Abstract
The baseline platelet count (BPC) in patients with acute ST elevation myocardial infarction (STEMI) may reflect the baseline anjiografic finding and may also predic long-term outcomes after primary percutaneous coronary intervention (PPCI). Available data for the value of BPC in patients with STEMI treated with PPCI are still questionable. Therefore, we sought to determine the prognostic value of BPC for baseline angiographic finding and the impact of BPC on clinical outcomes of patients treating with PPCI. Blood sample for BPC was obtained on admission in 140 consecutive patients undergoing PPCI. Patients were divided 2 groups that group-1 (104 patients): TIMI flow-grade 0 and group-2 (36 patients): TIMI flow-grade 1-3. Follow-up was performed at 1-9 months. Baseline demographics were comparable, but, BPC was significantly higher in group-1 comparing 2 (293.7±59.8x109/L vs. 237.7±50.9x109/L, p<0.0001), pre-procedural lesion length longer in group-1 comparing 2 (13.6±3.6 mm vs. 11.4±3.9 mm, p:0.003). Distal embolization (19.0% vs. 0.0%, p:0.001), slow-flow (15.2% vs. 2.9%, p:0.033) were more common in group-1 and mean maximum troponin-I level (9.1±4.2 μg/L vs. 5.1±3.9 μg/L, p<0.0001) and mean maximum creatinin kinase (2077.6±1378.4 U/L vs. 1163.4±869.7 U/L, p:<0.0001) were higher in group-1. In-hospital and 30-days major cardiac adverse events (MACEs) (16.5% vs. 5.7%), p:0.14) were similarly in both groups, but, at 6-months target vessel revascularization (13.9% vs. 0.0%, p:0.017) and MACEs significantly higher in the group-1 (24.1% vs. 2.9%, p:0.013). Conclusion: A higher BPC without any antithrombotic agent is a strongly predictor of total occlusion of IRA in STEMI treated with PPCI. And a higher BPC associated with poor clinical outcomes at 9-months. Apart from prognostic value, measuring of a BPC on admission may also provide further practical and therapeutic profits.
Keywords: Baseline platelet count, ST-elevation myocardial infarction, primary PCI, angiographic findings, infarct related artery patency, percutaneous coronary intervention, clinical outcome
Introduction
Acute coronary syndrome is a clinical manifestation of coronary atherosclerosis and plaque disruption with superimposed thrombosis [1,2]. Platelets play a significant role in pathogenesis of acute coronary syndromes [3,4]. So far, there have been few study evaluating the relation between baseline platelet count (BPC) and outcomes in acute ST-elevation myocardial infarction (STEMI), and results under debate. Althought the HERO-1 trial shown a correlation of higher platelet count with an increased rate of Thrombolysis In Myocardial Infarction (TIMI) grade 3 flow at 90 minutes after fibrinalytic therapy, no assosiation between platelet count and mortality at 35 days was found [5]. Further, a pooled analysis from 7 clinical trials of intravenous fibrinolytic teraphy for acute STEMI, a higher initial platelet count was associated with the presence of residual thrombus in the infarct related artery and higher rates of adverse composite cardiac events at one month [6]. Furthermore, Nikolsky et al [7] found that higher baseline platelet count predict long-term results in patients with acute myocardial in-farction.
However, there is no study to evaluate the relation between admision platelet count and angiographic finding and its impact on outcomes in STEMI treating PPCI.
Materials and methods
Study population
Between January 2006 and 2007, emergency cardiac catheterization was performed in 193 consecutive patients who referred to our hospital with AMI of ≤12h duration. After the baseline angiographic examination, 23 patients were treated as medical and/or only balon dilatation oving to less than 2 mm referance vessel diameter and 17 patients required urgent coronary artery bypass surgery oving to significant three-vessels or left main coronary artery disease. Also, we excluded patients in whom extremely severe concomitant disease (n=13 with severe hepatic and renal dysfunction and advanced malignancy and severe anemia). Primary PCI was performed on the remaining 140 patients constituted the study population. Each patient was followed for revascularization or cardiac death for 1 and 9 months after the procedure either outpatient clinic or telephone interviews or electronic letters. Informed consent was obtained from all participating patients.
Laboratory metods
In all cases, venous peripheral blood samples for the platelet count were drawn on admission. Blood samples were taken into standardized tubes containing dipotassium ethylenedinitro tetraacetic acid and stored in room temperature. All measurements were performed 30 min after blood collection on Beckman Coulter LH-750 Haematology System (Miami, USA), with time to result approximately 5 min. Platelet counts were measured without clopidogrel, heparin or glycoprotein 2b/3a inhibitors in emergency service.
Patients menagement
All patients received 300 mg chewable aspirin, 300-600 mg loading dose clopidogrel and a 5000-U heparin bolus before intervention. The glycoprotein IIb/IIIa inhibitors (tirofiban hidro-klorür) were administered during and after PCI, at the discretion of the operator, as a 0.25 mg/kg bolus and a 0.125 μg/kg/min 24h infusion. Heparin dosing was adjusted to achieve an activated clotting time ≥250 seconds. Before the PPCI procedure, standard left and right coronary angiograms with at least two best projections were obtained. Two experienced interventional cardiologists (SK and AK) assessed the angiograms with unknown to patients’ clinical data and they had consensus on the findings. The angiographic data recorded for each case were as follows: angiographic morphology of the target lesion, thrombus burden of the IRA and preprocedural and postprocedural TIMI flow grades [8]. The reference vessel diameter and target lesion length were determined using a digital edge detection algorithm [9] and selecting enddiastolic frames that demonstrated the stenosis in its most severe and nonforeshortened projection. In cases of total occlusion, target lesion length was determined after initial predilatation. All measurements were done using a quantitative angiography system (Axiom Artis QCA system, Siemens, Germany). Primary PCI was performed using standard technique. Direct stent implantation was strongly encouraged unless the IRA was heavily calcified or reference vessel diameter <2.5 mm. Bare metal stents were used for all stenting procedures. After PPCI, medical therapy included aspirin 300 mg/day for forever and clopidogrel for at least 30 days in addition to ß blockers, statins and angiotensin-converting enzyme inhibitors if not contraindicated were prescriped.
Definitions
Myocardial infarction was defined as typical chest pain at rest followed by an increase in creatine phosphokinase (CK and CK-MB beyond 2 times the upper limit of normal or >50% higher than the previous value, and 5 times the upper limit of normal after coronary artery bypass grafting), or new Q waves in the electrocardiogram. Reperfusion time was defined as the time from onset of chest pain to first balloon inflation. On angiography before PPCI, a thrombus was identified as an intraluminal-filling defect with contrast outlining two or more of its edges. The PCI procedure was considered successful if stent deployment was carried out at the desired site yielding residual stenosis of <20% diameter and TIMI grade 3 flow. No-reflow was defined as TIMI flow grade <3 on the final angiogram, in spite of residual stenosis <20%; absence of significant disection; visible thrombus; or prolonged spasm on in IRA. Target lesion revascularization was defined as reintervention on stented segment (stent + 5 mm proximal and distal). Target vessel revascularization was defined as reintervention on infarct related artery.
Follow-up
All patients were scheduled for outpatient visits at 1 and 9 months. In addition, patients were contacted by questionnaire. For patients reporting cardiac symptoms, at least one clinical and electrocardiographic examination was performed at the outpatient clinic or by the referring physician.
Study end points
The primary end point in this study were (i) the relation of BPC with initial angiographic finding, (ii) its impact on MACEs, defined as death from all causes, any myocardial infarction and target vessel revascularization with either repeat PCI or coronary artery bypass graft surgery.
Statistical analysis
The statistical analysis were performed using the SPSS/PC (version 13.0, SPSS Inc, Chicago, IL) software package. A statistical significance level of <0.05 was used. The study population was divided into 2 groups based on the baseline angiographic finding: (Group 1) complete occlusion of infarct related artery (TIMI grade flow 0) and (Group 2) severe stenosis of infarct related artery with TIMI grade flow 1-3. Summary statistics for the continuous variables were presented as means ± SD. Differences in Continuous variables between groups were determined by Student’s t-test. Categorical data were summarized as percentages, and comparisons between groups were performed with the Pearson chi-square test or Fisher’s exact test.
Multivariate logistic regression model was used to derive independent variables for total occlusion of IRA. Baseline platelet count, age, diabetes, hypertension, hypercholesterolemia, smoke, 3-vessel disease, left anterior descending artery, door-baloon time, pre-procedure reference vessel diameter, target lesion length and baseline killips class were included in the analysis. ROC curve analysis was done to determine the best cut-off value for BPC that predicted total occlusion of IRA.
Results
Baseline characteristics
Table 1 shows the baseline characteristics of the 140 patients, including the 104 with and 36 without total coronary occlusion recorded at presentation. No significant differences between the groups with respect to mean age, frequencies of coronary risk factors, proportions of patients who had had previous myocardial infarction and frequency of reperfusion time were found. However, baseline platelet count was detected significantly higher (287.2±62.4x109/L vs. 239.3±53.1x109/L, p<0.0001) and baseline ejection fraction was found significantly lower (38.6±6.5% vs. 44.1±6.1%, p≤0.0001) in patients with total occlusion.
Table 1.
Clinical features for the groups
| Group-1 | Group-2 | p | |
|---|---|---|---|
|
|
|||
| n=104 | n=36 | ||
| Age (years) | 59.8±10.7 | 60.8±12.4 | 0.66 |
| Men, n (%) | 91 (87.5) | 32 (88.9) | 1.0 |
| Diabetes mellitus, n (%) | 16 (15.4) | 2 (5.6) | 0.15 |
| Hypertension, n (%) | 54 (51.9) | 16 (44.4) | 0.56 |
| Hypercholesterolemia, n (%) | 38 (36.5) | 7 (19.4) | 0.09 |
| Family history of CAD, n (%) | 42 (40.4) | 9 (25.0) | 0.14 |
| Current smoker, n (%) | 63 (60.6) | 18 (50.0) | 0.36 |
| Previous MI, n (%) | 7 (6.7) | 0 (0.0) | 0.19 |
| Previous PCI, n (%) | 8 (7.7) | 1 (2.8) | 0.44 |
| Periferic vascular disease, n (%) | 0 (0.0) | 2 (5.6) | 0.06 |
| Initial Killip class ≥II, n (%) | 6 (5.8) | 0 (0.0) | 0.33 |
| Multivessel disease, n (%) | 63 (60.6) | 22 (61.1) | 1.0 |
| Baseline hemoglobin (g/dl) | 14.3±1.7 | 14.0±1.5 | 0.49 |
| Baseline hematocrit (%) | 41.5±5.3 | 41.0±4.7 | 0.65 |
| Baseline WBC count (x109/L) | 10962.7±3166.2 | 10141.6±3633.6 | 0.19 |
| Baseline platelet count (x109/L) | 287.2±62.4 | 239.3±53.1 | <0.0001 |
| Baseline serum creatinine (mg/dl) | 0.8±0.5 | 0.7±0.4 | 0.09 |
| Symptom onset to ballon inflation (h) | 4.4±2.4 | 4.9±2.7 | 0.2 |
| No. Of coronary arteries narrowed | |||
| 1 vessel | 41 (39.4) | 14 (38.9) | 1.0 |
| 2 vessel | 37 (35.6) | 9 (25.0) | 0.33 |
| 3 vessel | 26 (25.0) | 13 (36.1) | 0.28 |
| Left ventricular ejection fraction (%) | 38.6±6.5 | 44.1±6.1 | <0.0001 |
MI: Myocard Infarction; PCI: Percutaneous Coronary Intervention; WBC: White Blood Cell.
Procedural and in-hospital outcomes
Angiographic characteristics including minmal lumen diameter and referance vessel diameter before and after PCI, presence of thrombus and/or dissection, no of stent implanted, defibrillation and/or cardiac arrest and rates of angiographic and procedural success did not differ significantly both groups (Table 2). However, patients with complete occlusion had more slow flow and/or no-reflow (12.5% vs. 0.0%, p=0.022), more distal embolization (25.0% vs. 2.8%, p=0.008), higher peak throponin levels (7.5±4.2 U/L vs. 5.5±3.7 U/L, p=0.016), higher peak creatine kinase levels (2076.3±1297.1 U/L vs. 1423.1 vs. 978.1 U/L, p=0.008).
Table 2.
Angiographic findings and angioplasty results
| Group-1 | Group-2 | p | |
|---|---|---|---|
|
|
|||
| n=104 | n=36 | ||
| Infarct-related artery, n (%) | |||
| Left anterior descending aretry | 60 (57.7) | 15 (41.7) | 0.14 |
| Left circumflex artery | 10 (9.6) | 5 (13.9) | 0.53 |
| Right coronary artery | 34 (32.7) | 16 (44.4) | 0.28 |
| Baseline mean RVD (mm) | 2.8±0.6 | 2.8±0.5 | 0.79 |
| Baseline mean lesion length (mm) | 13.8±3.5 | 10.7±2.0 | <0.0001 |
| Final mean MLD (mm) | 2.9±0.5 | 2.8±0.5 | 0.18 |
| Final mean RVD (mm) | 2.9±1.1 | 2.5±0.5 | 0.53 |
| Glicoprotein 2b/3a inhibitors, n (%) | 78 (75.0) | 23 (63.9) | 0.28 |
| Slow no-reflow, n (%) | 17 (16.3) | 0 (0.0) | 0.006 |
| Distal embolization, n (%) | 14 (13.5) | 0 (0.0) | 0.021 |
| Direct stenting, n (%) | 93 (89.4) | 18 (50.0) | <0.0001 |
| No of stent implanted | 1.2±0.4 | 1.1±0.2 | 0.07 |
| Disection, n (%) | 6 (5.8) | 0 (0.0) | 0.33 |
| Only defibrillation, n (%) | 4 (3.8) | 2 (5.6) | 1.0 |
| Cardiovascular arrest, n (%) | 4 (3.8) | 0 (0.0) | 0.57 |
| Angiographic success, n (%) | 103 (99.0) | 36 (100.0) | 1.0 |
| Procedural success, n (%) | 96 (92.3) | 36 (100.0) | 0.11 |
| Peak troponin level, μg/L | 7.5±4.2 | 5.5±3.7 | 0.016 |
| Peak creatine kinase level, U/L | 2076.3±1297.1 | 1423.1±978.1 | 0.008 |
MLD: Minimal Luminal Diameter; RVD: Referance Vessel Diameter.
By multivariate analysis, variables that were indepently asociated with total occlusion of IRA were target lesion legth, hypercholesterolemia and BPC: (OR 1.43, 95% confidence interval 1.21-1.69, p<0.0001 for target lesion length; OR 3.00, 95% confidence interval 1.03-872, p=0.044 for hypercholesterolemia and OR 3.88, 95% confidence interval 1.43-10.48, p=0.007 for BPC. On the ROC curve analysis, the best cut-off value about BPC was 230x109/L (Figure 1).
Figure 1.
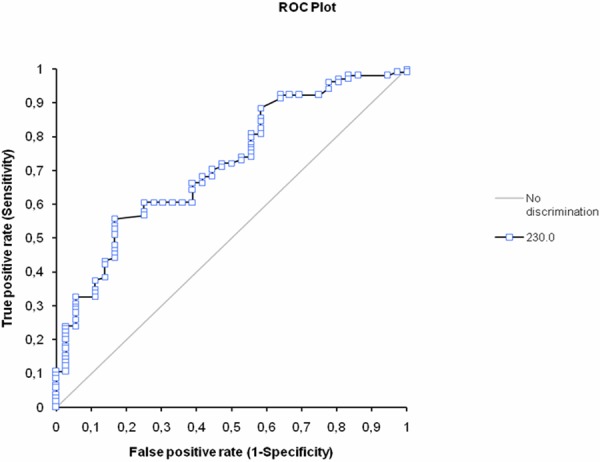
The receiver-operating characteristic (ROC) curve for mean baseline platelet count (BPC) for predicting baseline angiographic total occlusion of infarct related artery. The area under the ROC curve: 0.72 (95% confident interval 0.62 to 0.81).
Clinical outcomes at 9 month
There were no differneces in the in-hospital or 30-days (not showed) rates of death, target lesion revascularization, target vessel revascularization or any composite major adverse clinical events according to the presence of baseline total occlusion of the infarct related artery (Table 3). At the 9 months, hovewer, target lesion revascularization (18.3% vs. 2.8%, p=0.044), target vessel revascularization (18.3% vs. 2.8%, p=0.044), and composite MACEs (23.1% vs. 5.6%, p=0.037).
Table 3.
Clinical outcomes
| Group-1 | Group-2 | p | |
|---|---|---|---|
|
|
|||
| n=104 | n=36 | ||
| In-hospital | |||
| TLR | 8 (7.7) | 0 (0.0) | 0.1 |
| Death | 5 (4.8) | 1 (2.8) | 0.96 |
| MACE | 13 (12.5) | 1 (2.8) | 0.1 |
| 6-month | |||
| Non-Q-MI | 2 (1.9) | 0 (0.0) | 0.98 |
| Q-MI | 0 (0.0) | 0 (0.0) | - |
| TLR | 19 (18.3) | 1 (2.8) | 0.044 |
| TVR | 19 (18.3) | 1 (2.8) | 0.044 |
| Death | 5 (4.8) | 1 (2.8) | 1.0 |
| MACE | 24 (23.1) | 2 (5.6) | 0.037 |
MI: Myocardial infarction; TLR: Target Lesion Revascularization; TVR: Target Vessel Revascularization; MACE: Major Adverse Cardiac Event.
Discussion
This single center prospective study in consecutive unselected patients with acute STEMI treating contemporary treatmant with PPCI has two major findings. First, the BPC was strongly predict the initial angiographic findings that a patient on admission has a higher platelet count, the IRA might be total occlude. Second, a higher platelet count on admission, as Nicolsky et al [7] recently reported, was a strong independent predictor for long-term poor outcomes. In the interventional cardiology traditionally, total occlude coronary IRA had several pitfall such as the guidewire failure, distal embolization and slow-flow so on. Platelet count is a simple available laboratory test and has been associated with different clinical and epidemiologic factors. Previous studies have scrutinized platelet counts as related to clinical events. For the impact of platelet amount on mortality in unstable angina and non-ST-elevation miyocardial infarction Mueller et al [10] were done a prospective study that including 1616 consequtive patients. They found a nonlinear association between platelet count and long-term mortality. The lowest mortality was observed in patients with a platelet count between 181 and 210x109/L.
In the setting of STEMI, a high platelet count on presentation are independently associated with increased risk of adverse outcomes, particularly the risk of reinfarction and mortality [6,7,10]. More recently, Nikolsky et al [7] performed a large prospective randomise studied (from the CADILLAC Trial) about the effect of BPC on long-term results in acute STEMI. However, they did not study relation between the admission platelet amount and the initial angiographic findings. The 30-day incidence of subacute thrombosis including reocclusion increased at higher quartiles of initial platelet count (p=0.027). Also reinfarction and target vessel revascularization rates at 30 days were significantly higher in patients with increased baseline platelet counts, resulting in higher rates of cumulative MACEs (p=0.024). In addition to, in the same study at 1-year follow-up, patients in the highest platelet count quartile had the highest rates of all-cause and cardiac mortality, reinfarction, ischemic TVR, and composite major adverse cardiac events (p=0.048). A baseline platelet amount of 234x109/L was identified as the cut-off point that corresponded to the most significant relation between platelet count and 1-year death/reinfarction (p=0.0001). At 1 year, patients with a BPC ≥234 versus <234x109/L had higher rates of death or reinfarction (p=0.0001), death (p=0.002), and reinfarction (p=0.008).
Turakhia MP et al [6] reported that a higher platelet count, even after multivariate adjustment, is independently associated with the presence of residual thrombus in the infarct-related artery after administration of fibrinolytic therapy for STEMI. In additional to, they demonstrated that the mechanisms underlying the association of high platelet counts and poorer clinical outcomes are likely multifactorial. For instance, high platelet counts may reflect a state of thromboresistance.
Not only admision platelet amount but mean platelet volum also plays an important badly rol on clinical outcomes [12,13]. In this view, Martin JF et al [12] studied 1716 men six months after myocardial infarction and shown that mean platelet volum was greater in men who had a further ischaemic event (fatal or non-fatal) than in men who had no further myocardial infarction and also, the mean platelet volum was larger in men who died than in those who did not.
Clincal implications
In the paper, on admission without any antiagregans and antitrombotik agent such as heparin, higher BPC were associated with poor baseline angiographic findings such as total occlusions. İf platellet amount be higher, the IRA might be total occlusion. Total coronary artery occlusions are having badly clinical results [14,15]. Moreover, increased platelet counts were associated with more thrombus burden and thrombus embolization to distal arterial bed, slow or no-re-flow (which may explain the angiographic and procedural success) and larger infarct size as estimated by peak cardiac enzyme level, which these factors may also partly explain the poor prognosis in these patients [16].
Limitations
The present study had some limitation. First, the sample size was relatively small. Second, the follow-up time limited to 9 months. The fact that, increased mean platelet volume and platelet size, which might contribute to poorer clinical outcomes in acute coronary sydromes, [6-10,17] were not measured in this study. Even if these limitations, statistical significant was achieved. After all our results are need to confirm with larger studies.
Conclusions
A higher BPC without any antithrombotic agent is a strongly predict of total occlusion of IRA in STEMI treated with PPCI. And a higher BPC associated with poor clinical outcomes at 9-months. Apart from prognostic value, measuring of a BPC on admission may also provide further practical and therapeutic profits.
Disclosure of conflicts of interest
None.
References
- 1.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 3.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes. N Engl J Med. 1992;326:242–250. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- 4.Ly HQ, Kirtane AJ, Murphy SA, Buros J, Cannon CP, Braunwald E, Gibson CM TIMI Study Group. Association of platelet counts on presentation and clinical outcomes in ST-elevation myocardial infarction (from the TIMI Trials) Am J Cardiol. 2006;98:1–5. doi: 10.1016/j.amjcard.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 5.Wong CK, French JK, Gao W, White HD. Relation of initial platelet counts to Thrombolysis In Myocardial Infarction-3 flow rates at 90 minutes after commencing fibrinolytic therapy in patients with acute myocardial infarction. Am J Cardiol. 2002;90:54–57. doi: 10.1016/s0002-9149(02)02388-3. [DOI] [PubMed] [Google Scholar]
- 6.Turakhia MP, Murphy SA, Pinto TL, Antman EM, Giugliano RP, Cannon CP, Braunwald E, Gibson CM. Thrombolysis in Myocardial Infarction Study Group. Association of platelet count with residual thrombus in the myocardial infarct-related coronary artery among patients treated with fibrinolytic therapy for ST-segment elevation acute myocardial infarction. Am J Cardiol. 2004;94:1406–1410. doi: 10.1016/j.amjcard.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Nikolsky E, Grines CL, Cox DA, Garcia E, Tcheng JE, Sadeghi M, Mehran R, Lansky AJ, Na Y, Stone GW. Impact of baseline platelet count in patients undergoing primary percutaneous coronary intervention in acute myocardial infarction (from the CADILLAC trial) Am J Cardiol. 2007;99:1055–1061. doi: 10.1016/j.amjcard.2006.11.066. [DOI] [PubMed] [Google Scholar]
- 8.TIMI Study Group. The Thrombolysis in Myocardial Infarction (TIMI) trial, phase I findings: TIMI Study Group. N Eng J Med. 1985;312:932–936. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- 9.Hermiller JB, Cusma JT, Spero LA, Fortin DF, Harding MB, Bashore TM. Quantitative and qualitative coronary angiographic analysis: review of methods, utility, and limitations. Cathet Cardiovasc Diagn. 1992;25:110–131. doi: 10.1002/ccd.1810250207. [DOI] [PubMed] [Google Scholar]
- 10.Mueller C, Neumann FJ, Hochholzer W, Trenk D, Zeller T, Perruchoud AP, Buettner H. The impact of platelet count on mortality in unstable angina/non-ST-segment elevation myocardial infarction. Am Heart J. 2006;151:1214, e1–7. doi: 10.1016/j.ahj.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Gibson CM, Ly HQ, Murphy SA, Ciaglo LN, Southard MC, Stein EB, Buros JL, Sabatine MS, Cannon CP TIMI Study Group. Usefulness of clopidogrel in abolishing the increased risk of reinfarction associated with higher platelet counts in patients with ST-elevation myocardial infarction (results from CLARITY-TIMI 28) Am J Cardiol. 2006;98:761–763. doi: 10.1016/j.amjcard.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 12.Martin JF, Bath PM, Burr ML. Influence of platelet size on outcome after myocardial infarction. Lancet. 1991;338:1409–1411. doi: 10.1016/0140-6736(91)92719-i. [DOI] [PubMed] [Google Scholar]
- 13.Pabon Osuna P, Nieto Ballesteros F, Morinigo Munoz JL, Sanchez Fernandez PL, Arribas Jimenez A, Diego Dominguez M, Martin Luengo C. The effect of the mean platelet volume on the short-term prognosis of acute myocardial infarct. Rev Esp Cardiol. 1998;51:816–822. [PubMed] [Google Scholar]
- 14.Stone GW, Rutherford BD, McConahay DR, Johnson WL Jr, Giorgi LV, Ligon RW, Hartzler GO. Procedural outcome of angioplasty for total coronary occlusion: an analysis of 971 lesions in 905 patients. J Am Coll Cardiol. 1990;15:849–856. doi: 10.1016/0735-1097(90)90285-w. [DOI] [PubMed] [Google Scholar]
- 15.Ivanhoe RJ, Weintraub WS, Douglas JS Jr, Lembo NJ, Furman M, Gershony G, Cohen CL, King SB 3rd. Percutaneous transluminal coronary angioplasty of chronic total occlusions: primary success, restenosis, and long-term clinical follow-up. Circulation. 1992;85:106–115. doi: 10.1161/01.cir.85.1.106. [DOI] [PubMed] [Google Scholar]
- 16.Halkin A, Stone GW, Grines CL, Cox DA, Rutherford BD, Esente P, Meils CM, Albertsson P, Farah A, Tcheng JE, Lansky AJ, Mehran R. Prognostic implications of creatine kinase elevation after primary percutaneous coronary intervention for acute myocardial infarction. J Am Coll Cardiol. 2006;47:951–961. doi: 10.1016/j.jacc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Huczek Z, Kochman J, Filipiak KJ, Horszczaruk GJ, Grabowski M, Piatkowski R, Wilczynska J, Zielinski A, Meier B, Opolski G. Mean platelet volume on admission predicts impaired reperfusion and long-term mortality in acute myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol. 2005 Jul 19;46:284–290. doi: 10.1016/j.jacc.2005.03.065. [DOI] [PubMed] [Google Scholar]


