The formation and “transformation” of a tissue is guaranteed by a dynamic interaction between cells and surrounding microenvironment which results to be constituted by extracellular matrix, fibroblasts, vascular cells, and immune cells.
Inflammation that is characterized by tumor-infiltrating lymphocytes (TIL), macrophages tumor-associated (TAM), cells of myeloid origin with suppressive capacity (Myeloid derived suppressor cells MDSC) and dendritic cells (DC), plays a major role in cancer progression. Several are the mechanisms of tumor immune escape. Recent clinical trials have demonstrated that administration of monoclonal antibodies (mAb) which inhibit the interaction of immune regulatory checkpoint molecules such as Cytotoxic T-Lymphocyte Antigen 4 (CTLA-4) and Programmed cell death protein 1 (PD-1) with their ligands CD80, CD86 and Programmed cell death 1 ligand 1 (PD-L1), can have a major and lasting effect on the clinical course of several malignant diseases including renal cell carcinoma. Based on these findings, besides the classical diagnostic parameters, it is becoming essential to identify immunological biomarkers in order to predict the clinical course of the disease and especially the response to the treatment.
Renal cell carcinoma (RCC) is one of the most common disease in the urologic tract with more than 30% of patients with metastatic disease at diagnosis.
In RCC, as well as in other immunogenic tumors such as melanoma [1], the role of the microenvironment in the regulation of the mechanisms of tumor progression is well determined. RCC is defined as an immunogenic tumor since several evidence indicate a tumor immune response. Furthermore many therapeutic strategies based on the depletion of immune suppressive regulatory cell types which operate in RCC microenvironment as well as the inhibition of immune regulatory checkpoint molecules have been thoroughly described.
RCC immune infiltrate may be characterized by different subpopulations of leukocyte infiltrate. Specifically, DCs have been shown to play an important role in RCC tumor immunity. Infiltration of DCs into RCC tumor has been associated with improved patients’ survival and reduced risk of disease recurrence [2].
Moreover, immune infiltrate of RCC has been also characterized by the presence of a large numbers of MDSCs and TAMs. TAM infiltration has been involved in cancer progression by stimulating angiogenesis, tumor growth, cellular migration and invasion as well as metastatic potential and resistance to targeted agents [2].
In addition as well as for many types of solid tumors TILs have been also described in RCC tumor microenvironment being an important prognostic factor. TILs may be represented by CD20+ T cells. CD20 is a specific antigen for B lymphocytes. It is a non-glycosylated phosphoprotein expressed on the surface of all B cells starting from the phase of pro-B and progressively increasing in concentration until the mature B cells. Other subsets of lymphocytes which may be present in RCC tumors are represented by regulatory T (Treg) cells. Treg cells are able to suppress the immune responses. Expression of FOXP3, a transcription factor, is currently considered as the most specific and reliable marker for the identification and isolation of human Treg. In a recent study, identification of Treg cells by FOXP3 immunohistochemical staining has shown that in RCC Treg cell infiltrate is localized in the area of tumor invasion which represents the major tumor site of immune escape linked to progression of the disease [3].
Lastly TIL can be also be represented by CD45RO+ or CD8+ T cells. In human, CD45RO is a marker of memory T cells and in RCC its overexpression represents an independent prognostic factor for overall survival [4]. On the other hand in an in vitro study it has been demonstrated that CD8+ cells (cytotoxic T cells) react against tumor cells of RCC. The proportion of activated CD8+ T cells is represented by cells expressing the Granzyme B, a protein that plays a major role in the cytolytic function of cytotoxic T cells. Decrease of Granzyme B+ T cell infiltrate has been shown to correlate with a poor prognosis of the RCC patients [5].
The presence of these distinct subpopulations of tumor infiltrating leukocytes, their different expression as well as their correlation with clinical course of the disease demonstrate that evaluation of a tumor immune score may represents an important new diagnostic and prognostic tool. A tumor immune score can be defined by an immunohistochemical analysis utilizing immune cell type specific- antibodies such as CD45-, Granzyme B-, FOXP3- and CD20-specific antibodies. The evaluation of tumor immune infiltrate and the definition of the tumor immune score may take advantage by the analysis of Tissue Micro Array (TMA). Application of TMA has completely revolutionized biomedical research mainly for its ability to rapidly and simultaneously test new molecular biomarkers on a series of tumor samples incorporated in a single slide.
Since the characterization of the elements involved in cancer immune regulation needs the analysis of several markers, the use of a TMA containing cores adequately representing the inflammatory infiltrates in the intra-tumoral and peri-tumoral area can be a useful tool.
In support of this hypothesis we randomly selected 15 cases of RCCs from our tissue banking at Istituto Nazionale Tumori Fondazione “G. Pascale” in order to build a TMA containing, for each sample, 4 cores of intra-tumoral and 4 cores of peri-tumoral tissues with a diameter of 0.6 mm.
Utilizing CD3-, CD8-, CD20-, CD45-, FOXP3- and Granzyme B- specific antibodies as markers we performed an immunohistochemical analysis on both TMA and matched whole tissue sections with the aim to compare the obtained results in the definition of the inflammatory infiltrate.
The comparison of the studied immunological biomarker expression in the peri-tumoral area has shown a concordance rate for most of the represented lymphocyte populations (Figure 1A). Specifically, expression of CD3+ cells (highly expressed) in whole tissue section is found to have a concordance rate of 95% for 4 spots of tissue in the TMA, while expression of Granzyme B+ cells reaches 80%. However the concordance rate is definitely higher considering all 4 cores as compared with only 2 (Figure 2).
Figure 1.
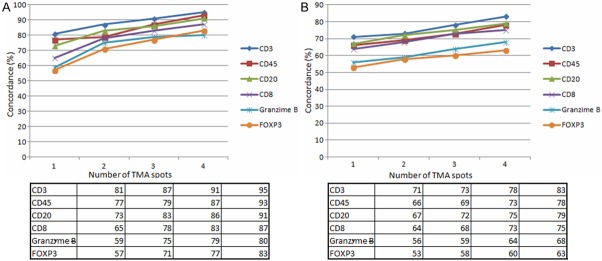
Graphical representation of the immunohistochemical staining concordance in the peri-tumoral (A) and intra-tumoral (B) area: percentage of concordance rate derived from the comparison between 1, 2, 3, 4 cores of tissue and whole sections are shown.
Figure 2.
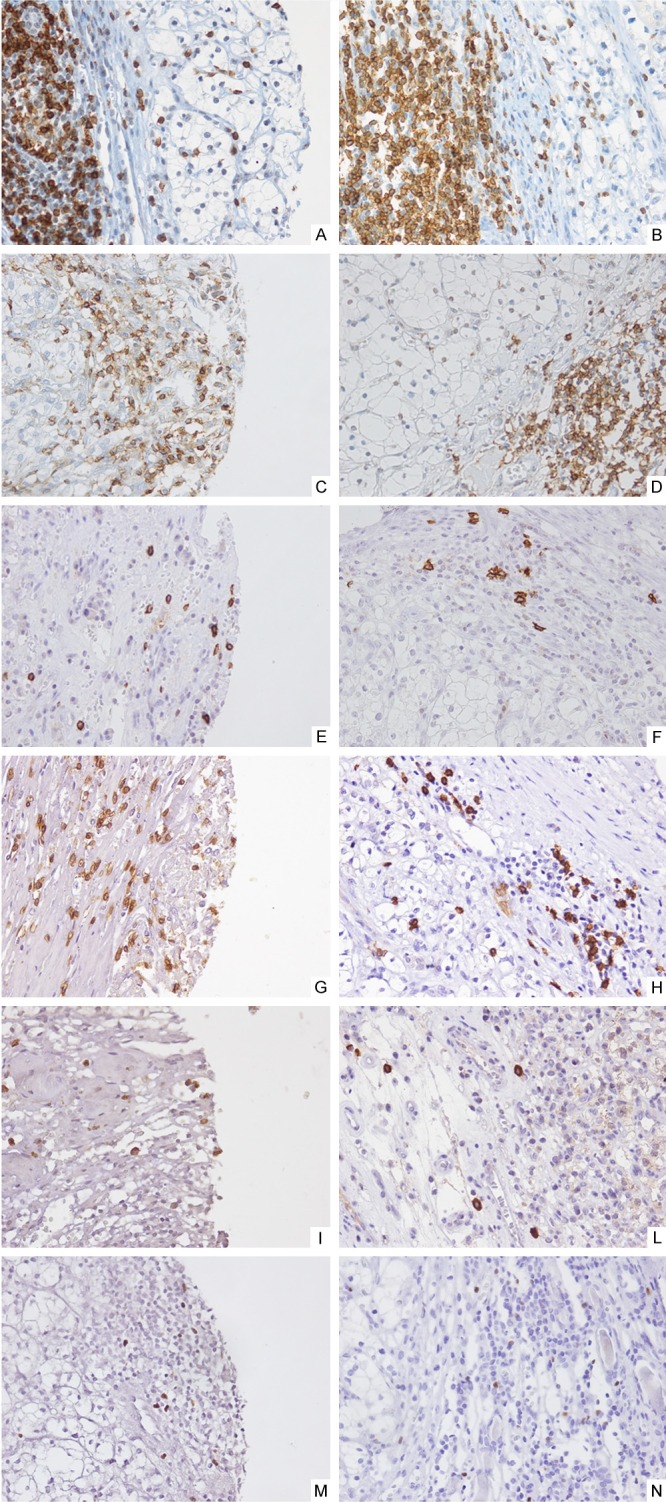
Immunohistochemical staining (40x) of leukocyte infiltrate in peri-tumoral RCC areas. Tissue sections were stained with CD3 (A, B), CD45RO (C, D), CD20 (E, F), CD8 (G, H), Granzyme B (I, L), FOXP3 (M, N) specific antibodies. Representative results are shown.
In addition, the comparison between cores of tissues on TMA and whole sections in the intra-tumoral area has revealed that the concordance rate increases for the most represented lymphocyte populations (Figure 1B). The expression of CD3+ cells in whole tissue section is found to have a concordance rate of 83% for 4 spots of tissue while the FOXP3 reaches 63%. Also in this case, the concordance rate is definitely higher considering all 4 cores compared with only 2 (Figure 3).
Figure 3.
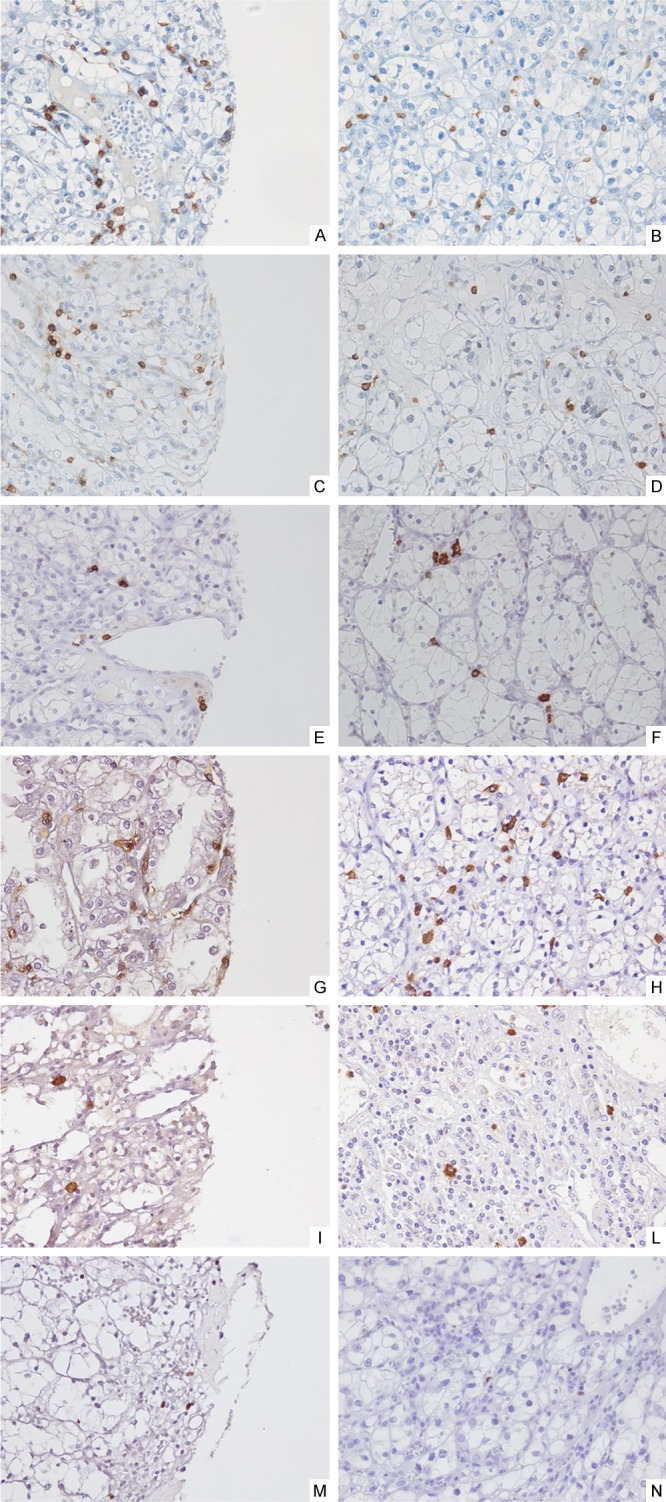
Immunohistochemical staining (40x) of leukocyte infiltrate in intratumoral RCC areas: Tissue sections were stained with CD3 (A, B), CD45RO (C, D), CD20 (E, F), CD8 (G, H), Granzyme B (I, L), FOXP3 (M, N) specific antibodies. Representative results are shown.
In conclusion, our data demonstrate that the TMA can be considered a useful tool for the study of immune cell populations in RCC tumor microenvironment. However an adequate number of cores is need. Specifically the optimal number of cores should be defined for each tumor types. In RCC as shown by our data a TMA should include at least 4 cores of tissue, for the most representative immune cell populations, in order to adequately represent both the intra-tumoral and peri-tumoral areas.
Disclosure of conflict of interest
None.
References
- 1.Cantile M, Collina F, Scognamiglio G, Di Bonito M, Franco R, Botti G. Inadequacy of tissue microarrays for the immunohistochemical detection of cancer stem cells in solid tumors: a viewpoint. Expert Rev Anticancer Ther. 2013;13:1139–41. doi: 10.1586/14737140.2013.845341. [DOI] [PubMed] [Google Scholar]
- 2.Hamada I, Kato M, Yamasaki T, Iwabuchi K, Watanabe T, Yamada T, Itoyama S, Ito H, Okada K. Clinical effects of tumor-associated macrophages and dendritic cells on renal cell carcinoma. Anticancer Res. 2002;22:4281–4. [PubMed] [Google Scholar]
- 3.Sell K, Barth PJ, Moll R, Thomas MA, Zimmer N, Oplesch E, Gudo M, Schrader M, Hofmann R, Schrader AJ. Localization of FOXP3-positive cells in renal cell carcinoma. Tumour Biol. 2012;33:507–13. doi: 10.1007/s13277-011-0283-1. [DOI] [PubMed] [Google Scholar]
- 4.Hotta K, Sho M, Fujimoto K, Shimada K, Yamato I, Anai S, Konishi N, Hirao Y, Nonomura K, Nakajima Y. Prognostic significance of CD45RO+ memory T cells in renal cell carcinoma. Br J Cancer. 2011;105:1191–6. doi: 10.1038/bjc.2011.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lionello I, Mangia P, Gattinoni L, Pende D, Cippone A, Sensi M, Rigatti P, Traversari C. CD8 (+) T lymphocytes isolated from renal cancer patients recognize tumour cells through an HLA- and TCR/CD3-independent pathway. Cancer Immunol Immunother. 2007;56:1065–76. doi: 10.1007/s00262-006-0268-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


