada H, Kobayashi S, Eguchi H, Tanemura M, Ito T, Doki Y, Mori M, Ishii H. CD90-(Thy-1-) High Selection Enhances Reprogramming Capacity of Murine Adipose-Derived Mesenchymal Stem Cells. Dis Markers. 2013;35:573–9. doi: 10.1155/2013/392578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu C. Transplantation of neural stem cells and bone marrow mesenchymal stem cells in treatment of spinal cord injury. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2012;26:186–9. [PubMed] [Google Scholar]
- 6.Gross P, Pfitzer EA, Tolker E. Experimental emphysemaits production with papain in normal and silicotic rats. Arch Envrion Health. 1965;11:50–8. doi: 10.1080/00039896.1965.10664169. [DOI] [PubMed] [Google Scholar]
- 7.Song YP, Cui DJ, Mao PY. Establishment and drug intervention of chronic obstructive pulmonary disease. Chin J Intern Med. 2000;39:556. [Google Scholar]
- 8.Abe S, Lauby G, Boyer C, Rennard SI, Sharp JG. Transp lanted BM and BM side population cells contribute progeny to the lung and liver in irradiated mice. Cytotherapy. 2003;5:523. doi: 10.1080/14653240310003576. [DOI] [PubMed] [Google Scholar]
- 9.Gao F, Wei D, Wu B, Zhou M, Zhang J, Chen JY. Bilateral lung transplantation for bronchiolitis obliterans after allogeneic bone marrow transplantation: a case report and literature review. Zhonghua Xue Ye Xue Za Zhi. 2013;34:669–72. doi: 10.3760/cma.j.issn.0253-2727.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Suga A, Ueda K, Takemoto Y, Nishimoto A, Hosoyama T, Li TS, Hamano K. Significant role of bone marrow-derived cells in compensatory regenerative lung growth. J Surg Res. 2013;183:84–90. doi: 10.1016/j.jss.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Krause DS, Theise ND, Collector MI. Multiorgan, multilineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]


