Abstract
The aim of this study was to investigate the effects of citicoline on the development of colitis and antioxidant parameters in rats subjected to tribenzene sulfonic acid (TNBS)-induced colitis. Twenty four Wistar Albino female rats were divided into four subgroups (n=6) (control, colitis control, colitis + 50 mg/kg citicoline, colitis + 250 mg/kg citicoline). Colitis was induced using an enema of TNBS and ethanol; following which citicoline was administrated for 3 days and effects of citicoline was subsequently evaluated. Based on microscopic damage scores, there was no difference between rats of the TNBS-colitis and 50 mg/kg citicoline treated groups, whereas treatment with 250 mg/kg citicoline, caused significant reduction in colon injury compared to that observed in rats of TNBS-colitis group. In terms of the biochemical analyses, myeloperoxidase (MPO), malondialdehyde (MDA), reduced glutathione (GSH), and IL-6 levels in rats from 250 mg/kg citicoline group were significantly different from that TNBS-colitis group. The levels of MPO, MDA, GSH and IL-6 in control rats were also significantly different those of rats in the TNBS-colitis group. Citicoline may have a positive protective effect on the inflammatory bowel disease treatment process and could, therefore, be used as an adjunct therapy in colitis. These effects of citicoline may exist through anti-inflammatory and antioxidant mechanism.
Keywords: TNBS, antioxidant system, citicoline, inflammatory bowel disease
Introduction
Inflammatory bowel disease (IBD) is characterized by chronic inflammatory process of the gastrointestinal system and the major types are ulcerative colitis (UC) and Crohn’s disease (CD) [1,2]. Although the etiology of IBD has not yet been defined, but it is widely accepted that interactions between genetic and environmental and intestinal immune factors could be responsible for the development of chronic intestinal inflammation [2,3]. Chronic inflammation is associated with the generation of reactive oxygen species (ROS) that may be capable of contributing to or even initiating an inflammatory response [4-6].
Several animal models of intestinal inflammation have been established, and these can lead to provide new insights into the pathology and immune response of IBD. The 2,4,6 trinitrobenzene sulfonic acid (TNBS)-induced colitis model applied by intrarectal administration of TNBS, initiate events in the development of mucosal inflammation, resembling many of the clinical, histological and immune characteristics of IBD [7-9].
The mucus of the colon serves a barrier towards invasion of bacteria in stools to prevent inflammation [10]. Phosphatidyl-choline (PC) is essential element of the gastrointestinal mucus layer which represents more than 90% of the phospholipids [11]. In IBD, the mucus PC content is reduced markedly independent of the state of inflammation [12,13]. Stremmel et al. stated that abnormal PC layer to contribute to the development of inflammation [14,15].
Citicoline (CDP-choline or cytidine 5’-diphosphocholine) is synthesized in vivo and serve as substrate for the synthesis of PC [16]. Following parenteral or oral administration, citicoline is metabolized in the circulation to cytidine and choline [17-19], which are then used for phospholipids synthesis [20]. Several researchers reported the beneficial outcome of citicoline treatment in neurological disorders [21-23].Recently, Cetinkaya et al. showed favorable effects of citicoline in neonatal rat model of necrotizing enterocolitis [24]. To our knowledge, this is the first study to examine the effects of citicoline histopathologically and biochemically in experimental colitis model in rats induced by TNBS.
Materials and methods
Chemicals
Citicoline and TNBS were purchased from Sigma Chemical Company (St. Louis, MO, USA). Prior to use citicoline was dissolved in 0.9% saline; it was given intraperitoneally in final volume of 0.2 ml.
Animals
Wistar Albino female rats (250-300 g) were kept in a light-[12:12-h (light:dark) photoperiod] and temperature-(22 ± 0.5°C controlled room maintained at a constant relative humidity of 65-70% and fed a standard diet and water ad libitum. All experiments were performed in accordance with the guidelines for animal research from the National Institutes of Health (NIH publication No. 86-23, revised 1985) and were approved by the Committee on Animal Research at Adnan Menderes University.
Study groups
Rats were randomly divided into four groups: (1) control + intraperitoneal saline (control, n=6), (2) intrarectal TNBS + intraperitoneal saline (colitis control, n=6), (3) intrarectal TNBS + intraperitoneal citicoline (50 mg/kg) (n=6), (4) intrarectal TNBS + intraperitoneal citicoline (250 mg/kg) (n=6).
Induction of colitis
After fasting the animals overnight, we emptied on the morning of experiment by hitting the hind legs of the animals to the in an up-down motion while holding onto the tail with one hand. Following intraperitoneal anesthesia with ketamine (50 mg/kg) and xylazine (5 mg/kg), inflammation was induced in the colon by intrarectal administration of 0.8 ml of a 25 mg TNBS solution dissolved in 37% ethanol in saline using an 8 cm-long cannula. Rats were treated intraperitoneally with either citicoline (50 and 250 mg/kg) or saline for 3 days following the induction of colitis, after which time they were decapitated.
Histological procedure and assessment
After decapitation, the last 10 cm of colon (2-10 cm from the anus verge) was excised, opened longitudinally, rinsed with saline solution, and fixed in 10% neutral formaldehyde solution, at 4°C for 24 hours. The tissues then underwent routine histological procedure (dehydration in ethanol and clearing in xylene) and embedded in paraffin blocks, and the blocks randomly cut in 5 μm sections by a microtome (Leica RM 2135; Leica, Germany). These sections were stained with hematoxylin-eosin and mounted with entellan. Screen shots were taken with Olympus DP20 Digital camera attached onto an Olympus BX51 microscope.
A histological grading scale (minimum score: 0, maximum score: 12) was used to determine the extent of TNBS induced colitis. Each of the individual parameters estimated was graded on a scale from 0-3 depending upon the severity of changes (0, no change; 1, mild; 2, moderate; 3, severe). The evaluated parameters were: damage/necrosis, inflammatory cell infiltration, submucosal edema, hemorrhage of mucosa [25,26]. Microscopic scoring of tissue samples were performed by an observer unaware of the treatment groups.
Biochemical study
Tissues were homogenized in 50 mM phosphate buffer, pH 7.0 at 0-4°C (w/v=1/10). A portion of the homogenate was removed for malondialdehyde (MDA), GSH, protein assays, then it was centrifuged at 700 g for 10 min and the supernatant was used for the assay of TBARS, MDA and GSH. Protein levels were determined using the method of Lowry [27].
Determination of MPO activity
We used a slightly modified method of Suzuki et al. [28] to determine MPO activity. Briefly, tissue samples were homogenized in 1:10 potassium phosphate buffer (50 mM, pH 7.4). The homogenate was then centrifuged at 15,000 g and the pellet resuspended in an equal volume of a detergent-containing buffer (50 mM potassium phosphate, pH 6, 0.5% hexadecyltrimethylammonium bromide, 10 mmol/L EDTA). A standard reaction mixture contained 1.6 mmol/l tetramethyl benzidine. The reaction was started by the addition of H2O2 to a final concentration of 0.88 mmol/l (0.003%). The rate of the MPO-catalyzed oxidation of tetramethyl benzidine was followed by recording the increase in absorbance at 655 nm. Knowing the initial linear phase of the reaction, we were then able to calculate the absorbency change per minute and subsequently expressed enzyme activity as the amount of enzyme producing one absorbency change per minute under assay conditions. Data are expressed as units per gram wet tissue.
Determination of MDA activity
The breakdown product of lipid peroxidation, TBARS, was measured using the method of Buege and Aust [29]. Briefly, the stock solution contained equal volumes of 15% (w/v) trichloroacetic acid in 0.25 N hydrochloric acid and 0.37% (w/v) 2-thiobarbituric acid in 0.25 N hydrochloric acid. One volume of the sample and two volumes of stock reagent were mixed in a tube, vortexed, and heated for 15 min in a boiling water bath. After cooling on ice, the precipitate was removed by centrifugation at 1000 g for 15 min, and absorbance was measured at 532 nm against a blank containing all the reagents except test sample. The values expressed as nmoles per gram wet tissue.
Determination of total GSH
The levels of GSH were estimated by monitoring the reduction of DTNB (dithiobis-2-nitrobenzoic acid), which forms a yellow colored anion at 412 nm [30].
Determination of tissue IL-6 activity
IL-6 levels were quantified using enzyme-linked immunosorbent assay (ELISA) kits specific for the rat cytokines (Biosource International, Camarillo, CA).
Statistical analysis
All data are expressed as means ± SEM. The Mann-Whitney U test was used for the analytic assessment of comparisons between the groups. Values of p<0.05 were regarded as significant.
Results
There was significant difference between the microscopic damage scores of the control (0 ± 0) and TNBS-induced control groups (10.4 ± 0.89, p<0.05) (Figures 1A, 1B and 2). There was no difference between the microscopic damage scores of the TNBS-induced colitis and 50 mg/kg citicoline treated groups (9.67 ± 1.03, p>0.05) (Figures 1B, 1C and 2). On the other hand, treatment with 250 mg/kg citicoline (5.00 ± 2.18) caused significant reduction compared to the TNBS-induced colitis group (p<0.05) (Figures 1B, 1D and 2). There was no significant difference between the microscopic damage scores of the 50 mg/kg and 250 mg/kg citicoline treated groups (p>0.05) (Figures 1C, 1D and 2).
Figure 1.
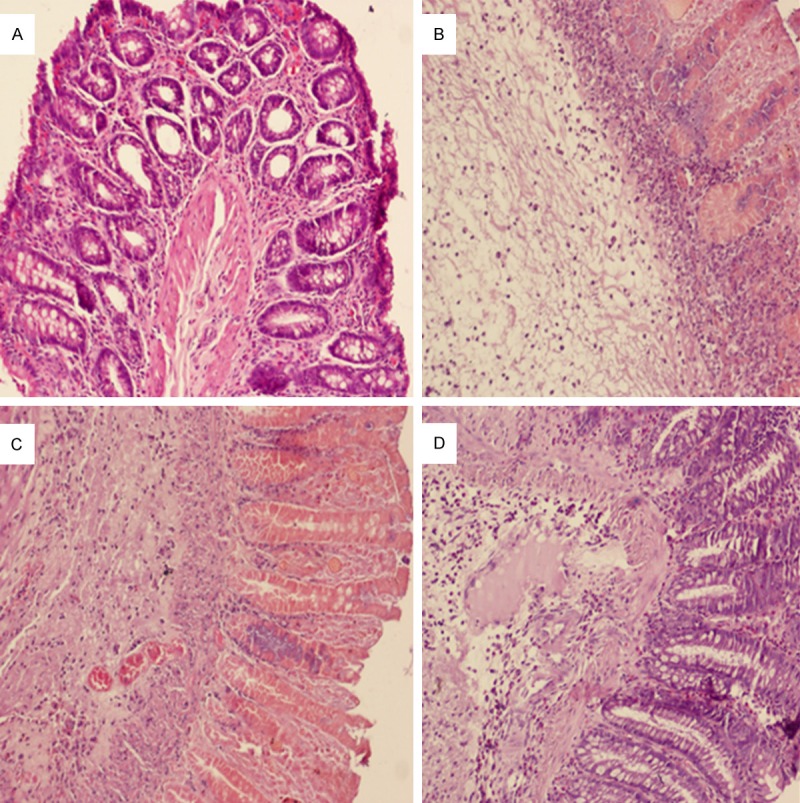
Histopathological features of the colon associated with colitis and the effects of citicoline on colon injury. Histological appearance in rat colonic mucosa of (A) normal (noncolitis), (B) Trinitrobenzene sulfonic acid (TNBS)-induced colitis, (C) TNBS-colitis + citicoline (50 mg/kg), (D) TNBS-colitis + citicoline (250 mg/kg). Colitis was produced after TNBS administration and was characterized by necrosis of the mucosa, diffuse infiltration of inflammatory cells in the mucosa and submucosa, and sub-mucosal edema (B). Treatment with citicoline 50 mg/kg did not change the morphological signs of cell damage associated with TNBS administration (C); treatment with cticoline 250 mg/kg greatly reduced the morphological alterations associated with TNBS administration, protecting the mucosal structure (D). Original magnification was 10x (A-D) on hematoxylin and eosin stained preparations.
Figure 2.
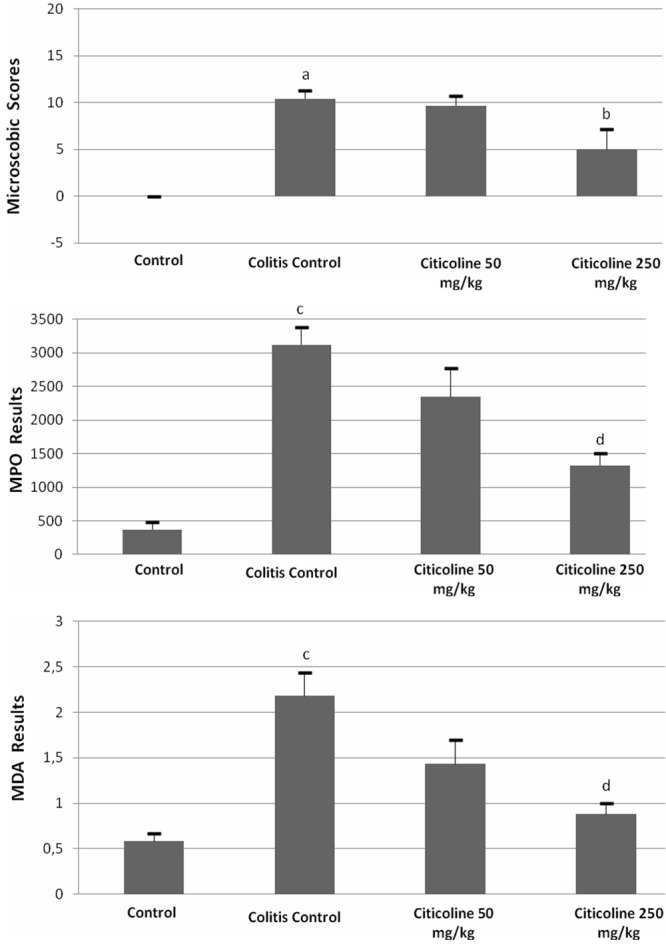
Effects of citicoline (50 and 250 mg/kg) administered intraperitoneally for three consecutive days after induction of colitis on microscopic damage scores, myeloperoxidase (MPO) and malondialdehyde (MDA) activity in rat colonic mucosa. Data are presented as means ± SEM. ap<0.05 versus control, bp<0.05 colitis control, cp<0.001 versus control, dp<0.01 versus colitis control.
To investigate the anti-inflammatory activity of the citicoline, we measured the activity of MPO from colonic tissues of all groups. The results are shown in Figure 2. The data indicate that the activity of MPO in control group (369.2 ± 115.3) dramatically increased in TNBS-induced colitis group (3118 ± 262.1) (p<0.001). Treatment with 50 mg/kg citicoline reduced the MPO activity (2343.4 ± 430.5) (p>0.05). On the other hand, treatment with 250 mg/kg citicoline markedly reduced the MPO activity (1326.6 ± 178.49) (p<0.01).
Compared with control group, higher MDA levels were found in TNBS-induced colitis group (0.58 ± 0.09 vs 2.17 ± 0.26) (p<0.001) (Figure 2). However, MDA levels were significantly decreased in 250 mg/kg citicoline (0.88 ± 0.12) treated group compared with TNBS-induced colitis group (p<0.01) (Figure 2). On the contrary, no significant anti-inflammatory effect was observed in treatment with 50 mg/kg citicoline (p>0.05).
GSH level in TNBS-induced colitis group (68.9 ± 10.9) was significantly decreased compared with control group (148.4 ± 11.5) (p<0.001) (Figure 3). Treatment with 50 mg/kg citicoline showed higher GSH levels (91.4 ± 5.9) (p>0.05). Furthermore, treatment with 250 mg/kg citicoline markedly increased the levels of GSH (110.5 ± 3.3) (p<0.05) (Figure 3).
Figure 3.
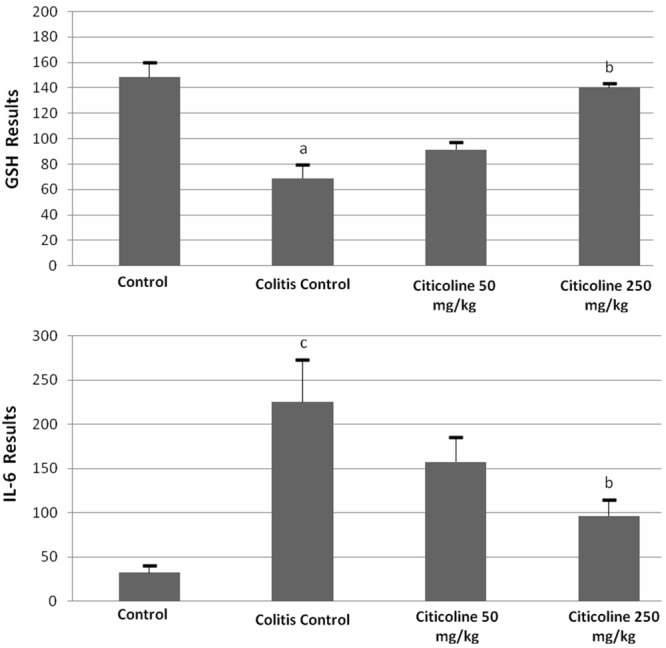
Effects of citicoline (50 and 250 mg/kg) administered intraperitoneally for three consecutive days after induction of colitis on glutathione (GSH) and IL-6 levels in rat. Data are presented as means ± SEM. ap<0.001 versus control, bp<0.05 versus colitis control, cp<0.01 versus control.
Compared with the control group, higher IL-6 levels was found in TNBS-induced colitis group (32.4 ± 8.2 vs 225.01 ± 48.5) (p<0.01) (Figure 3). IL-6 levels were significantly decreased in 250 mg/kg citicoline (96.6 ± 18.4) treated group compared with TNBS-induced colitis group (p<0.05) (Figure 3). On the other hand, no significant anti-inflammatory effect was observed in 50 mg/kg citicoline treatment (p>0.05).
Discussion
Although the etiology of IBD remains unknown, several factors in the initiation of human colitis, such as prolonged neutrophil infiltration and increased production of inflammatory mediators, are involved [31-33]. The tissue injury produced by neutrophils and macrophages has been attributed to their ability to release ROS, nitrogen metabolites and cytokines, as well as their disrupting effects on epithelial integrity [34,35].
The TNBS-induced colitis model has been widely used as an experimental model and has led to its widespread use in the investigation of IBD pathogenesis and has adopted as an approach to observe the effects of drugs because of its similarity to human IBD. This model is characterized by oxidative stress and mucosal infiltration by polymorphonuclear cells [7-9]. Therefore, in this study we have attempted to mimic pathophysiological changes in IBD.
Phospholipids are fundamental ingredients of cellular membranes and are involved in membrane functions such as homeostasis, enzymatic activities and receptor functioning. Ischemia or hypoxia to tissues disturbs membrane complexity by several mechanisms including phospholipids disintegration [36]. Therefore, phospholipids production is essentially important under such circumstances. Mucus layer in the gastrointestinal tract is a key factor for conservation of the epithelia from luminal invaders, intestinal pathogens and their products [37]. Even though, phospholipids accounts for a minor part of gastrointestinal mucus, they are crucial in the maintenance of an intact barrier activity. PC that represents 35-72% of the phospholipids is responsible for the formation of the hydrophobic surface of the gastrointestinal mucus [12]. Several investigators stated that PC interferes with cell signaling by attenuating apoptosis and has anti-inflammatory properties [38,39]. Therefore, luminal PC had two possible functions, to modulate the inflammatory signaling system and to establish hydrophobic surface [40]. Several investigators showed that exogenous PC protects gastrointestinal mucosa from acids and other toxic agents [41-43]. In the present study we found that citicoline prevents TNBS-induced colitis in rat. These data are in accordance with the previous studies that suggest therapeutic effect of citicoline in TNBS-induced colitis model.
In the present experiment, histological analyses showed that TNBS administration yields submucosal infiltration of leukocytes, which have been suggested contribute markedly to tissue damage. As leukocytes are contributors of inflammatory mediators and a major source of ROS in inflamed colon mucosa, infiltration of these cells into mucosa has been indicated to provide significantly to tissue damage and mucosal dysfunction [6,7]. In our study, we observed significant reduction in the severity of tissue injury at microscopic damage score, especially in rats treated with 250 mg/kg citicoline. Consequently, we conclude that the citicoline treatment reduced the inflammatory cell infiltration.
Neutrophil infiltration is one of the most remarkable histological findings in the inflamed colonic tissue of IBD. MPO is an enzyme existing in neutrophils which catalyzes formation of potent cytotoxic oxidants such as hypochlorous acid from H2O2 and chloride ions and N-chloramines. Furthermore, MPO is an indicator for leukocyte infiltration, which is commonly found in inflamed tissue [44,45]. As expected, MPO activity was significantly induced by stimulation with TNBS. On the other hand, the reduction of colonic MPO activity as well as the absence of cellular infiltration following treatment with citicoline, indicate that citicoline does possess antioxidant and anti-inflammatory properties that function in the prevention of TNBS-induced colitis.
Oxidative stress and its consequent lipid peroxidation disrupt the integrity of intestinal mucosa barrier, and activate inflammatory mediators, resulting in increased colonic MDA contents shown in several studies [46-48]. Several studies have demonstrated that the MDA levels in rats with IBD decreases as a result of antioxidant and anti-inflammatory agents [49-51]. In accordance with previous reports, therapy with citicoline for 3 days resulted decrease in MDA levels in colonic tissue in a dose dependent manner, suggesting that citicoline successfully inhibited lipid peroxidation induced by TNBS.
GSH depletion after intestinal injury in rats characteristic of colitis induced by TNBS [33,51]. GSH plays an important role in controlling the redox state of the cell by acting as a scavenger of ROS and keeping the enzyme GSH peroxidase in a reduced state [52,53]. In our study, we found a significant decrease in the activity of GSH in the colonic tissue of the TNBS-induced colitis group and that the administration of citicoline (250 mg/kg) prevented this depletion.
Studies showed that activated macrophages accumulate in colon mucosa and secrete many pro-inflammatory cytokines such as IL-6 in IBD. IL-6 could stimulate neutrophil chemotaxis and relate to the presence of injury in the colon which led to tissue destruction [54-56]. In the present study, it was observed that the levels of IL-6 were elevated in rat serum with TNBS-induced colitis. Furthermore, the treatment of citicoline significantly reduced the serum levels of IL-6. This result indicated that citicoline ameliorates TNBS colitis by suppressing pro-inflammatory mediator IL-6.
In conclusion, we have shown for the first time that citicoline can prevent TNBS-induced colitis in rats. Citicoline significantly reduced microscopic and histopathological injury and decreased inflammation. Citicoline treatment also decreased lipid peroxidation and oxidant status. This would appear to be a promising approach that may be considered as a complementary treatment of ulcerative colitis and Crohn’s disease.
References
- 1.Loftus EV. Clinical epidemiology of inflammatory bower disease: incidence, prevalence, and environmental, influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 2.Katz JA, Itoh J, Fiocchi C. Pathogenesis of inflammatory bowel disease. Curr Opin Gastroenterol. 1999;15:291–297. doi: 10.1097/00001574-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Kucharzik T, Maaser C, Lügering A, Kagnoff M, Mayer L, Targan S, Domschke W. Recent understanding of IBD pathogenesis: implications for future therapies. Inflamm Bowel Dis. 2008;12:1068–1083. doi: 10.1097/01.mib.0000235827.21778.d5. [DOI] [PubMed] [Google Scholar]
- 4.Lih-Brody L, Powell SR, Collier KP, Reddy GM, Cerchia R, Kahn E, Weissman GS, Katz S, Floyd RA, McKinley MJ, Fisher SE, Mullin GE. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig Dis Sci. 1996;41:2078–2086. doi: 10.1007/BF02093613. [DOI] [PubMed] [Google Scholar]
- 5.Kruidenier L, Kuiper I, Van Duijn W, Mieremet-Ooms MA, van Hogezand RA, Lamers CB, Verspaget HW. Imbalanced secondary mucosal antioxidant response in inflammatory bowel disease. J Pathol. 2003;201:17–27. doi: 10.1002/path.1408. [DOI] [PubMed] [Google Scholar]
- 6.Grisham MB. Oxidants and free radicals in inflammatory bowel disease. Lancet. 1994;344:859–861. doi: 10.1016/s0140-6736(94)92831-2. [DOI] [PubMed] [Google Scholar]
- 7.Grisham MB, Volkmer C, Tso P, Yamada T. Metabolism of trinitrobenzene sulfonic acid by the rat colon produces reactive oxygen species. Gastroenterology. 1991;101:540–547. doi: 10.1016/0016-5085(91)90036-k. [DOI] [PubMed] [Google Scholar]
- 8.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- 9.Neurath M, Fuss I, Strober W. TNBS-colitis. Int Rev Immunol. 2000;19:51–62. doi: 10.3109/08830180009048389. [DOI] [PubMed] [Google Scholar]
- 10.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 11.Ehehalt R, Jochims C, Lehmann WD, Erben G, Staffer S, Reininger C, Stremmel W. Evidence of luminal phosphatidylcholine secretion in rat ileum. Biochim Biophys Acta. 2004;1682:63–71. doi: 10.1016/j.bbalip.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Braun A, Treede I, Gotthardt D, Tietje A, Zahn A, Ruhwald R, Schoenfeld U, Welsch T, Kienle P, Erben G, Lehmann WD, Fuellekrug J, Stremmel W, Ehehalt R. Alterations of phospholipid concentration and species composition of the intestinal mucus barrier in ulcerative colitis; a clue to pathogenesis. Inflamm Bowel Dis. 2009;15:1705–1720. doi: 10.1002/ibd.20993. [DOI] [PubMed] [Google Scholar]
- 13.Ehehalt R, Wagenblast J, Erben G, Lehmann WD, Hinz U, Merle U, Stremmel W. Phosphatidylcholine and lysophosphatidylcholine in intestinal mucus of ulcerative colitis patients. A quantitative approach by nano electrospray-tandem mass spectrometry. Scand J Gastroenterol. 2004;39:737–742. doi: 10.1080/00365520410006233. [DOI] [PubMed] [Google Scholar]
- 14.Stremmel W, Ehehalt R, Staffer S, Stoffels S, Mohr A, Karner M, Braun A. Mucosal protection by phosphatidylcholine. Dig Dis. 2012;30:85–91. doi: 10.1159/000342729. [DOI] [PubMed] [Google Scholar]
- 15.Stremmel W. Mucosal protection by phosphatidylcholine as new therapeutic concept in ulcerative colitis. Z Gastroenterol. 2013;51:384–389. doi: 10.1055/s-0033-1335042. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy EP, Weiss SB. The function of cytidine coenzymes in the biosynthesis of phospholipids. J Biol Chem. 1956;222:193–214. [PubMed] [Google Scholar]
- 17.Wurtman RJ, Regan M, Ulus I, Yu L. Effect of oral CDP-choline on plasma choline and urine levels in humans. Biochem Pharmacol. 2000;60:989–992. doi: 10.1016/s0006-2952(00)00436-6. [DOI] [PubMed] [Google Scholar]
- 18.Cansev M, Yılmaz MS, Ilcol YO, Hamurtekin E, Ulus IH. Cardiovascular effects of CDP-choline and its metabolites; involvement of peripheral autonomic nervous system. Eur J Pharmacol. 2007;577:129–142. doi: 10.1016/j.ejphar.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 19.Wurtman RJ, Cansev M, Ulus IH. Choline and its products acetylcholine and phosphatidylcholine. Berlin: Springer-Verlag; 2009. [Google Scholar]
- 20.Lopez-Coviella I, Agut J, Savci V, Ortiz JA, Wurtman RJ. Evidence that 5’-cytidinediphosphocholine can affect brain phospholipids composition by increasing choline and cytidine plasma levels. J Nerochem. 1995;65:889–894. doi: 10.1046/j.1471-4159.1995.65020889.x. [DOI] [PubMed] [Google Scholar]
- 21.Fiedorowicz M, Makarewicz D, Stanczak-Mrozek K, Grieb P. CDP-choline (citicoline) attenuates damage in a rat model of birth asphyxia. Acta Neurobil Exp. 2008;68:389–397. doi: 10.55782/ane-2008-1705. [DOI] [PubMed] [Google Scholar]
- 22.Ozay R, Bekar A, Kocaeli H, Karlı N, Filiz G, Ulus IH. Citicoline improves functional recovery, promotes nerve regeneration, and reduces postoperative scarring after peripheral nerve surgery in rats. Surgical Neurology. 2007;68:615–622. doi: 10.1016/j.surneu.2006.12.054. [DOI] [PubMed] [Google Scholar]
- 23.Adibhatia RM, Hatcher FJ. Cytidine 5’-diphosphocholine (CDP-choline) in stroke and other CNS disorders. Neurochem Res. 2005;30:15–23. doi: 10.1007/s11064-004-9681-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cetinkaya M, Cansev M, Cekmez F, Tayman C, Canpolat FE, Kafa IM, Uysal S, Tunc T, Sarici SU. CDP-choline reduces severity of intestinal injury in a neonatal rat model of necrotizing enterocolitis. J Surg Res. 2013;183:119–128. doi: 10.1016/j.jss.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 25.González R, Rodríguez S, Romay C, Ancheta O, González A, Armesto J, Remirez D, Merino N. Anti-inflammatory activity of phycocyanin extract in acetic acid-induced colitis in rats. Pharmacol Res. 1999;39:55–59. [PubMed] [Google Scholar]
- 26.Gué M, Bonbonne C, Fioramonti J, Moré J, Del Rio-Lachèze C, Coméra C, Buéno L. Stress-induced enhancement of colitis in rats: CRF and arginine vasopressin are not involved. Am J Physiol. 1997;272:84–91. doi: 10.1152/ajpgi.1997.272.1.G84. [DOI] [PubMed] [Google Scholar]
- 27.Lowry OH, Risebrough NJ, Farr AL, Randal RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193:265–270. [PubMed] [Google Scholar]
- 28.Suzuki K, Ota H, Sasagawa S, Sakatani T, Fujikura T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal Biochem. 1983;132:345–352. doi: 10.1016/0003-2697(83)90019-2. [DOI] [PubMed] [Google Scholar]
- 29.Buege JA, Aust SD. Lactoperoxidase catalyzed lipid peroxidation of microsomal and artificial membranes. Biochem Biophys Acta. 1976;444:192–201. doi: 10.1016/0304-4165(76)90236-1. [DOI] [PubMed] [Google Scholar]
- 30.Fairbanks VF, Klee GG. Biochemical aspects of hematology. In: Burtis CA, Ashwood ER, editors. Tietz Textbook of Clinical Chemistry. 1999. pp. 1642–1710. [Google Scholar]
- 31.Babbs CF. Oxygen radicals in ulcerative colitis. Free Radic Biol Med. 1992;13:169–181. doi: 10.1016/0891-5849(92)90079-v. [DOI] [PubMed] [Google Scholar]
- 32.Keshavarzian A, Sedghi S, Kanofsky J, List T, Robinson C, Ibrahim C, Winship D. Excessive production of reactive oxygen metabolites by inflamed colon: analysis by chemiluminescence probe. Gastroenterology. 1992;103:177–185. doi: 10.1016/0016-5085(92)91111-g. [DOI] [PubMed] [Google Scholar]
- 33.Nieto N, Torres MI, Fernández MI, Girón MD, Ríos A, Suárez MD, Gil A. Experimental ulcerative colitis impairs antioxidant defense system in rat intestine. Dig Dis Sci. 2000;45:1820–1827. doi: 10.1023/a:1005565708038. [DOI] [PubMed] [Google Scholar]
- 34.McKenzie SJ, Baker MS, Buffington GD, Doe WF. Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J Clin Invest. 1996;98:136–141. doi: 10.1172/JCI118757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grisham MB, Yamada T. Neutrophils, nitrogen oxides, and inflammatory bowel disease. J Clin Invest. 1996;98:136–141. doi: 10.1111/j.1749-6632.1992.tb39753.x. [DOI] [PubMed] [Google Scholar]
- 36.Secades JJ. Citicholine: pharmacological and clinical review, 2010 update. Rev Neurol. 2011;52:S1–S62. [PubMed] [Google Scholar]
- 37.Allen A, Leonard AJ, Sellers LA. The mucus barrier. It’s role in gastroduodenal mucosal protection. J Clin Gastroenterol. 1988;10:93–98. [PubMed] [Google Scholar]
- 38.Lichtenberger LM. The hydrophobic barrier properties of gastrointestinal mucus. Ann Rev Physiol. 1995;57:565–583. doi: 10.1146/annurev.ph.57.030195.003025. [DOI] [PubMed] [Google Scholar]
- 39.Treede I, Braun A, Sparla R, Kühnel M, Giese T, Turner JR, Anes E, Kulaksiz H, Füllekrug J, Stremmel W, Griffiths G, Ehehalt R. Anti-inflammatory effects of phosphatidylcholine. J Biol Chem. 2007;282:27155–27164. doi: 10.1074/jbc.M704408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ehehalt R, Braun A, Karner M, Füllekrug J, Stremmel W. Phosphatidylcholine as a constituent in the colonic mucosal barrier- physiological and clinical relevance. Biochim Biophys Acta. 2010;1801:983–993. doi: 10.1016/j.bbalip.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Eros G, Kaszaki J, Czobel M, Boros M. Systemic phosphatidylcholine pretreatment protects canine esophagel mucosa during acute experimental biliary reflux. World J Gastroenterol. 2006;12:271–279. doi: 10.3748/wjg.v12.i2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Demirbilek S, Aydin G, Yucesan S, Vural H, Bitiren M. Polyunsaturated phosphatidylcholine lowers collagen deposition in a rat model of corrosive esophageal burn. Eur J Pediatr Surg. 2002;12:8–12. doi: 10.1055/s-2002-25082. [DOI] [PubMed] [Google Scholar]
- 43.Stremmel W, Ehehalt R, Autschbach F, Karner M. Phosphatidylcholine for steroid-refractory chronic ulcerative colitis: a randomized trial. Ann Intern Med. 2007;147:603–610. doi: 10.7326/0003-4819-147-9-200711060-00004. [DOI] [PubMed] [Google Scholar]
- 44.Islam MS, Murata T, Fujisawa M, Nagasaka R, Ushio H, Bari AM, Hori M, Ozaki H. Anti-inflammatory effects of phytosteryl ferulates in colitis induced by dextran sulphate sodium in mice. Br J Pharmacol. 2008;154:812–824. doi: 10.1038/bjp.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abreu MT. The pathogenesis of inflammatory bowel disease: translational implications for clinicians. Curr Gastroenterol Rep. 2002;4:481–489. doi: 10.1007/s11894-002-0024-0. [DOI] [PubMed] [Google Scholar]
- 46.Stark G. Functional consequences of oxidative membrane damage. J Membr Biol. 2005;205:1–16. doi: 10.1007/s00232-005-0753-8. [DOI] [PubMed] [Google Scholar]
- 47.Isozaki Y, Yoshida N, Kuroda M, Takagi T, Handa O, Kokura S, Ichikawa H, Naito Y, Okanoue T, Yoshikawa T. Effect of a novel water-soluble vitamin E derivative as a cure For TNBS induced colitis in rats. Int J Mol Med. 2006;17:497–502. [PubMed] [Google Scholar]
- 48.Colón AL, Madrigal JL, Menchén LA, Moro MA, Lizasoain I, Lorenzo P, Leza JC. Stress increases susceptibility to oxidative/nitrosative mucosal damage in an experimental model of colitis in rats. Dig Dis Sci. 2004;49:1713–1721. doi: 10.1023/b:ddas.0000043391.64073.e4. [DOI] [PubMed] [Google Scholar]
- 49.Liu X, Wang J. Anti-inflammatory effects of iridoid glycosides fraction of Folium syringae leaves on TNBS-induced colitis in rats. J Ethnopharmacol. 2011;133:780–787. doi: 10.1016/j.jep.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 50.Isik F, Tunali Akbay T, Yarat A, Genc Z, Pisiriciler R, Caliskan-Ak E, Cetinel S, Altıntas A, Sener G. Protective effects of Black Cumin (Nigella sativa) oil on TNBS-induced experimental colitis in rats. Dig Dis Sci. 2011;3:721–730. doi: 10.1007/s10620-010-1333-z. [DOI] [PubMed] [Google Scholar]
- 51.Ek RO, Serter M, Ergin K, Yildiz Y, Cecen S, Kavak T, Yenisey C. The effects of Caffeic Acid Phenethyl Ester (CAPE) on TNBS-induced colitis in ovariectomized rats. Dig Dis Sci. 2008;53:1609–1617. doi: 10.1007/s10620-007-0056-2. [DOI] [PubMed] [Google Scholar]
- 52.Witaicenis A, Seito LN, Di Stasi LC. Intestinal anti-inflammatory activity of esculetin and 4-methylesculetin in the tribenzensulphonic acid model of rat colitis. Chemico-Biol Interac. 2010;186:211–218. doi: 10.1016/j.cbi.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 53.Sies H. Glutathione and its role in cellular functions. Free Rad Biol Med. 1999;27:916–921. doi: 10.1016/s0891-5849(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 54.Ito H, Takazoe M, Fukuda Y, Hibi T, Kusugami K, Andoh A, Matsumoto T, Yamamura T, Azuma J, Nishimoto N, Yoshizaki K, Shimoyama T, Kishimoto T. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn’s disease. Gastroenterology. 2004;126:989–996. doi: 10.1053/j.gastro.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 55.Luo JY, Cao JC, Jiang XL, Cui HF. Effect of low molecular weight heparin rectal suppository on experimental ulcerative colitis in mice. Biomed Pharmacother. 2010;64:441–445. doi: 10.1016/j.biopha.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 56.Mahida YR. The key role of macrophages in the immunopathogenesis of inflammatory bowel disease. Inflamm Bowel Dis. 2000;6:21–33. doi: 10.1097/00054725-200002000-00004. [DOI] [PubMed] [Google Scholar]


