Abstract
This study was aimed to comparison of the effects of the chronic use of the Ribavirin and caffeic acid phenethyl ester (CAPE) on the pancreatic damage and hepatotoxicity in rats. Methods: The rats were given orally 30 mg/kg/day doses of Ribavirin for 30 days, and intraperitoneally 10 μmol/kg doses of CAPE. The 37 rats were divided into 4 groups: (I) Control (n=7), (II) Ribavirin (R) (n=10), (III) CAPE (n=10), and (IV) R+CAPE (n=10). Results: Ribavirin and CAPE yielded similar results in terms of Serum, total antioxidant status (TAS), total oxidant status (TOS), amylase, lipase, and insulin compared to the control group. However, while Ribavirin provided similar results with the control group in terms of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) enzymes, the CAPE group had elevated AST and ALT levels compared to the control group. Histopathologic evaluations revealed that CAPE or Ribavirin had no degenerative effects on both the pancreas and liver tissues. In this way, the biochemical results were confirmed by the histopathologic results. Conclusion: It can be concluded that Ribavirin does not lead to any pancreatic damage and hepatotoxicity, and has more beneficial effects than CAPE on especially liver tissue.
Keywords: CAPE, ribavirin, insulin, hepatotoxicity, pancreatic damage, oxidative stress
Introduction
The most common drawbacks of antiviral drugs are their side effects. These effects often bring about some other problems including toxic effects, inadequacy of the treatment, the development of drug resistance, and the absence of further treatments. Although antiviral drugs are commonly and routinely taken by the patients with chronic infection, very few studies can be found in the literature that discuss whether these antiviral drugs lead to degenerative disorders like pancreatic and hepatic damage.
Ribavirin (1-β-d-ribofuranosyl-1,2,4-triazole-3-carboxamide) is a synthetic nucleoside analog with in-vitro antiviral activity, which has been suggested as an inhalation treatment for severe respiratory syncytial virus infections in children [1,2] and also in combination with interferon-α for the treatment of chronic hepatitis C virus (HCV) infection [2,3]. Moreover, Ribavirin has been reported to perform direct antiviral activity against many DNA and RNA viruses [2,4,5].
The clinical advantages of Ribavirin are hindered by its side-effects, especially causing the curable dose-reliant hemolytic anemia as a result of long-term administration. The mechanism of hemolytic anemia has yet to be proven; however, it is reported that a metabolite of Ribavirin, called Ribavirin triphosphate, is likely to accumulate in erythrocytes and thus rival the erythrocyte ATP to function as a source of energy, which in turn reduces the lifespan of the erythrocytes [2,4,6].
Ribavirin has been reported in several studies to have an antiproliferative effect on lymphocytes [2,6,7] and to elongate skin allograft survival in rats [7,8]. Moreover, it has been suggested that Ribavirin is likely to intermediate immunomodulation by modifying the network of cytokines. In particular, the immunomodulatory function of Ribavirin resulting from cytokine alteration has recently been shown in some investigational models of viral hepatitis, contact hypersensitivity, or inflammatory reaction to bacterial superantigen [6,8].
Caffeic acid phenethyl ester (CAPE) is a biologically active ingredient of bee honey propolis and has long been used as a conventional therapy. Recent studies have reported that CAPE has antiinflammatory, antioxidant, immunomodulatory, antimycotic, and anticarcinogenic effects [9,10]. Also, many studies have reported that CAPE has protective effects against hepatotoxicity. For example, Tomur et al [11] reported that CAPE attenuates bile duct ligation (BDL) induced cholestatic liver injury and the hepatoprotective effect of CAPE is associated with antioxidative potential. Albukhari et al [12] reported that CAPE protects against tamoxifen induced hepatotoxicity. Saavedra-Lopes et al [13] reported that CAPE was able to protect the liver against normothermic ischemia-reperfusion (I/R) injury in rats. Gokcimen et al [14] reported that CAPE has protective effects on doxorubicin- induced hepatocellular damage. Kus et al [15] and Lee et al [16] reported that CAPE treatment prevents carbon tetrachloride (CCl4) induced liver damage in rats. Also, Koyu et al [17] reported that CAPE reduces vancomycin induced pancreatic damage. Turkyilmaz et al [18] revealed that CAPE had beneficial effects on the course of acute necrotizing pancreatitis (ANP) in rats, and shows promise as a treatment for ANP.
Because these studies clearly show that CAPE has protective effects against hepatotoxicity and pancreatic damage, we aimed to comparison of the chronic effects of Ribavirin and CAPE on the hepatotoxicity and pancreatic damage in this present study.
A literature review spanning over the last few decades has revealed no publication reporting the effects of chronic use of Ribavirin on the pancreatic and hepatic damage. Based on this scarcity, the present study is aimed to contribute to the literature with comparison of the chronic effects of Ribavirin and CAPE on the pancreas and liver tissues.
Materials and methods
Animals, care, and nutrition
A total of 37 female Wistar Albino rats weighing 200-250 g were randomly divided into four groups. The rats were provided with appropriate laboratory conditions with a 12-hour light/dark cycle and a room temperature of 21±3°C. The study was approved by the Necmettin Erbakan University Experimental Medical Research Center’s Experimental Animals Ethics Committee, Konya, Turkey.
Animals and treatment
The rats were given orally 30 mg/kg/day doses of Ribavirin for 30 days, and intraperitoneally 10 μmol/kg doses of CAPE. The 37 rats were divided into 4 groups: (I) Control (n=7), (II) Ribavirin (R) (n=10), (III) CAPE (n=10), and (IV) R+CAPE (n=10). At the end of the experiment, all the rats were sacrificed using a Ketamine/Xylazine combination and shortly thereafter, blood and tissue samples were taken for biochemical and histopathologic examination.
Biochemical analysis
The lipase, amylase, aspartate aminotransferase (AST), alanine aminotransferase (ALT) parameters were photometrically measured using an Abbott ARCHITECT c16000 device. Insulin levels were measured with enzyme-linked immunosorbent assay (ELISA) (Cayman Cat. No A05105). For the evaluation of the total antioxidant status (TAS) of supernatant fractions, a new, programmable and colorimetric measurement method developed by Erel was used. By this method, the most effective biological radicals called Hydroxyl radicals are produced. During the test, the hydrogen peroxide, which is present in reagent 2, is mixed with the ferrous ion solution that is available in reagent 1. The radicals produced in this way, including brown-colored dianisidinyl radical cations which are produced by the hydroxyl radicals, are also effective radicals. This method enables the measurement of the antioxidative effect of the sample against the ineffective radical reactions that are initiated by the resultant hydroxyl radicals. The test yields perfect precision values lower than 3%. The TAS results are stated as nmol Trolox equivalent/mg protein. The total oxidant status (TOS) of supernatant fractions was evaluated by using a newly developed, programmable and colorimetric measurement method developed by Erel. The ferrous ion-o-dianisidine complex is oxidized to ferric ion by the oxidants present in the sample. The oxidation reaction is elevated by glycerol molecules, which are plentifully available in the reaction agent. In an acidic medium, a colored complex is formed with xylenol orange by the ferric ion. The total amount of oxidant molecules available in the sample is the determining factor for the color intensity, which can be measured spectrophotometrically. Hydrogen peroxide is then used to calibrate the test, and the results are expressed in terms of nmol H2O22 equivalent/mg protein [19].
Histopathologic analysis
Hematoxylin and eosin procedure
At the first stage, the tissues were dipped into a solution of 10% formaldehyde and were then embedded into paraffin blocks. With the aid of a microtome, 4-micron sections were cut from the blocks. Under standard protocols, all the tissues were stained with hematoxylin and eosin. The slides were evaluated using an Olympus (Tokyo, Japan) BX51 microscope.
Histopathologic examination
All the pancreatic tissues were evaluated for edema formation, inflammatory cell infiltration, and acinar cell necrosis [20,21].
Hepatic injury was evaluated for cytoplasmic vacuolation, focal nuclear pyknosis, cytoplasmic hypereosinophilia, loss of intercellular borders, and severe necrosis with disintegration of hepatic cords, hemorrhage and neutrophil infiltration [22].
Immunohistochemical procedures
Immunohistochemical examination was performed on a Leica Bond-Max automated IHC/ISH platform (Leica Microsystems Inc, Buffalo Grove, Illinois). Four micrometer paraffin sections were dewaxed in a Bond Dewax solution, rehydrated in alcohol and Bond Wash solution (Leica Microsystems). Antigen retrieval was performed using a high-pH (ER2) retrieval solution for 15 minutes followed by endogenous peroxidase blocking for 5 minutes on the machine. Anti-mouse monoclonal antibody Bcl-2 (C-2: sc-7382, Santa Cruz Biotechnology, Inc. in dilution 1:200), anti-mouse monoclonal antibody Bax (B-9: sc-7480, Santa Cruz Biotechnology, Inc. in dilution 1:100) and anti-mouse caspase-3 (CPP32) monoclonal antibody (clone JHM62, Leica Biosystems Ltd, Newcastle) was applied at 1:50 dilution for 60 minutes at room temperature. Detection was performed using the Bond Polymer Refine Red Detection system (Leica Microsystems) with a 15 minute post primary step followed by 25 minutes incubation with alkaline phosphatase-linked polymers. Sections were then counterstained with hematoxylin on the machine, dehydrated in alcohols, and mounted with mounting medium (Sakura Finetek USA Inc, Torrance, California). Prepared tissues were observed by histopathologists unaware of the experimental study groups. The numbers of apoptotic cells were counted in ten randomly selected microscope fields under a 400× magnification in a blind fashion. We calculated the average number of stained neurons for each set of ten fields and expressed as the number of the positive cells/high-power field.
Statistical analysis
The data for the biochemical parameters were analyzed by ANOVA, followed by the post hoc Tukey test and Dunnet T3. All the data were evaluated using SPSS Windows 20.0 (IBM, Statistical Package for the Social Sciences). A p value of <0.05 was considered statistically significant.
Results
Biochemical results revealed that Ribavirin and CAPE yielded similar results in terms of Serum TAS, TOS, amylase, lipase and insulin compared to the control group. However, while Ribavirin provided similar results with the control group in terms of AST and ALT, the CAPE group had increased AST and ALT levels compared to the control group (Tables 1, 2 and 3).
Table 1.
Comparison of Lipase, Amylase, AST, ALT levels between groups
| Dependent Variable | (I) GROUPS | (J) GROUPS | Mean Difference (I-J) | Std. Error | Sig. |
|---|---|---|---|---|---|
| LYPASE | CAPE | K | -0.12286 | 0.73906 | 0.998 |
| R | -0.36571 | 0.73906 | 0.959 | ||
| R+CAPE | -0.35143 | 0.73906 | 0.964 | ||
| K | R | -0.24286 | 0.67466 | 0.984 | |
| R+CAPE | -0.22857 | 0.67466 | 0.986 | ||
| R | R+CAPE | 0.01429 | 0.67466 | 1 | |
| AMYLASE | CAPE | K | -37.97143 | 79.36116 | 0.637 |
| R | -65.68571 | 79.36116 | 0.417 | ||
| R+CAPE | -53.82857 | 79.36116 | 0.505 | ||
| K | R | -27.71429 | 72.44649 | 0.706 | |
| R+CAPE | -15.85714 | 72.44649 | 0.829 | ||
| R | R+CAPE | 11.85714 | 72.44649 | 0.871 | |
| AST | CAPE | K | 389.28571* | 21.75174 | 0 |
| R | 399.71429* | 21.75174 | 0 | ||
| R+CAPE | 390.85714* | 21.75174 | 0 | ||
| K | R | 10.42857 | 19.85654 | 0.952 | |
| R+CAPE | 1.57143 | 19.85654 | 1 | ||
| R | R+CAPE | -8.85714 | 19.85654 | 0.97 | |
| ALT | CAPE | K | 170.54286* | 14.54516 | 0 |
| R | 159.68571* | 14.54516 | 0 | ||
| R+CAPE | 146.82857* | 14.54516 | 0 | ||
| K | R | -10.85714 | 13.27785 | 0.845 | |
| R+CAPE | -23.71429 | 13.27785 | 0.306 | ||
| R | R+CAPE | -12.85714 | 13.27785 | 0.769 |
The mean difference is significant at the 0.05 level.
Table 2.
Comparison of TAC and TOS levels between groups
| Dependent Variable | (I) GROUP | (J) GROUP | Mean Difference (I-J) | Std. Error | Sig. |
|---|---|---|---|---|---|
| TOS | CAPE | K | 0.79429 | 26.31215 | 1 |
| R | -14.97429 | 26.31215 | 0.94 | ||
| R+CAPE | -10.67571 | 26.31215 | 0.977 | ||
| K | R | -15.76857 | 24.0196 | 0.912 | |
| R+CAPE | -11.47 | 24.0196 | 0.963 | ||
| R | R+CAPE | 4.29857 | 24.0196 | 0.998 | |
| TAS | CAPE | K | 0.35151 | 0.13637 | 0.075 |
| R | 0.30209 | 0.13637 | 0.15 | ||
| R+CAPE | 0.38023* | 0.13637 | 0.049 | ||
| K | R | -0.04943 | 0.12449 | 0.978 | |
| R+CAPE | 0.02871 | 0.12449 | 0.996 | ||
| R | R+CAPE | 0.07814 | 0.12449 | 0.922 |
The mean difference is significant at the 0.05 level.
Table 3.
Comparison of insulin levels between groups
| (I) GROUPS | (J) GROUPS | Mean Difference (I-J) | Std. Error | Sig. |
|---|---|---|---|---|
| K | R | -0.04457 | 0.24994 | 1 |
| R+CAPE | 0.04443 | 0.32793 | 1 | |
| R | R+CAPE | 0.089 | 0.33006 | 1 |
| CAPE | K | 0.27257 | 0.21166 | 0.732 |
| R | 0.228 | 0.21494 | 0.862 | |
| R+CAPE | 0.317 | 0.3021 | 0.865 |
*The mean difference is significant at the 0.05 level.
Histopathologic results
Pancreas
In the Ribavirin + CAPE group the pancreatic tissues (H&E, x100) had normal histomorphological appearance (Figure 1A) similar to control group (Figure 1D), whereas in the Ribavirin (Figure 1B) and CAPE groups (Figure 1C) the pancreases (H&E, x100) were normal and had minimal edema formation and inflammation with no acinar necrosis (Figure 1).
Figure 1.
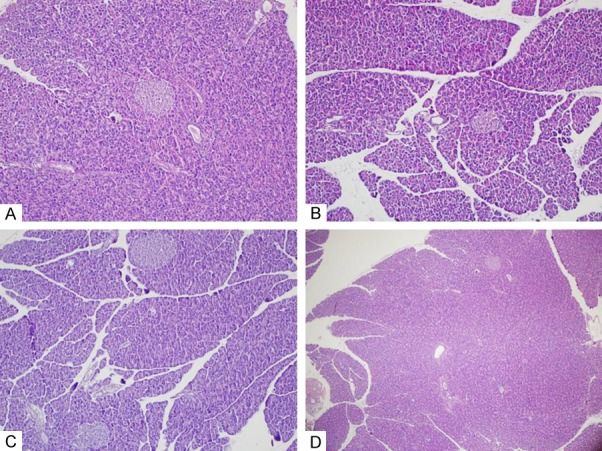
Pancreas tissue (Hematoxylin and Eosin Staining). In the Ribavirin + CAPE group the pancreatic tissues (H&E, x100) had normal histomorphological appearance (A) similar to control group (D), whereas in the Ribavirin (B) and CAPE groups (C) the pancreases (H&E, x100) were normal and had minimal edema formation and inflammation with no acinar necrosis.
Liver
In the Ribavirin (Figure 2A) and Ribavirin + CAPE groups (Figure 2B), the livers (H&E, x100) had normal margins with no evidence of purulent infiltration, nuclear pyknosis, cytoplasmic hypereosinophilia, hemorrhage, or necrosis whereas in the CAPE group (Figure 2C) the livers (H&E, x200) presented with dilated sinusoids – which reflected mild hepatic injury – along with mild cytoplasmic vacuolization and nuclear pyknosis (Figure 2).
Figure 2.

Liver Tissue (Hematoxylin and Eosin Staining & Immunohistochemistry). In the Ribavirin (A) and Ribavirin + CAPE groups (B), the livers (H&E, x100) had normal margins with no evidence of purulent infiltration, nuclear pyknosis, cytoplasmic hypereosinophilia, hemorrhage, or necrosis whereas in the CAPE group (C) the livers (H&E, x200) presented with dilated sinusoids – which reflected mild hepatic injury – along with mild cytoplasmic vacuolization and nuclear pyknosis. Immunohistochemical (IHC) results were similar to hematoxylin and eosin staining results. Thus; in the Ribavirin (D) and Ribavirin + CAPE groups (E) the livers (IHC, x100) had normal with no evidence of apoptosis, whereas in the CAPE group (F) the livers (IHC, x100) presented slight apoptosis increase.
Immunohistochemical (IHC) results were similar to hematoxylin and eosin staining results. Thus; in the Ribavirin (Figure 2D) and Ribavirin + CAPE groups (Figure 2E) the livers (IHC, x100) had normal with no evidence of apoptosis, whereas in the CAPE group (Figure 2F) the livers (IHC, x100) presented slight apoptosis increase (Figure 2).
Discussion
The present study is an attempt to add the Ribavirin to World Health Organization (WHO) Model List of Essential Medicines for the treatment of viral hemorrhagic fevers (VHF), specifically for Lassa fever, Argentine hemorrhagic fever (AHF), Crimean-Congo hemorrhagic fever (CCHF) and hemorrhagic fever with renal syndrome (HFRS) [4,23-27].
Sidwell et al [4] was the first to suggest that Ribavirin is a wide-spectrum antiviral drug which conveys minimal toxicity and is active against a variety of RNA and DNA viruses in culture and in animals. Since then, Ribavirin has been reported effective for the treatment of 1) respiratory syncytial virus (RSV) infection in immunosuppressed and high-risk children, and adults, 2) viral hemorrhagic fevers (VHFs) caused by Arenaviridae and Bunyaviridae [23-27] and 3) hepatitis C virus (HCV) infection. However, for Hantavirus pulmonary syndrome [27,28], Rift Valley fever, or Filoviruses [27,29], Ribavirin has not been reported effective.
Although the exact mechanism of this drug has yet to be proven, the drug seems to be intervening the intracellular RNA and DNA synthesis and then constraining the protein synthesis and viral reproduction of the RNA or DNA viruses that are susceptible to Ribavirin [27,30]. The use of Ribavirin has been approved by the United States Food and Drug Administration (FDA) for the treatment of respiratory syncytial virus and hepatitis C virus infection. Yet, no antiviral drug has been confirmed by FDA for the treatment of viral hemorrhagic fevers.
There is a scarcity in the numbers of animal and human studies on the clinical effects of Ribavirin. Although they are limited, these studies demonstrate a clear advantage of Ribavirin treatment, especially in terms of tolerability and safety [27].
A literature review on relevant studies revealed that only a few publications have been conducted related to the effects of Ribavirin on hepatotoxicity and pancreatic injury.
Single use of Ribavirin has been tried in the treatment of hepatitis C virus infection such as in patients with hepatitis C recurring after liver transplant and in the patients with renal transplant; however, the results have often been inconsistent. In most of these studies, Ribavirin monotherapy was reported to improve liver enzyme levels and not to have any remarkable effect on HCV viremia. It was also reported that this therapy leads to significant histological development while reducing hepatic inflammation and necrosis but has no effect on necroinflammation or even exacerbates the fibrosis. Salam et al [31], for example, reported that Ribavirin, as an antiviral drug, provides protective effects for the liver in the carbon tetrachloride (CCl4) model of hepatic toxicity. They also reported that the amount of leakage of hepatocellular enzymes ALT and AST into plasma was significantly decreased and the histological degree of hepatocyte necrosis was reduced [31-37].
In our study as well, Ribavirin yielded similar AST and ALT levels as the control group did. It was also found that Ribavirin has positive effects on these enzymes, likely because it does not have any toxic effects on the liver. We considered that this was because both the Ribavirin and Ribavirin + CAPE groups presented with normal-margin hepatic tissues with no evidence of purulent infiltration, nuclear pyknosis, cytoplasmic hypereosinophilia, hemorrhage, and necrosis.
Interestingly, we also found that CAPE – but not Ribavirin – led to an increase in AST and ALT levels. Furthermore, the CAPE group yielded dilated sinusoids, which reflected mild hepatic injury, along with mild cytoplasmic vacuolization and nuclear pyknosis.
Fang et al reported that 8-day dosing of Ribavirin (120 mg/kg/day), levovirin (2000 mg/kg/day) and viramidine (120 mg/kg/day) did not induce any of the main Cytochromes P450 (CYPs) (including CYP1A, 2B, 3A and 4A) at the protein level and had no remarkable effect on the mRNA expression of hepatic toxicology genes in rats. This means that the use of Ribavirin does not lead to any effect on hepatic enzymes even when applied at a four times higher dose than our level. Accordingly, it is clear that a high dose of Ribavirin does not lead to any significant changes at the transcription level of most of the hepatic toxicological genes [4].
Irena et al demonstrated that Ribavirin is likely to constrain the progress of Experimental autoimmune encephalomyelitis (EAE) by inhibiting the development of inflammatory mediators including tumor necrosis factor and IL-1, as previously reported in an experimental model of viral hepatitis by Ning et al [6,34]. On the other hand, Chaudhari et al [38] reported that the combined use of Ribavirin and interferon may cause drug-induced pancreatitis in patients with chronic HCV.
In the present study we investigated the effects of Ribavirin on pancreatic injury and found normal pancreatic tissues with no inflammation of acinar necrosis as a result of concurrent Ribavirin therapy on liver and pancreas tissues. Also, Banting and Best found that insulin is secreted from Langerhans islets, in 1921. With this discovery, the pancreas-insulin relationship has been an important factor in the evaluation of pancreatic injuries resulting from nonsurgical methods or chemical toxic substances [39,40]. In the present study we found that Ribavirin and CAPE groups yielded similar results in terms of serum insulin levels, compared to the control group.
Levent et al [41] reported that the chronic hepatitis C (CHC) patients who undertook pegylated interferon alfa-2b and Ribavirin therapy yielded significantly higher values of SOD, GSH-Px than pretreatment levels. Also, the MDA levels were significantly reduced while SOD and GSH-Px levels increased and the ALT, AST levels were reduced to the control levels after treatment.
In our study, the Ribavirin and CAPE groups yielded similar results in terms of Serum TAS and TOS, compared to the control group, as reported by Levent et al [40], and while Ribavirin provided similar results with the control group in terms of AST and ALT, the CAPE group had elevated AST and ALT levels compared to the control group.
Conclusions
It can be concluded that Ribavirin does not lead to any pancreatic damage and hepatotoxicity, and has effects more beneficial than CAPE on liver tissue. Further studies are warranted to analyze the effects of Ribavirin in longer periods and with different doses.
Acknowledgements
This study with 1206 M 0124 (395) number was supported by Mustafa Kemal University Scientific Research Project Coordination.
Disclosure of conflict of interest
None.
References
- 1.Hall CB, McBride JT, Walsh EE, Bell DM, Gala CL, Hildreth S, Ten Eyck LG, Hall WJ. Aerosolized ribavirin treatment of infants with respiratory syncytial viral infection. A randomized doubleblind study. N Engl J Med. 1983;308:1443–1447. doi: 10.1056/NEJM198306163082403. [DOI] [PubMed] [Google Scholar]
- 2.Fang C, Srivastava P, Lin CC. Effect of ribavirin, levovirin and viramidine on liver toxicological gene expression in rats. J Appl Toxicol. 2003;23:453–459. doi: 10.1002/jat.938. [DOI] [PubMed] [Google Scholar]
- 3.Reichard O, Norkrans G, Fryden A, Braconier JH, Sonnerborg A, Weiland O. Randomised, double-blind, placebo-controlled trial of interferon alpha-2b with and without ribavirin for chronic hepatitis C. The Swedish Study Group. Lancet. 1998;351:83–87. doi: 10.1016/s0140-6736(97)06088-1. [DOI] [PubMed] [Google Scholar]
- 4.Sidwell RW, Huffman JH, Khare GP, Allen LB, Witkowski JT, Robins RK. Broad-spectrum antiviral activity of Virazole: 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science. 1972;177:705–706. doi: 10.1126/science.177.4050.705. [DOI] [PubMed] [Google Scholar]
- 5.Patterson JL, Fernandez-Larsson R. Molecular mechanisms of action of ribavirin. Rev Infect Dis. 1990;12:1139–1146. doi: 10.1093/clinids/12.6.1139. [DOI] [PubMed] [Google Scholar]
- 6.Milicevic I, Pekovic S, Subasic S, Mostarica-Stojkovic M, Stosic-Grujicic S, Medic-Mijacevic L, Pejanovic V, Rakic L, Stojiljkovic M. Ribavirin reduces clinical signs and pathological changes of experimental autoimmune encephalomyelitis in Dark Agouti rats. J Neurosci Res. 2003;72:268–278. doi: 10.1002/jnr.10552. [DOI] [PubMed] [Google Scholar]
- 7.Peavy DL, Koff WC, Hyman DS, Knight V. Inhibition of lymphocyte proliferative responses by ribavirin. Infect Immun. 1980;29:583–589. doi: 10.1128/iai.29.2.583-589.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heagy W, Crumpacker C, Lopez PA, Finberg RW. Inhibition of immune functions by antiviral drugs. J Clin Invest. 1991;87:1916–1924. doi: 10.1172/JCI115217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alp H, Aytekin I, Esen H, Başarılı K, Kul S. Effects of Caffeic Acid Phenethyl Ester, Ellagic Acid, Sulforaphane and Curcumin on Diazinon Induced Damage to the Lungs, Liver and Kidneys in An Acute Toxicity Rat Model. Kafkas Univ Vet Fak Derg. 2011;17:927–933. [Google Scholar]
- 10.Alp A, Buyukbas S, Alp H, Gergerlioglu HS, Oz M, Basarali MK, Kiyici A. Effects of exercise and caffeic acid phenethyl ester after chronic exercise rat model. J Sports Sci Med. 2011;10:649–654. [PMC free article] [PubMed] [Google Scholar]
- 11.Tomur A, Kanter M, Gurel A, Erboga M. The efficiency of CAPE on retardation of hepatic fibrosis in biliary obstructed rats. J Mol Histol. 2011;42:451–458. doi: 10.1007/s10735-011-9350-6. [DOI] [PubMed] [Google Scholar]
- 12.Albukhari AA, Gashlan HM, El-Beshbishy HA, Nagy AA, Abdel-Naim AB. Caffeic acid phenethyl ester protects against tamoxifen-induced hepatotoxicity in rats. Food Chem Toxicol. 2009;47:1689–1695. doi: 10.1016/j.fct.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 13.Saavedra-Lopes M, Ramalho FS, Ramalho LN, Andrade-Silva A, Martinelli AL, Jordao AA Jr, Castro-e-Silva O, Zucoloto S. The protective effect of CAPE on hepatic ischemia/reperfusion injury in rats. J Surg Res. 2008;150:271–277. doi: 10.1016/j.jss.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 14.Gokcimen A, Cim A, Tola HT, Bayram D, Kocak A, Ozguner F, Ayata A. Protective effect of N-acetylcysteine, caffeic acid and vitamin E on doxorubicin hepatotoxicity. Hum Exp Toxicol. 2007;26:519–525. doi: 10.1177/0960327107076885. [DOI] [PubMed] [Google Scholar]
- 15.Kus I, Colakoglu N, Pekmez H, Seckin D, Ogeturk M, Sarsilmaz M. Protective effects of caffeic acid phenethyl ester (CAPE) on carbon tetrachloride-induced hepatotoxicity in rats. Acta Histochem. 2004;106:289–297. doi: 10.1016/j.acthis.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Lee KJ, Choi JH, Khanal T, Hwang YP, Chung YC, Jeong HG. Protective effect of caffeic acid phenethyl ester against carbon tetrachloride-induced hepatotoxicity in mice. Toxicology. 2008;248:18–24. doi: 10.1016/j.tox.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Koyu A, Gokalp O, Gumral N, Oktem F, Karahan N, Yilmaz N, Saygin M. Impact of caffeic acid phenethyl ester treatment on vancomycin-induced pancreatic damage in rats. Toxicol Ind Health. 2013 doi: 10.1177/0748233713501708. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Turkyilmaz S, Alhan E, Ercin C, Kural Vanizor B, Kaklikkaya N, Ates B, Erdogan S, Topaloglu S. Effects of caffeic acid phenethyl ester on pancreatitis in rats. J Surg Res. 2008;145:19–24. doi: 10.1016/j.jss.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 19.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Onur OE, Guneysel O, Akoglu H, Denizbasi A, Onur E. Adrenomedullin reduces the severity of cerulein-induced acute pancreatitis. Peptides. 2007;28:2179–2183. doi: 10.1016/j.peptides.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 21.Virlos I, Mazzon E, Serraino I, Di Paola R, Genovese T, Britti D, Thiemerman C, Siriwardena A, Cuzzocrea S. Pyrrolidine dithiocarbamate reduces the severity of cerulein-induced murine acute pancreatitis. Shock. 2003;20:544–550. doi: 10.1097/01.shk.0000093543.78705.aa. [DOI] [PubMed] [Google Scholar]
- 22.Kesik V, Guven A, Vurucu S, Tunc T, Uysal B, Gundogdu G, Oztas E, Korkmaz A. Melatonin and 1400 W ameliorate both intestinal and remote organ injury following mesenteric ischemia/ reperfusion. J Surg Res. 2009;157:e97–e105. doi: 10.1016/j.jss.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 23.Snell NJ. Ribavirin--current status of a broad spectrum antiviral agent. Expert Opin Pharmacother. 2001;2:1317–1324. doi: 10.1517/14656566.2.8.1317. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez H, Banks G, Smith R. Ribavirin: a clinical overview. Eur J Epidemiol. 1986;2:1–14. doi: 10.1007/BF00152711. [DOI] [PubMed] [Google Scholar]
- 25.McCormick JB, King IJ, Webb PA, Scribner CL, Craven RB, Johnson KM, Elliott LH, BelmontWilliams R. Lassa fever. Effective therapy with ribavirin. N Engl J Med. 1986;314:20–26. doi: 10.1056/NEJM198601023140104. [DOI] [PubMed] [Google Scholar]
- 26.Huggins JW. Prospects for treatment of viral hemorrhagic fevers with ribavirin, a broad-spectrum antiviral drug. Rev Infect Dis. 1989;11(Suppl 4):S750–761. doi: 10.1093/clinids/11.supplement_4.s750. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization (WHO) The Secretaryn of the Expert Committee on the Selection and Use of Essential Medicines Policy, Access and Rational Use Department of Medicines Policy and Standards. 20 Avenue Appia CH-1211 Geneva 27, Ribavirin.doc. 1/31 pp. Switzerland email: emlsecretariat@who.int. 07 Nov. 06. [Google Scholar]
- 28.Chapman LE, Mertz GJ, Peters CJ, Jolson HM, Khan AS, Ksiazek TG, Koster FT, Baum KF, Rollin PE, Pavia AT, Holman RC, Christenson JC, Rubin PJ, Behrman RE, Bell LJ, Simpson GL, Sadek RF. Intravenous ribavirin for hantavirus pulmonary syndrome: safety and tolerance during 1 year of open-label experience. Ribavirin Study Group. Antivir Ther. 1999;4:211–219. doi: 10.1177/135965359900400404. [DOI] [PubMed] [Google Scholar]
- 29.Borio L, Inglesby T, Peters CJ, Schmaljohn AL, Hughes JM, Jahrling PB, Ksiazek T, Johnson KM, Meyerhoff A, O’Toole T, Ascher MS, Bartlett J, Breman JG, Eitzen EM Jr, Hamburg M, Hauer J, Henderson DA, Johnson RT, Kwik G, Layton M, Lillibridge S, Nabel GJ, Osterholm MT, Perl TM, Russell P, Tonat K. Hemorrhagic fever viruses as biological weapons: medical and public health management. JAMA. 2002;287:2391–2405. doi: 10.1001/jama.287.18.2391. [DOI] [PubMed] [Google Scholar]
- 30.Parker WB. Metabolism and antiviral activity of ribavirin. Virus Res. 2005;107:165–171. doi: 10.1016/j.virusres.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Abdel Salam OM, Sleem AA, Omara EA, Hassan NS. Effect of ribavirin alone or combined with silymarin on carbon tetrachloride induced hepatic damage in rats. Drug Target Insights. 2007;2:19–27. [PMC free article] [PubMed] [Google Scholar]
- 32.Quadri R, Giostra E, Roskams T, Pawlotsky JM, Mentha G, Rubbia-Brandt L, Perrin L, Hadengue A, Negro F. Immunological and virological effects of ribavirin in hepatitis C after liver transplantation. Transplantation. 2002;73:373–378. doi: 10.1097/00007890-200202150-00010. [DOI] [PubMed] [Google Scholar]
- 33.Di Bisceglie AM, Conjeevaram HS, Fried MW, Sallie R, Park Y, Yurdaydin C, Swain M, Kleiner DE, Mahaney K, Hoofnagle JH. Ribavirin as therapy for chronic hepatitis C. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1995;123:897–903. doi: 10.7326/0003-4819-123-12-199512150-00001. [DOI] [PubMed] [Google Scholar]
- 34.Ning Q, Brown D, Parodo J, Cattral M, Gorczynski R, Cole E, Fung L, Ding JW, Liu MF, Rotstein O, Phillips MJ, Levy G. Ribavirin inhibits viral-induced macrophage production of TNF, IL-1, the procoagulant fgl2 prothrombinase and preserves Th1 cytokine production but inhibits Th2 cytokine response. J Immunol. 1998;160:3487–3493. [PubMed] [Google Scholar]
- 35.Cattral MS, Hemming AW, Wanless IR, Al Ashgar H, Krajden M, Lilly L, Greig PD, Levy GA. Outcome of long-term ribavirin therapy for recurrent hepatitis C after liver transplantation. Transplantation. 1999;67:1277–1280. doi: 10.1097/00007890-199905150-00014. [DOI] [PubMed] [Google Scholar]
- 36.Stanimirovic V, Nikolic D, Stanimirovic B, Nikolic A, Cucak S. Evaluation of ribavirin efficacy and tolerance in subjects with chronic hepatitis C virus infection. Vojnosanit Pregl. 2002;59:479–484. doi: 10.2298/vsp0205479s. [DOI] [PubMed] [Google Scholar]
- 37.Hoofnagle JH, Ghany MG, Kleiner DE, Doo E, Heller T, Promrat K, Ong J, Khokhar F, Soza A, Herion D, Park Y, Everhart JE, Liang TJ. Maintenance therapy with ribavirin in patients with chronic hepatitis C who fail to respond to combination therapy with interferon alfa and ribavirin. Hepatology. 2003;38:66–74. doi: 10.1053/jhep.2003.50258. [DOI] [PubMed] [Google Scholar]
- 38.Chaudhari S, Park J, Anand BS, Pimstone NR, Dieterich DT, Batash S, Bini EJ. Acute pancreatitis associated with interferon and ribavirin therapy in patients with chronic hepatitis C. Dig Dis Sci. 2004;49:1000–1006. doi: 10.1023/b:ddas.0000034562.17003.50. [DOI] [PubMed] [Google Scholar]
- 39.Bliss M. The Discovery of Insulin. Chicago: University of Chicago Press; 2007. [Google Scholar]
- 40.Kurcer Z, Karaoglu D. The use of Alloxan and Streptozotocin in Experimental Diabetes Models. Turk Jem. 2012;16:34–40. [Google Scholar]
- 41.Levent G, Ali A, Ahmet A, Polat EC, Aytac C, Ayse E, Ahmet S. Oxidative stress and antioxidant defense in patients with chronic hepatitis C patients before and after pegylated interferon alfa-2b plus ribavirin therapy. J Transl Med. 2006;4:25. doi: 10.1186/1479-5876-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]


