Abstract
To investigate the expression of Ataxin-3 in human gastric cancer tissues and cell lines, and explore its clinical pathologic significance. Methods: The expression of Ataxin-3 in gastric cancer (n=536) and noncancerous gastric mucosa (n=312) was determined by immunohistochemistry and correlated to clinicopathologic features such as histologic differentiation and tumor size. The expression of Ataxin-3 protein in the human gastric cancer cell lines MKN45, SGC7901 and in normal human gastric epithelial cells (GES-1) was also evaluated by Western blot analysis. Quantitative real-time PCR was used to determine Ataxin-3 mRNA expression in human gastric cancer cell lines and tissues. Results: The expression of Ataxin-3 protein was decreased in the gastric cancer compared to noncancerous gastric tissue, and correlated with tumor size, Lauren classification, histologic differentiation, and mutant p53 protein (P < 0.05). Similarly, Ataxin-3 mRNA expression was decreased in the gastric cancers compared to the noncancerous gastric tissue. Ataxin-3 protein and mRNA expression was lower in MKN45, SGC7901 cells than in the normal GES-1 cells. Conclusion: Decreased expression of Ataxin-3 may play an important role in gastric carcinogenesis and development of gastric cancer.
Keywords: Ataxin-3, gastric cancer, clinicopathologic features
Introduction
Over the last several decades, there has been a decline in both the incidence and mortality of gastric cancer (GC), but the prevalence of gastric cancer in China remains higher than in most of the western countries [1-3]. The prognosis for patients with advanced gastric cancer is poor, and the overall 5-year survival rate is less than 30% after surgery [4]. Thus, additional research investigating the molecular mechanisms involved in gastric cancer initiation and progression is necessary to identify valuable biomarkers for early diagnosis and to develop novel therapeutic strategies.
The ubiquitin proteasome system (UPS) plays an important role in cellular homeostasis by degrading damaged proteins and preventing the abnormal accumulation of misfolded proteins. In some cases, the amount of proteins to be degraded exceeds the degrading capacity of the UPS, which can result in the abnormal accumulation of ubiquitinated proteins and may eventually cause cell dysfunction, cells death, or possibly major diseases, such as cancer [5]. Deubiquitinating enzymes (DUB) are an important part of the UPS, and function in the deubiquitination of proteins by recognizing specific sequences in ubiquitin (Ub) and proteasome substrates. The process of deubiquitination is closely related to the occurrence of many kinds of tumors [6].
The human Ataxin-3 protein is encoded by the ATXN3 gene located on chromosome 14q21, and is expressed by cells throughout the body [7]. Ataxin-3 is a deubiquitinating enzyme (DUB) that interacts with poly-Ub chains (≥ 4 ubiquitin subunits) through its Josephin domain (JD) in the N-terminus and its Ub interaction motifs (UIMs) in the C-terminus [8]. It can cleave ubiquitin from unneeded proteins. Ataxin-3 binds and trims long poly-Ub chains and can prevent further chain extension, thus restricting the length of Ub chains on its substrate proteins. This enzymatic function targets proteins to specific pathways, such as proteasomal degradation, and is also important for the maintenance of Ub recycling [9,10]. Similarly to other DUBs in the UPS, Ataxin-3 is important for many cellular functions, such as protein homeostasis [11], the regulation of transcription [12], cytoskeleton regulation [13], myogenesis [14], degradation of mis-folded proteins [15] and cell cycle progression and cell death [16-18]. DUB dysregulation is a frequent event in cancer [19]. By comprehensive screening of human cancer for DUB dysregulation, Chiara L et al reported gastric carcinomas as examples of tumors with DUB downregulation [6].
Defects in the Ataxin-3 protein are the major cause of a neurologic disease called spinocerebellar ataxia type 3 (SCA3) (also known as Machado-Joseph disease (MJD)). A feature of the disease is the presence of inclusions of aggregated pathological protein, due to an abnormally expanded polyglutamine (PolyQ) region of Ataxin-3 [20]. Ataxin-3 normally contains 12-41 glutamines near the C-terminus, but in Ataxin-3 mutants there is an expansion of poly-Q repeats to 62-84 glutamines. Both normal and mutant Ataxin-3 proteins are degraded by the ubiquitin-proteasome pathway (UPP) [21].
However, the cellular functions of Ataxin-3 are currently poorly understood and the expression of Ataxin-3 in cancer tissues has not yet been examined. Therefore, we designed a study to investigate the levels of Ataxin-3 expression in human gastric adenocarcinoma tissues and gastric cancer cell lines to determine if the expression level of Ataxin-3 correlates with clinicopathologic features and prognosis of gastric cancer patients.
Materials and methods
Patients and tissue samples
A total of 536 patients with primary gastric adenocarcinoma, who underwent curative surgery at the Guangxi tumor hospital between January 2001 and December 2011, were selected in this research. The research study was approved by the hospital’s Ethics Committee. All patient tissue samples were formalin-fixed, paraffin-embedded, and clinically and histopathologically diagnosed. No patients had received treatment prior to admission. Patient data were retrieved from the operative reports. Follow-up data were obtained by phone and from the outpatient clinical database. This study comprised of samples from 375 men and 161 women aged 22 to 88 years old. The median age was 50 years. Based on the Lauren classification, 220 tumors were intestinal, 172 were diffuse, and 144 were mixed gastric cancers. According to the tumor, nodes, and metastasis 7th UICC/AJCC TNM Staging for Gastric Cancer, 50 cancers were categorized as stage I, 197 were stage II, 221 were stage III, and 68 were stage IV. There were 10 highly differentiated tumors, 100 moderately differentiated and 426 poorly differentiated. A total of 312 samples of noncancerous gastric mucosa were obtained from adjacent gastrectomy margins. The deadline for follow-up was July 2013. The survival time was counted from the date of surgery to the end of the follow-up or to the date of death. Deaths mainly occurred due to recurrence or metastasis.
The other patient cohort included 14 patients whose tumors were excised during November 2012 at the First Affiliate Hospital of Guangxi Medical University. These GCs and companion noncancerous gastric mucosa tissues were collected for quantitative real-time RT-PCRs.
Immunohistochemistry
Ataxin-3 and p53 were detected by immunohistochemical staining. Anti-Ataxin-3 polyclonal rabbit antibody (1:200; Abnova Corporation, Taipei) and Anti-p53 mouse monoclonal antibody (1:200; Zhongsanjinqiao, Beijing, China) were used to stain tissues. Immunohistochemical staining was performed using the Super Vision Two Steps method, on 10% formalin-fixed, paraffin-embedded, 4-5 μm serial paraffin sections. Immunohistochemical analysis was used to evaluate protein expression in 312 noncancerous gastric tissues and 536 GC tissues. According to the protocol (Abnova Corporation) for immunohistochemistry on paraffin-embedded tissue sections, slides were baked at 60°C for 12 h followed by deparaffinization with xylene and rehydration. The sections were submerged into 10 mM sodium citrate (pH 6.0) antigen retrieval buffer and microwaved for antigen retrieval, after which they were treated with 3% hydrogen peroxide for 10 minutes to block endogenous peroxidase activity. Sections were incubated with primary antibody (diluted 1:200) overnight at 4°C. Tissue sections were then treated with broad-spectrum secondary antibody (Changdao, Shanghai) for 1 hour at room temperature, stained with diaminobenzidine (DAB) at room temperature, and examined with a microscope. All tissue sections were counterstained with hematoxylin, dehydrated, and mounted. For p53 staining ethylenediaminetetraacetic acid (EDTA, pH 8.0) and high pressure were used for antigen retrieval. Negative control sections, without primary antibody, were subjected to the same procedures. No positive staining was observed in the negative control slides. The staining was predominantly cytoplasmic for Ataxin-3 and nuclear for p53.
Evaluation of immunohistochemical staining
The percentage of positive cells and the staining intensities of each sample were evaluated and scored independently by at least two observers. The percentage of positive cells was graded on a scale of 0-4: 0-5% stained cells scored as 0, 6-25% positive cells scored as 1, 26-50% positive as 2, 51-75% stained cells as 3 and 76%-100% positive cells 4. Staining intensity was graded from 0-3 according to the following criteria: 0 (no staining), 1 (weak staining, light yellow), 2 (moderate staining, yellow brown), and 3 (strong staining, brown). The raw data were converted to an immunohistochemical score (IHS) by multiplying the quantity and intensity scores [22]. An IHS score of 9-12 was considered strong immunoreactivity (+++), 5-8 as moderate (++), 1-4 as weak (+), and 0 as negative (-) [23].
Cell lines
Human GC cell lines MKN45 and SGC7901, as well as normal human gastric epithelial cells (GES-1) were purchased from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin and 10% fetal bovine serum. The cells were grown at 37°C in the presence of 5% CO2 and 95% O2 in a humidified incubator.
Western blot analysis
Untreated cells were seeded in 6-well plates at a density of 0.2×106 cells/ml with fresh complete culture medium. SGC7901, MKN45 and GES-1 cells were lysed with cell lysis buffer (50 mM Tris [pH 7.2], 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, and 500 mM NaCl, 10 mM MgCl2) in the presence of protease inhibitors (Roche). Equal quantities of proteins were then separated by 12% SDS-PAGE and transferred onto a PVDF membrane (Millipore) using the Bio-Rad microarray system (Bio-Rad). Membranes were blocked with 5% non-fat milk in TBS-T (120 mM Tris-HCl [pH 7.4], 150 mM NaCl, and 0.05% Tween 20) for 1 hour and then incubated with antibodies against Ataxin-3 (1:500, Genetex) or β-Actin (1:1000, Santa Cruz) overnight at 4°C. After antibody incubation, membranes were washed and then incubated with HRP-conjugated secondary antibodies (Santa Cruz) for 1 hour at room temperature. Luminometric detection of target proteins was obtained with SuperSignal West Dura Chemiluminescent Substrate (Pierce, Thermo Scientific) and visualized by a CCD camera imager (Bio-Rad).
Quantitative real-time RT-PCR assay
Total RNA was extracted from the cells and tissues using the Trizol reagent (Life, USA). Approximately 1 μg of RNA was used for the reverse transcription reaction with Oligo dT (18T) (Omega). The cDNA was amplified with the following primers: 5’- CCAGAACATCATCC-CTGCCT -3’ (forward) and 5’- CCTGCTTCACCA-CCTTCTTG -3’ (reverse) for GAPDH; 5’- GGAGTCCATCTTCCACGAGA -3’ (forward) and 5’- GATCGATCCTGAGCCTCTGA -3’ (reverse) for Ataxin-3; Quantitative real-time PCR was carried out in triplicate with SYBR Green PCR Master Mix using a 7900HT qPCR system thermal cycler (Applied Biosystems. Foster City, CA). GAPDH mRNA was used as an internal control for each sample, and the Ct value for each sample was normalized to GAPDH mRNA. The experiments were repeated three times.
Statistical analysis
All statistical analyses were performed using the SPSS16.0 software. Correlation of Ataxin-3 protein expression with immunohistochemistry and clinicopathologic parameters was evaluated by χ2 test or Fisher’s exact probability test. The follow-up time was calculated from the date of surgery to the date of death, or the last known follow-up. Independent prognostic factors were analyzed by the Cox proportional hazards regression model. P < 0.05 was considered statistically significant.
Results
Expression of Ataxin-3 in gastric cancer and noncancerous mucosa
Ataxin-3 protein was detected in all 316 (100%) noncancerous mucosa. The majority of the positive cells (by IHC) were located in fundic gland epithelium and the basal parts of the glands displayed the most intense immunostaining, from ++ to +++ (Figure 1 and Table 1). Positive Ataxin-3 expression was detected in 390 of the 536 (72.76%) human GCs examined by IHC, while 146 (27.24 %) GCs were negative (Figure 1 and Table 1). Ataxin-3 staining was detected in the majority of normal gastric mucosal epithelia cells, ganglioneures, and in part of the smooth muscle cells. Ataxin-3 was also found in the cytoplasm of GC cells but the staining was weaker (+ to ++) than in the fundic gland epithelial cells. The difference in Ataxin-3 expression between gastric cancer and noncancerous mucosa was statistically significant (P < 0.001, Table 1).
Figure 1.
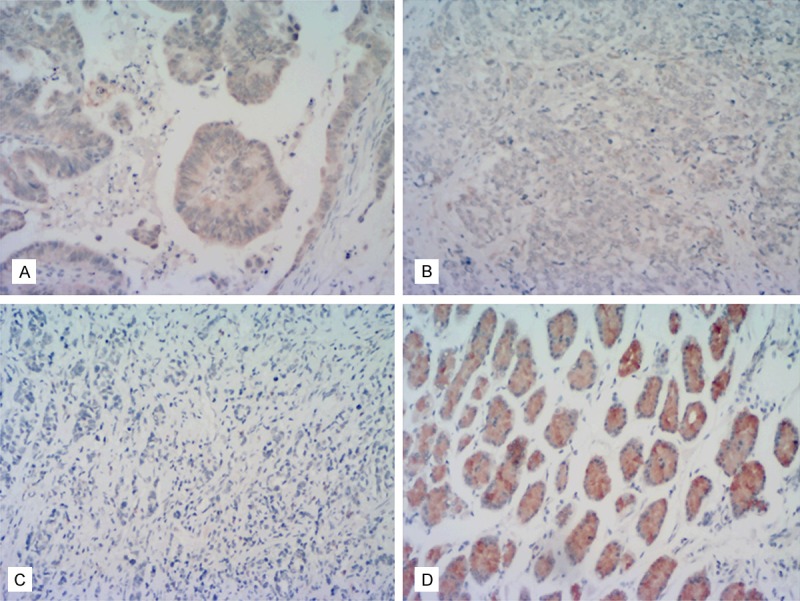
Expression of Ataxin-3 protein in human gastric cancer and noncancerous tissues. A: Representative tissue section showing moderate immunoreactivity (++) in GC tissue. B: An example of weak immunoreactivity (+) in GC tissue. C: An example of a GC tumor with negative immunoreactivity (-). D: Noncancerous gastric mucosa exhibiting strong Ataxin-3 immunoreactivity (+++). (All slides ×200 magnification).
Table 1.
Expression of Ataxin-3 protein (IHC) in gastric cancer and noncancerous gastric mucosa tissues
| Groups | n | Ataxin-3 | |||
|---|---|---|---|---|---|
|
| |||||
| - | + | ++ | +++ | ||
| Gastric cancer tissue | 536 | 146 | 283 | 107 | 0 |
| Noncancerous gastric mucosa | 312 | 0 | 22 | 236 | 54 |
Ataxin-3 protein expression and clinicopathologic characteristics
Ataxin-3 expression correlated with tumor size (Spearman’s rho = -0.087, P < 0.05), Lauren classification (Spearman’s rho = -0.384, P < 0.000), histologic differentiation (Spearman’s rho = -0.230, P < 0.000) and p53 expression (Spearman’s rho = +0.108, P < 0.05). And it has no Ataxin-3 expression did correlate with age, gender, tumor site, Bormann type, invasion depth, lymph node metastasis, distant metastasis, and TNM stages (Table 2). The factors for possible prognostic effects in gastric cancer were analyzed by Cox regression analysis (Table 3). The multivariate analysis suggested that tumor site, tumor size, Lauren classification, histologic differentiation, TNM stages and the expression of p53 (P < 0.05) were independent prognostic factors for patients with GC. However, age, gender, Bormann type, smoking and drinking were not prognostic indicators for GC.
Table 2.
The correlation of Ataxin-3 expression (IHC) with clinicopathological parameters
| parameters | Ataxin-3 | ||||
|---|---|---|---|---|---|
|
| |||||
| - | + | ++ | F/X2 | P | |
| Age (y) | |||||
| < 60 | 85 | 164 | 67 | 0.744 | 0.689 |
| ≥ 60 | 61 | 119 | 40 | ||
| Gender | |||||
| male | 101 | 199 | 75 | 0.061 | 0.970 |
| female | 45 | 84 | 32 | ||
| Tumor site | |||||
| Upper 1/3 | 34 | 60 | 26 | 4.097 | 0.661 |
| Middle 1/3 | 37 | 66 | 31 | ||
| Lower 1/3 | 71 | 143 | 48 | ||
| Two-thirds or more | 4 | 14 | 2 | ||
| Tumor size (cm) | |||||
| < 5 | 76 | 140 | 71 | 9.079 | 0.011 |
| ≥ 5 | 70 | 143 | 36 | ||
| Borrmann type | |||||
| Borrmann 0 | 9 | 9 | 3 | 8.308 | 0.078 |
| Borrmann I~II | 48 | 69 | 36 | ||
| Borrmann III~IV | 89 | 205 | 68 | ||
| Lauren’s classification | |||||
| Intestinal | 28 | 120 | 72 | 83.952 | 0.000 |
| Mixed | 45 | 100 | 27 | ||
| Diffuse | 73 | 63 | 8 | ||
| Histological differentiation | |||||
| Well | 1 | 5 | 4 | 30.117 | 0.000 |
| Moderate | 10 | 57 | 33 | ||
| Poor | 135 | 221 | 70 | ||
| Invasion depth | |||||
| T1 | 14 | 14 | 5 | 7.849 | 0.249 |
| T2 | 10 | 13 | 10 | ||
| T3 | 34 | 79 | 29 | ||
| T4 | 88 | 177 | 63 | ||
| Lymph node metastasis | |||||
| N0 | 62 | 110 | 43 | 1.473 | 0.961 |
| N1 | 25 | 55 | 19 | ||
| N2 | 30 | 57 | 19 | ||
| N3 | 29 | 61 | 26 | ||
| Distant metastasis | |||||
| Yes | 124 | 245 | 98 | 2.604 | 0.272 |
| No | 22 | 38 | 9 | ||
| TNM stage | |||||
| I | 16 | 22 | 12 | 5.413 | 0.492 |
| II | 55 | 105 | 37 | ||
| III | 53 | 119 | 49 | ||
| IV | 22 | 37 | 9 | ||
| p53 | |||||
| - | 65 | 86 | 33 | 9.729 | 0.045 |
| + | 29 | 69 | 23 | ||
| ++ to +++ | 52 | 128 | 51 | ||
Table 3.
Multivariate Cox regression survival analysis of clinicopathological parameters and Ataxin-3 expression
| Parameters | B | SE | Wald | Exp (B) | P |
|---|---|---|---|---|---|
| Tumor site | 9.082 | 0.028 | |||
| Tumor site (1) | -0.478 | 0.279 | 2.925 | 0.620 | |
| Tumor site (2) | -0.595 | 0.278 | 4.571 | 0.551 | |
| Tumor site (3) | -0.747 | 0.274 | 7.446 | 0.474 | |
| Tumor size | 0.301 | 0.130 | 5.401 | 1.352 | 0.020 |
| Lauren’s classification | 11.307 | 0.004 | |||
| Lauren’s classification (1) | -0.444 | 0.138 | 10.389 | 0.641 | 0.001 |
| Lauren’s classification (2) | -0.076 | 0.146 | 0.272 | 0.927 | 0.602 |
| *TNM stage | 168.231 | 0.000 | |||
| TNM stage (1) | -3.199 | 0.443 | 52.094 | 0.041 | 0.000 |
| TNM stage (2) | -2.281 | 0.185 | 152.277 | 0.102 | 0.000 |
| TNM stage (3) | -1.238 | 0.158 | 61.362 | 0.290 | 0.000 |
| P53 | 11.356 | 0.003 | |||
| P53 (1) | -0.451 | 0.136 | 11.054 | 0.637 | 0.001 |
| P53 (2) | -0.091 | 0.146 | 0.395 | 0.913 | 0.530 |
TNM, tumor-node-metastasis.
Expression of Ataxin-3 protein in normal gastric cells and cancer cell lines
Of the three cell lines examined, Ataxin-3 protein expression was highest in human normal gastric epithelial cells (GES-1) and higher in the human GC cell line SGC7901 than in MKN45. β-Actin was used as a loading control (Figure 2).
Figure 2.
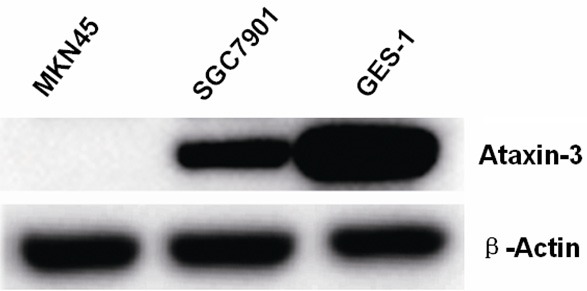
Western blot analysis of Ataxin-3 protein expression in human GC cell lines (MKN45 and SGC7901) and human normal gastric epithelial cells (GES-1). Ataxin-3 protein expression was highest in the GES-1 cells and higher in SGC7901 than MKN45.
Ataxin-3 mRNA expression in gastric cancer tissue and gastric cancer cell lines
Ataxin-3 mRNA was higher in the noncancerous control tissues and cell line than in the gastric cancer tissue and gastric cancer cell lines (Figure 3).
Figure 3.
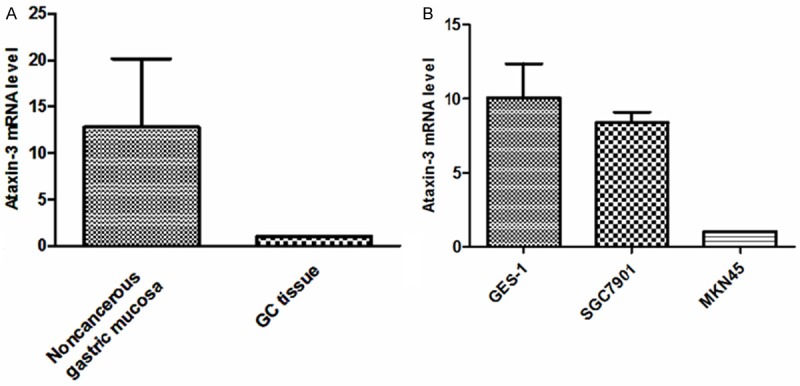
Quantitative RT-PCR analysis of Ataxin-3 mRNA expression. Ataxin-3 mRNA expression was lower in gastric cancer tissues and gastric cancer cell lines than in the control noncancerous gastric tissues and cell line. A: Expression of Ataxin-3 mRNA in human tissue samples (GC - n = 14; noncancerous gastric mucosa - n = 14; Standard Error of the Mean (SEM), experiment performed in triplicate; Ataxin-3 mRNA expression relative to GAPDH). B: Expression of Ataxin-3 mRNA in human GC cell lines (MKN45 and SGC7901) and in normal gastric epithelial cells (GES-1).
Discussion
We found that Ataxin-3 is expressed in the majority of normal gastric mucosal epithelia and also in ganglioneures, in the center cells of lymph follicles, and in a portion of smooth muscle cells. Our observation that Ataxin-3 protein expression is widespread in human tissues is consistent with results previously reported [7,24]. Additionally, Ataxin-3 mRNA expression in gastric cancers was lower than in the control noncancerous mucosa. We found similar results in the gastric cancer cell lines and a normal gastric epithelial cell line. As a deubiquitinating enzyme, Ataxin-3 can interact with ubiquitin, polyubiquitin chains, ubiquitin-like protein Nedd8, and ubiquitinylated proteins [9]. We hypothesize that Ataxin-3 expression decreases in cells where protein metabolism becomes disordered and damaged proteins are produced which can lead to changes in the biological half-life of some important proteins, for example proteins required for cellular transformation may accumulate inside the cells resulting in malignant transformation and tumor initiation and progression. Our research suggests that Ataxin-3 might be associated with the progression of gastric cancer, but the underlying molecular mechanisms for this process need to be further examined.
According to the Lauren’s classification [25], there are three types (intestinal, diffuse and mixed types) of gastric carcinoma. The intestinal type is well differentiated tubular or papillary adenocarcinoma and is more common in older patients. The intestinal type often follows a process of multifocal atrophic gastritis to intestinal epithelial metaplasia and then malignant change to a cancer. The diffuse type is the most undifferentiated carcinoma and it is more common in young patients. There is no correlation with chronic atrophic gastritis and intestinal epithelial metaplasia in the diffuse type. The mixed histological type is a combination of the two types. The Lauren classification may represent different molecular mechanisms involved in the occurrence and development of GC. For example, micro satellite instability and methylation of promoter regions are usually present in the intestinal subtype, but the methylation of specific genes, such as E-cadherin (CDH1), are more common in the diffuse subtype [26]. It is not clear about how Ataxin-3 has the selectivity to the ubiquitination substrate? Our results showed that Ataxin-3 protein expression was lower in the diffuse subtype than in the intestinal type. The cell lines were derived from moderate to well differentiated gastric cancer tissues. Ataxin-3 expression was higher than that derived from poor differentiation of cell line. Ataxin-3 has deubiquitinylating activity through its JD domain and is able to bind and cleave some poly-ubiquitin chains, which are a common recognition motifs for proteasomal degradation, therefore Ataxin-3 can interact with some subunits of the proteasome [9,27]. Expression analysis of some of the putative Ataxin-3 substrates that are involved in the occurrence of the diffuse type of gastric cancer, may be an important next step for understanding how Ataxin-3 may play a role in malignant transformation.
The p53 tumor suppressor gene is a gene of the uppermost relationship to human tumors [28]. p53 gene mutations frequently occur in GC [29,30]. Wild type p53 gene is a tumor suppressor gene. Mutations in p53 changes its normal protein structure and was decreased its suppressive function, resulting in the transformation of cells. In our research, Ataxin-3 protein expression in gastric cancer tissues was significantly correlated with the expression of mutated p53, suggesting that Ataxin-3 may be related to the role of p53 in the oncogenic process of GC. Chou AH [16] report that polyglutamine expanded Ataxin-3 upregulates mRNA expression of Bax and PUMA and causes apoptotic death of affected neurons by enhancing phosphorylation and transcriptional activity of p53. They used the wild-type and SCA3 transgenic mice and found that wild and mutant types of Ataxin-3 have p53 transcriptional activity, by facilitating p53 phosphorylation at Ser15 residue and p53 DNA-binding activity to the Bax promoter sequence and formation of the DNA-protein complex. Some attempts to identify ubiquitin ligases that are responsible for the ubiquitination of mutant p53, suggested a role for the chaperone-associated ubiquitin ligase CHIP (C terminus of Hsc70-interacting protein); the research showed that CHIP can target both wild-type and mutant p53 for degradation [31,32]. As a deubiquitinating enzyme (DUB), Ataxin-3 participates in initiating, regulating and terminating the CHIP ubiquitination cycle. Ataxin-3 limits the length of ubiquitin chains on CHIP substrates and deubiquitinates Ub-CHIP, after polyubiquitinated substrates have formed [10]. These results provided molecular evidence for the interaction between p53 and Ataxin-3. Usually p53 mutation is a late event in the process of tumor formation. We have reason to speculate that the down-regulation of Ataxin-3 expression may be an early event in tumorigenesis; expression of Ataxin-3 can enhance the malignant features of cells by promoting the expression of mutant p53, such as in the carcinogenesis of gastric cancer.
Identification of prognostic factors for gastric cancer is of great importance for survival and treatment strategies. The patients’ survival results in our research show that tumor location, tumor size, Lauren’s classification, TNM stage, and the expression of mutant p53 protein were independent prognostic factors, which is consistent with previously published results about the prognosis of GC [33,34]. In this experiment, we did not find that Ataxin-3 related directly to the survival of the patients, so it may be a synergistic but not independent role in prognosis of GC patients. Some researchers proposed that Ataxin-3 may repress transcription by directly binding to chromatin, inhibiting histone acetylation, and recruiting repressor complexes [35]. For example, Ataxin-3 may regulate the transcription of matrix metalloproteinase-2 (MMP-2). Normally Ataxin-3 binds to target DNA sequences in specific chromatin regions of the MMP-2 gene promoter and represses transcription by recruitment of the histone deacetylase 3 (HDAC3), the nuclear receptor corepressor (NCoR), and deacetylation of histones bound to the promoter display the repressor function [12]. These indicate that Ataxin-3 has a complex molecule linked to tumor development and progression.
Because our study is presently the only examination of Ataxin-3 expression in human GC, more research into the cellular functions of Ataxin-3 should be done in the future.
In conclusion, Ataxin-3 abnormal expression may serve as a useful treatment target for GC. Thus, we believe that more research on Ataxin-3 may provide a base for identifying potential biomarkers for the occurrence, development and progression of GC.
Acknowledgements
This study was supported in part by the Guangxi Natural Science Foundation of China (2011GXNSFC018020, 2012GXNSFAA053163) and Foundation of Guangxi Provincial Department of Health (Z2012343). We would like to thank Dr. Laura Laviolette and Dr. David Pepin (Harvard University and Massachusetts General Hospital, Boston, USA) for their critical review of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Shin HR, Carlos MC, Varghese C. Cancer control in the Asia Pacific region: current status and concerns. Jpn $lxfS1$ 2012;42:867–881. doi: 10.1093/jjco/hys077. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Zhang S, Zhao P, Li G, Wu L, He J. Report of incidence and mortality in China cancer registries, 2009. Chin J Cancer Res. 2013;25:10–21. doi: 10.3978/j.issn.1000-9604.2012.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thun M, Jemal A, Desantis C, Blackard B, Ward E. An overview of the cancer burden for primary care physicians. Prim Care. 2009;36:439–454. doi: 10.1016/j.pop.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Shen M, Schmitt S, Buac D, Dou QP. Targeting the ubiquitin-proteasome system for cancer therapy. Expert Opin Ther Targets. 2013;17:1091–1108. doi: 10.1517/14728222.2013.815728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luise C, Capra M, Donzelli M, Mazzarol G, Jodice MG, Nuciforo P, Viale G, Di Fiore PP, Confalonieri S. An atlas of altered expression of deubiquitinating enzymes in human cancer. PLoS One. 2011;6:e15891. doi: 10.1371/journal.pone.0015891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albrecht M, Golatta M, Wullner U, Lengauer T. Structural and functional analysis of ataxin-2 and ataxin-3. Eur J Biochem. 2004;271:3155–3170. doi: 10.1111/j.1432-1033.2004.04245.x. [DOI] [PubMed] [Google Scholar]
- 8.Mao Y, Senic-Matuglia F, Di Fiore PP, Polo S, Hodsdon ME, De Camilli P. Deubiquitinating function of ataxin-3: insights from the solution structure of the Josephin domain. Proc Natl Acad Sci U S A. 2005;102:12700–12705. doi: 10.1073/pnas.0506344102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnett B, Li F, Pittman RN. The polyglutamine neurodegenerative protein ataxin-3 binds polyubiquitylated proteins and has ubiquitin protease activity. Hum Mol Genet. 2003;12:3195–3205. doi: 10.1093/hmg/ddg344. [DOI] [PubMed] [Google Scholar]
- 10.Scaglione KM, Zavodszky E, Todi SV, Patury S, Xu P, Rodríguez-Lebrón E, Fischer S, Konen J, Djarmati A, Peng J, Gestwicki JE, Paulson HL. Ube2w and ataxin-3 coordinately regulate the ubiquitin ligase CHIP. Mol Cell. 2011;43:599–612. doi: 10.1016/j.molcel.2011.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reina CP, Nabet BY, Young PD, Pittman RN. Basal and stress-induced Hsp70 are modulated by ataxin-3. Cell Stress Chaperones. 2012;17:729–742. doi: 10.1007/s12192-012-0346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evert BO, Araujo J, Vieira-Saecker AM, de Vos RA, Harendza S, Klockgether T, Wullner U. Ataxin-3 represses transcription via chromatin binding, interaction with histone deacetylase 3, and histone deacetylation. J Neurosci. 2006;26:11474–11486. doi: 10.1523/JNEUROSCI.2053-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodrigues AJ, do Carmo Costa M, Silva TL, Ferreira D, Bajanca F, Logarinho E, Maciel P. Absence of ataxin-3 leads to cytoskeletal disorganization and increased cell death. Biochim Biophys Acta. 2010;1803:1154–1163. doi: 10.1016/j.bbamcr.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 14.do Carmo Costa M, Bajanca F, Rodrigues AJ, Tome RJ, Corthals G, Macedo-Ribeiro S, Paulson HL, Logarinho E, Maciel P. Ataxin-3 plays a role in mouse myogenic differentiation through regulation of integrin subunit levels. PLoS One. 2010;5:e11728. doi: 10.1371/journal.pone.0011728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warrick JM, Morabito LM, Bilen J, Gordesky-Gold B, Faust LZ, Paulson HL, Bonini NM. Ataxin-3 suppresses polyglutamine neurodegeneration in Drosophila by a ubiquitin-associated mechanism. Mol Cell. 2005;18:37–48. doi: 10.1016/j.molcel.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 16.Chou AH, Lin AC, Hong KY, Hu SH, Chen YL, Chen JY, Wang HL. p53 activation mediates polyglutamine-expanded ataxin-3 upregulation of Bax expression in cerebellar and pontine nuclei neurons. Neurochem Int. 2011;58:145–152. doi: 10.1016/j.neuint.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Zhou L, Wang H, Wang P, Ren H, Chen D, Ying Z, Wang G. Ataxin-3 protects cells against H2O2-induced oxidative stress by enhancing the interaction between Bcl-X(L) and Bax. Neuroscience. 2013;243:14–21. doi: 10.1016/j.neuroscience.2013.03.047. [DOI] [PubMed] [Google Scholar]
- 18.Chou AH, Yeh TH, Kuo YL, Kao YC, Jou MJ, Hsu CY, Tsai SR, Kakizuka A, Wang HL. Polyglutamine-expanded ataxin-3 activates mitochondrial apoptotic pathway by upregulating Bax and downregulating Bcl-xL. Neurobiol Dis. 2006;21:333–345. doi: 10.1016/j.nbd.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Fraile JM, Quesada V, Rodriguez D, Freije JM, Lopez-Otin C. Deubiquitinases in cancer: new functions and therapeutic options. Oncogene. 2012;31:2373–2388. doi: 10.1038/onc.2011.443. [DOI] [PubMed] [Google Scholar]
- 20.La Spada AR, Taylor JP. Polyglutamines placed into context. Neuron. 2003;38:681–684. doi: 10.1016/s0896-6273(03)00328-3. [DOI] [PubMed] [Google Scholar]
- 21.Ying Z, Wang H, Fan H, Zhu X, Zhou J, Fei E, Wang G. Gp78, an ER associated E3, promotes SOD1 and ataxin-3 degradation. Hum Mol Genet. 2009;18:4268–4281. doi: 10.1093/hmg/ddp380. [DOI] [PubMed] [Google Scholar]
- 22.Hu BS, Yu HF, Zhao G, Zha TZ. High RSF-1 expression correlates with poor prognosis in patients with gastric adenocarcinoma. Int J Clin Exp Pathol. 2012;5:668–673. [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X, Cai H, Huang H, Long Z, Shi Y, Wang Y. The prognostic significance of apoptosis-related biological markers in Chinese gastric cancer patients. PLoS One. 2011;6:e29670. doi: 10.1371/journal.pone.0029670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitt I, Evert BO, Khazneh H, Klockgether T, Wuellner U. The human MJD gene: genomic structure and functional characterization of the promoter region. Gene. 2003;314:81–88. doi: 10.1016/s0378-1119(03)00706-6. [DOI] [PubMed] [Google Scholar]
- 25.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 26.Qiu MZ, Cai MY, Zhang DS, Wang ZQ, Wang DS, Li YH, Xu RH. Clinicopathological characteristics and prognostic analysis of Lauren classification in gastric adenocarcinoma in China. J Transl Med. 2013;11:58. doi: 10.1186/1479-5876-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicastro G, Masino L, Esposito V, Menon RP, De Simone A, Fraternali F, Pastore A. Josephin domain of ataxin-3 contains two distinct ubiquitin-binding sites. Biopolymers. 2009;91:1203–1214. doi: 10.1002/bip.21210. [DOI] [PubMed] [Google Scholar]
- 28.Sigal A, Rotter V. Oncogenic mutations of the p53 tumor suppressor: the demons of the guardian of the genome. Cancer Res. 2000;60:6788–6793. [PubMed] [Google Scholar]
- 29.Bellini MF, Cadamuro AC, Succi M, Proenca MA, Silva AE. Alterations of the TP53 gene in gastric and esophageal carcinogenesis. J Biomed Biotechnol. 2012;2012:891961. doi: 10.1155/2012/891961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Craanen ME, Blok P, Dekker W, Offerhaus GJ, Tytgat GN. Chronology of p53 protein accumulation in gastric carcinogenesis. Gut. 1995;36:848–852. doi: 10.1136/gut.36.6.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lukashchuk N, Vousden KH. Ubiquitination and degradation of mutant p53. Mol Cell Biol. 2007;27:8284–8295. doi: 10.1128/MCB.00050-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sisoula C, Trachana V, Patterson C, Gonos ES. CHIP-dependent p53 regulation occurs specifically during cellular senescence. Free Radic Biol Med. 2011;50:157–165. doi: 10.1016/j.freeradbiomed.2010.10.701. [DOI] [PubMed] [Google Scholar]
- 33.Reim D, Loos M, Vogl F, Novotny A, Schuster T, Langer R, Becker K, Höfler H, Siveke J, Bassermann F, Friess H, Schuhmacher C. Prognostic implications of the seventh edition of the international union against cancer classification for patients with gastric cancer: the Western experience of patients treated in a single-center European institution. J. Clin. Oncol. 2013;31:263–271. doi: 10.1200/JCO.2012.44.4315. [DOI] [PubMed] [Google Scholar]
- 34.Nagini S. Carcinoma of the stomach: A review of epidemiology, pathogenesis, molecular genetics and chemoprevention. World J Gastrointest Oncol. 2012;4:156–169. doi: 10.4251/wjgo.v4.i7.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li F, Macfarlan T, Pittman RN, Chakravarti D. Ataxin-3 is a histone-binding protein with two independent transcriptional corepressor activities. J Biol Chem. 2002;277:45004–45012. doi: 10.1074/jbc.M205259200. [DOI] [PubMed] [Google Scholar]


