Abstract
Titanium barriers have been used for guided bone regeneration in preclinical and preliminary clinical reports as a possible alternative to bone grafting. In two cases with lateral bone defects, rigid titanium barriers were used to provide a secluded space in conjunction with bone substitutes. Sufficient lateral bone volume was generated for implant placement, and no complications were observed during 2 years of follow up. In conclusion, space-making stiff titanium barriers may be applied successfully for lateral alveolar crest augmentation.
Keywords: Barriers, bone augmentation, titanium
Introduction
Insufficient bone volume for dental implant placement is a constant challenge in oral surgery. Usually, sites with extensive bone defects in the first stage receive bone grafts, with the implants being placed in the augmented alveolar site in the second stage [1]. As an alternative that increases the bone volume, the use of a subperiosteal barrier has been proposed to allow for spontaneous bone growth after the formation of a coagulum below the barrier, permitting guided bone regeneration [2]. However, some researchers have questioned the feasibility of titanium barriers for performing an alveolar ridge reconstruction prior to implant placement [3]. The osteoconductive capability of titanium and the implant stability are reported to play an important role in achieving successful bone augmentation with occlusive barriers [4] or titanium meshes. Essentially, the morphology of the defect is responsible for the clinical outcome and may strongly influence the final results [5,6].
Within the context of contradictory clinical reports [7], the aim of this case study was to demonstrate the use of a rigid titanium occlusive barrier to perform a lateral alveolar bone augmentation, with and without subperiosteal tunneling.
Clinical presentation and results
Case 1
A 44-year-old healthy man was admitted to our clinic with an implant fracture in the left mandibular first molar region (Figure 1A). Radiographically, a vertical bone resorption was observed adjacent to a fractured implant (Figure 1B). The implant was removed, identifying a defect in the buccal wall (Figure 1C). A rigid titanium occlusive barrier was placed and screwed in the defect region, and a pure β-Tricalcium phosphate (Cerasorb®, Curasan AG, Kleinostheim, Germany) biomaterial was placed underneath the barrier (Figure 1D). The wound was closed using a vestibular mucoperiosteal flap. Radiological control was completed at 1 week (Figure 2A), and the healing was uneventful. At 3 months, the rigid barrier was removed, and a 7.5 mm crestal width transversal bone was observed 5 mm below the crestal level. At this point, the implant was placed (Figure 2B). An implant-supported prosthetic rehabilitation was performed after full osseointegration of the implant was noted at 6 months. At the 24-month follow up (Figure 2C), healthy peri-implant tissues were noted.
Figure 1.
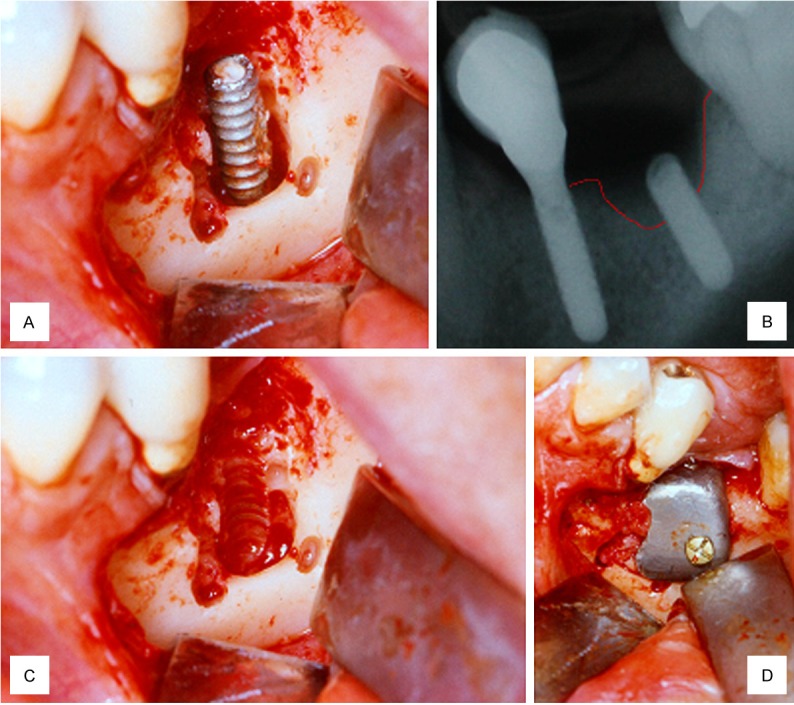
A. Peri-implantitis in first molar region; B. Vertical resorption around implant; C. Visualization of buccal defect after implant removal; D. Rigid titanium non-resorbable and screwed barrier placement.
Figure 2.
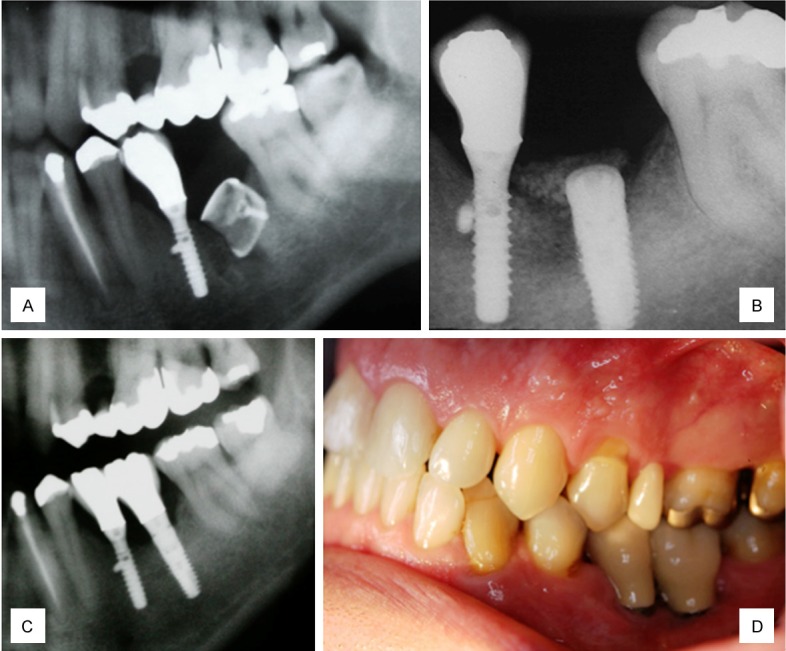
A. One-week control radiograph; B. 5-months control radiograph; C. 24-months radiograph control; D. 24-months clinical control.
Case 2
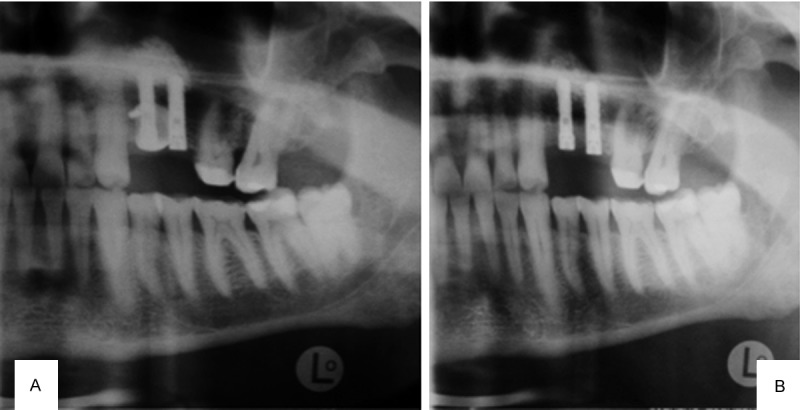
A 66-year-old healthy man was referred for implant placement in the left upper first and second premolar region (Figure 3A). The alveolar bone width at the implant site 24 was below 3 mm, and the vertical height was between 6 and 8 mm; these values indicate a need for both vertical and horizontal augmentation. A vertical incision allowed subperiosteal tunneling (Figure 3B) for the subantral sinus floor augmentation (SALSA, [8]) with simultaneous implant insertion. Subsequently, a rigid titanium occlusive barrier filled with a pure β-Tricalcium phosphate (Cerasorb®, Curasan AG, Kleinostheim, Germany) biomaterial (Figure 3C) was placed to cover the exposed buccal aspect of the implant 2.4 (Figure 4A). During the healing period, no exposure of the barrier was observed, and it was removed at the time of implant exposure (Figure 4B), showing a transverse dimension of 7 mm 5 mm below the crest. After prosthetic treatment, the patient was controlled clinically and radiographically during a 24-month follow-up period (Figure 5A and 5B).
Figure 3.
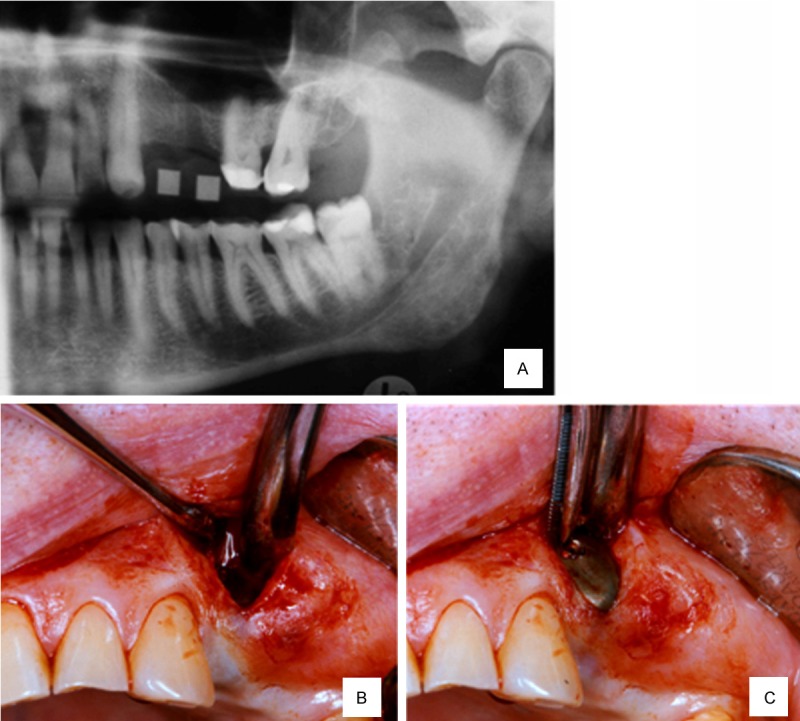
A. Edentulous region of 2.4 and 3.5 teeth; B. Subperiosteal tunneling procedure; C. Rigid titanium non-resorbable and screwed barrier placement.
Figure 4.
A. Control radiograph after sinus lift and barrier placement; B. 4-months control radiograph.
Figure 5.
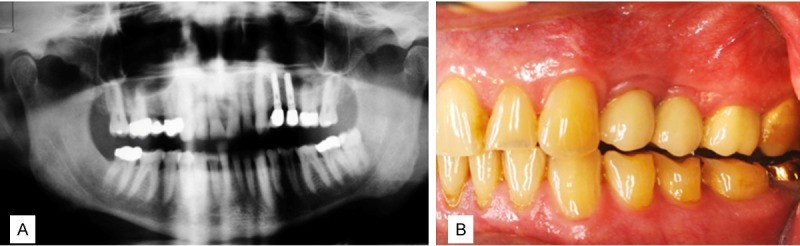
A. 24-months radiograph control; B. 24-months clinical control.
Discussion
Some authors have stated that the best way to allow guided bone augmentation is to use a stiff occlusive titanium barrier [9]. Schmid et al. [10] reported that the degree of mineralization of titanium dome-shaped barriers in rabbits was higher when compared with permeable Teflon barriers, achieving successful results with the model used here. Later, the research of van Steenberghe et al. [2] on humans and rabbits yielded results supporting that a large bone volume augmentation could be accomplished with this technique.
The use of dental implants may be limited by alveolar ridge deficiencies, considering that a minimum of 5 to 6 mm horizontal width is needed [11]. The use of occlusive titanium barriers provides sufficient space maintenance to allow the migration of angiogenic and osteogenetic cells into the regenerative space while stabilizing the bone substitute and the coagulum [12].
In both cases, a sufficient gain of bone mass was observed through the placement of implants with at least a 1.5 mm layer of mm lateral bone at the time of prosthetic loading. Van Steenberghe et al. [2] also reported a vertical and transverse gain of bone height up to 16 mm in severely reabsorbed maxillary arches, although many of the barriers were exposed early.
From the cases presented, either using an open approach (case 1) or a subperiosteal tunnel approach, as in case 2, it may be concluded that barrier exposure does not occur if the soft tissue is closed without tension and the barrier volume is not overextended. The latter tunnel technique represents a minimally invasive method that can be considered in alveolar ridge augmentation procedures to have a relatively low morbidity and high level of patient comfort [13].
β-Tricalcium phosphate represents an interesting bone graft for craniofacial tissue regeneration [14,15], although it remains unclear whether a scaffold providing the material is regularly required underneath an occlusive barrier. However, this may impede the volume contraction of the coagulum, provide calcium and apparently does not have negative effects on the bone regeneration.
Although allografts and xenografts provide less bone quantity and quality when compared with autografts [16], the purpose of bone augmentation was achieved in both cases treated with pure β-tricalcium phosphate. These observations are in accordance with the data of Molly et al [4], who reported that the behavior of allografts and xenografts could be different when these grafts are placed under a stiff occlusive titanium membrane.
It is important to note that some requirements are necessary for a successful barrier outcome, including stability and blood supply [9]. The stiffness of the barrier used in both cases allows for the maintenance of its shape and the creation of a space for graft placement, thus preventing the collapse of the biomaterial. Another important feature is the occlusivity of this barrier, which prevents fibrous tissue ingrowth [3]. Although the main disadvantage of these barriers are their tendency to become exposed early, as described by van Steenberghe et al. [2], this complication did not occur in any of the cases presented here. A possible explanation could be the favorable relation of the soft tissue coverage compared to the relatively small volume of the barrier.
The main advantage of the technique using a rigid titanium occlusive barrier is that the surgery is less traumatic compared with removing bone grafts and does not require a donor site [2].
Thus, both clinical cases confirm the previous reports [2,4] of clinically successful bone augmentations with rigid titanium barriers.
Conclusion
In conclusion, the use of a rigid titanium occlusive screwed barrier with pure β-tricalcium phosphate might be a reliable technique for lateral alveolar bone augmentation.
Disclosure of conflict of interest
None.
References
- 1.Chiapasco M, Zaniboni M, Boisco M. Augmentation procedures for the rehabilitation of deficient edentulous ridges with oral implants. Clin Oral Implants Res. 2006;17:136–159. doi: 10.1111/j.1600-0501.2006.01357.x. [DOI] [PubMed] [Google Scholar]
- 2.Van Steenberghe D, Johansson C, Quirynen M, Molly L, Albrektsson T, Naert I. Bone augmentation by means of a stiff occlusive titanium barrier. Clin Oral Implants Res. 2003;14:63–71. doi: 10.1034/j.1600-0501.2003.140109.x. [DOI] [PubMed] [Google Scholar]
- 3.Rakhmatia YD, Ayukawa Y, Furuhashi A, Koyano K. Current barrier membranes: titanium mesh and other membranes for guided bone regeneration in dental applications. J Prosthodont Res. 2013;57:3–14. doi: 10.1016/j.jpor.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Molly L, Quirynen M, Michiels K, Van Steenberghe D. Comparison between jaw bone augmentation by means of a stiff occlusive titanium membrane or an autologous hip graft: a retrospective clinical assessment. Clin Oral Implants Res. 2006;17:481–487. doi: 10.1111/j.1600-0501.2006.01286.x. [DOI] [PubMed] [Google Scholar]
- 5.Louis PJ. Vertical ridge augmentation using titanium mesh. Oral Maxillofac Surg Clin North Am. 2010;22:353–368. doi: 10.1016/j.coms.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Miyamoto I, Funaki K, Yamauchi K, Kodama T, Takahashi T. Alveolar ridge reconstruction with titanium mesh and autogenous particulate bone graft: computed tomography-based evaluations of augmented bone quality and quantity. Clin Implant Dent Relat Res. 2012;14:304–311. doi: 10.1111/j.1708-8208.2009.00257.x. [DOI] [PubMed] [Google Scholar]
- 7.Wyszkowski A. Starre Titanbarrieren als Augmentationshilfe - eine klinische Nachuntersuchung - Resultate einer retrospektiven Studie. Med Diss Georg. 2009 Aug; Universität Göttingen. [Google Scholar]
- 8.Engelke W, Schwarzäller W, Behnsen A, Jacobs HG. Subantroscopic Laterobasal Sinus Floor Augmentation (SALSA): An Up-to-5 Year Clinical Study. Int J Oral Maxillofac Implants. 2003;18:135–143. [PubMed] [Google Scholar]
- 9.Lundgren A, Lundgren D, Taylor A. Influence of barrier occlusiveness on guided bone augmentation. An experimental study in the rat. Clin Oral Implants Res. 1998;9:251–260. doi: 10.1034/j.1600-0501.1998.090406.x. [DOI] [PubMed] [Google Scholar]
- 10.Schmid J, Hammerle CH, Olah AJ, Lang NP. Membrane permeability is unnecessary for guided generation of new bone. An experimental study in the rabbit. Clin Oral Implants Res. 1994;5:125–130. doi: 10.1034/j.1600-0501.1994.050302.x. [DOI] [PubMed] [Google Scholar]
- 11.Kocyigit ID, Tuz HH, Alp YE, Atil F, Tekin U, Coskunses FM. Correction of postsurgical alveolar ridge defect with vertical alveolar distraction of the onlay block graft. J Craniofac Surg. 2012;23:1550–1552. doi: 10.1097/SCS.0b013e31825c74b4. [DOI] [PubMed] [Google Scholar]
- 12.Ozdemir H, Ezirganli S, Isa Kara M, Mihmanli A, Baris E. Effects of platelet rich fibrin alone used with rigid titanium barrier. Arch Oral Biol. 2013;58:537–544. doi: 10.1016/j.archoralbio.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Hasson O. Augmentation of deficient lateral alveolar ridge using the subperiosteal tunneling dissection approach. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:e14–19. doi: 10.1016/j.tripleo.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 14.Ricci JL, Clark EA, Murriky A, Smay JE. Three-dimensional printing of bone repair and replacement materials: impact on craniofacial surgery. J Craniofac Surg. 2012;23:304–308. doi: 10.1097/SCS.0b013e318241dc6e. [DOI] [PubMed] [Google Scholar]
- 15.Bulgin D, Hodzic E. Autologous bone marrow-derived mononuclear cells combined with beta-TCP for maxillary bone augmentation in implantation procedures. J Craniofac Surg. 2012;23:1728–1732. doi: 10.1097/SCS.0b013e31826cf177. [DOI] [PubMed] [Google Scholar]
- 16.Handschel J, Simonowska M, Naujoks C, Depprich RA, Ommerborn MA, Meyer U, Kübler NR. A histomorphometric meta-analysis of sinus elevation with various grafting materials. Head Face Med. 2009;5:12. doi: 10.1186/1746-160X-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]


