Abstract
Background
Cannabis is widely abused, and efficacies of therapeutics for cannabis dependence remain suboptimal. Magnetic resonance imaging (MRI) may aid in the identification of biological markers for successful treatment outcomes (i.e., abstinence).
Methods
Twenty men with cannabis dependence and twenty non-substance-using healthy comparison (HC) men underwent MRI scanning. Cannabis-dependent individuals then participated in a 12-week randomized clinical trial of behavioral treatments (contingency management (CM), cognitive behavioral therapy (CBT) or both). Pretreatment functional and structural data were compared between the cannabis-dependent and HC participants. In addition, individuals with cannabis dependence were subdivided based on the successful achievement of 21 days of consecutive abstinence during treatment to assess whether abstinent versus nonabstinent cannabis-dependent participants displayed different pretreatment functional and structural characteristics when compared to HC participants.
Results
In comparison to HC participants, cannabis-dependent participants demonstrated greater ventral striatal activation during the receipt of losing outcomes and smaller putamenal volumes. Cannabis-dependent participants who did not subsequently achieved 21 days of consecutive abstinence had increased activity within the striatum during the receipt of losing outcomes, relative to HC participants. Cannabis-dependent participants who did not achieve 21 days of abstinence had decreased bilateral putamen volumes prior to treatment, relative to HC participants.
Conclusions
Individual differences in pretreatment striatal function and structure may relate to individual differences in treatment responses for cannabis dependence. While mechanisms underlying these associations require further exploration, the striatum might mediate treatment responses via its role in associative reward-learning (e.g., through skills training in CBT or reinforcement of abstinence in CM).
Keywords: cannabis dependence, fMRI, FSL-FIRST, reward processing, contingency management, cognitive behavioral therapy
1. INTRODUCTION
Cannabis is widely abused worldwide (Hall and Degenhardt, 2009; Degenhardt and Hall, 2012). Long-term heavy cannabis use is associated with increased rates of mood, anxiety and psychotic disorders, risky sexual behaviors, and other measures of poor health (Kingree and Betz, 2003; Moore et al., 2007; Degenhardt et al., 2009; Mathews et al., 2011; Andrade et al., 2013; Degenhardt et al., 2013). Specific neurocognitive effects of long-term cannabis use may include alterations in IQ, executive functioning and verbal and visual memory (Bolla et al., 2002; Gruber et al., 2012; Meier et al., 2012). Despite the prevalence and negative consequences of cannabis use, the efficacy of current treatment options for cannabis dependence remains limited (Kadden et al., 2007; Carroll et al., 2012).
Current treatment options for cannabis dependence are predominantly nonpharmacological and include cognitive behavioral therapy (CBT; Denis et al., 2006) and contingency management (CM; Carroll et al., 2006). These treatments appear effective for some individuals with cannabis dependence; however, overall rates of abstinence during and subsequent to treatment remain suboptimal (Denis et al., 2006; Kadden et al., 2007; Carroll et al., 2012; Danovitch and Gorelick, 2012). While further research into how best to improve treatment interventions is needed (Danovitch and Gorelick, 2012), a complementary line of research involves the identification of behavioral and/or biological factors that might characterize treatment responders and which could predict optimal treatment responses on an individual basis (Potenza et al., 2011; Feldstein Ewing and Chung, 2013). Such factors may shed light on the mechanisms of action of existing treatments, which could inform treatment adaptations to enhance efficacy or guide individually-tailored treatment-assignment approaches.
Despite behavioral literature suggesting complex and relatively subtle neuropsychological alterations associated with long-term cannabis use (Rogers and Robbins, 2001; Bolla et al., 2002; van Holst and Schilt, 2011), relatively few studies have examined the relationship between neural function and treatment outcomes in cannabis dependence. However, pretreatment individual differences in functional neurocircuitry might impact treatment responses in cannabis-using youth (Feldstein Ewing and Chung, 2013), and less is known about such relationships among adults with cannabis dependence.
As with brain function, brain structure may also relate to substance-use-treatment outcomes (Xu et al., 2010; Froeliger et al., 2010). While the precise mechanism behind these associations remains unclear, it is possible that specific structural alterations might negatively impact individuals’ successful engagement in treatment (Chung et al., 2013). For example, preclinical data have demonstrated that structural damage to the putamen disrupts habit formation or the learning of new action-outcome contingencies (Yin et al., 2004). Thus, structural alterations within this region might impair an individual’s ability to modify previously-learned stimulus-response relationships (such as those relating to the reinforcing properties of cannabis) as is required for the development of new adaptive behaviors (e.g., skills training to deal with craving) aimed at reducing substance use. However, further research is needed to confirm this hypothesis, and to explore the relationship between pretreatment brain structure and function and treatment outcomes in cannabis dependence.
In particular, investigating how structure and function of brain regions involved in reward processing (e.g., ventral striatum; VS; Knutson, 2001) may relate to treatment outcomes is important in the study of addictions and their treatment (Thayer and Hutchison, 2013). To our knowledge, no studies have explored the relationship between pretreatment brain structure and responses to treatment in cannabis dependence. Such research may aid in the identification of biological markers which might eventually guide the selection of appropriate treatment interventions (Feldstein Ewing and Chung, 2013).
The ventral and dorsal striatum are involved in multiple aspects of reward processing (e.g., craving, anticipatory and outcome processing; Roitman et al., 2005; Liu et al., 2011; Everitt and Robbins, 2013; Goldman et al., 2013). Thus, the striatum may relate to important aspects of the pathophysiology of substance-use disorders and their treatment (Brewer et al., 2008).
In this study, we explored the relationship between pretreatment striatal function and brain structure and short-term abstinence in response to behavioral treatments for cannabis dependence. VS activity was examined using a monetary incentive delay (MID) task (Andrews et al., 2011) which is a well-established probe of reward-related neurocircuitry (Knutson, 2001; Andrews et al., 2011) previously used to study aspects of reward processing across a range of substance- and addiction-related disorders (Goldstein et al., 2007; Wrase et al., 2007; Beck et al., 2009; Peters et al., 2011; Balodis et al., 2012; Donovan et al., 2012)). In particular, amongst cocaine-dependent individuals performing the MID task, greater bilateral VS activation was observed (relative to non-substance-using comparison participants) when participants were presented with winning outcomes (e.g., WON $5), and greater right VS activation was related to poorer treatment outcome (less abstinence; Jia et al., 2011). These findings suggest that MID performance successfully recruits brain regions related to real-world clinical outcomes, although such relationships may differ across addictions (e.g., to cannabis versus cocaine). To investigate striatal volume, bilateral caudate and putamen volumes were compared using FSL’s FIRST, an automated segmentation tool for subcortical structures (Patenaude et al., 2011).
Two previously published fMRI studies employing MID tasks have studied reward processing among cannabis users, and both have reported increases in VS activity during reward anticipation (Nestor et al., 2010; Jager et al., 2013); however, neither study included treatment-seeking individuals or a formal assessment (e.g., SCID) of cannabis dependence. Based on these findings (Nestor et al., 2010; Jager et al., 2013), we hypothesized that, relative to non-substance-using HC participants, individuals with cannabis dependence would: (i) exhibit greater brain activity within the VS during reward processing (i.e., reward anticipation and reward receipt) during MID task performance; and (ii) have lower GM volume within the caudate and putamen. We also explored the hypothesis that among individuals with cannabis dependence, individual differences in GM volumes and brain activations within the striatum would relate to treatment responses, as has been observed functionally in studies of cocaine dependence (Jia et al., 2011) and structurally in studies of tobacco smoking (Froeliger et al., 2010).
2. METHODS
2.1. Participants and recruitment
Cannabis-dependent participants were recruited from a randomized clinical trial (RCT) of community-based, outpatient treatments for cannabis dependence exploring the relative efficacy of CM, CBT or combined CM and CBT (Carroll et al., 2012). Two-hundred-and-six individuals were screened for eligibility for participation in the trial. Forty-four individuals did not complete screening and a further 35 individuals were deemed ineligible for trial participation (Carroll et al., 2012). Exclusion criteria for the RCT included likely and imminent incarceration and physical dependence on any substance other than cannabis or nicotine; for further detail. Participants with cannabis dependence were not excluded for co-occurring disorders; see (Carroll et al., 2012). While both men and women were recruited for the RCT, the study sample was largely male (>80%; Carroll et al., 2012). The participants from the RCT who also participated in pretreatment neuroimaging consisted of 20 men and 1 woman with cannabis dependence. Given the possibility of gender-related differences in neural responses, the female participant was excluded from subsequent analyses. Thus, the final sample included 20 men with cannabis dependence (mean age=26.7 years; standard error=2.2) and 20 male HC participants (mean age=29.2; standard error=2.3) recruited from the community via advertisement. Exclusion criteria for HC participants included any past or current psychotropic medication (e.g., antidepressants, anxiolytics, antipsychotics, mood stabilizers), any Axis-I disorder, including lifetime alcohol or other substance-use disorder other than nicotine dependence, as assessed using a Structured Clinical Interview (SCID; First, 1995). Exclusion criteria for all participants additionally included claustrophobia, head trauma resulting in loss of consciousness or other contraindication to MRI scanning.
Demographic and clinical characteristics of cannabis-dependent and HC participants are shown in Table 1. The cannabis-dependent and HC groups did not differ in age (F=.66, p=.42); but differed in race (χ2=7.87, p=.05) and the cannabis-dependent group had lower IQ, on average (F=16.85, p<.001).
Table 1A.
Demographic and clinical characteristics of participants with cannabis dependence and healthy comparison participants.
| CB (n = 20) | HC (n= 20 ) | |||||
|---|---|---|---|---|---|---|
| Mean | St. Error | Mean | St. Error | F | p | |
| Age | 26.65 | 2.19 | 29.2 | 2.25 | 0.66 | 0.42 |
| Shipley IQ | 93.1 | 2.86 | 108.42 | 2.36 | 16.85 | 0.0002 |
| n | % | n | % | χ 2 | p | |
| Gender (male) | 20 | 100.00 | 20 | 100.00 | - | - |
| Ethnicity | 1 | 5.00 | 1 | 5.00 | 0.00 | 1.00 |
| Race | ||||||
| African American | 12 | 60.00 | 7 | 35.00 | 7.87 | 0.05 |
| Caucasian | 5 | 25.00 | 13 | 65.00 | ||
| Hispanic* | 1 | 5.00 | 0 | 0.00 | ||
| Biracial** | 2 | 10.00 | 0 | 0.00 | ||
| Married/Serious | ||||||
| Relationship | 1 | 5.00 | 1 | 5.00 | 0.00 | 1.00 |
| Employed *** | 10 | 50.00 | 7 | 63.16 | 0.69 | 0.41 |
| Tobacco user | 15 | 80.00 | 2 | 10.00 | 17.29 | <.0001 |
One cannabis-dependent participant reported their race as hispanic
Indicates half African American and half Caucasian
Information on employment status missing for one healthy comparison participant
2.2. Abstinence
Given the difficulty many cannabis users have in achieving abstinence (rather than reducing the frequency of their use) a sustained period of continuous abstinence – as opposed to proportion of (non-continuous) days of abstinence during treatment is considered a clinically-relevant outcome (Kadden et al., 2007). Thus, abstinence was defined based on the total number of consecutive days of self-reported abstinence during treatment. A threshold of 21 or more consecutive days of abstinence was selected, as this has been found to be a significant predictor of longer-term abstinence following treatment for substance use (Carroll et al., under review; Donovan et al., 2012).
Thirteen out of the 20 participants with cannabis dependence achieved 21 or more days of consecutive abstinence within treatment (65% abstinent; 35% nonabstinent). Participants who achieved 21 days of abstinence (hereafter referred to as abstinent participants) versus those who did not (hereafter referred to as non-abstinent participants) did not differ in age (F=1.07, p=.32), race (χ2=2.64, p=.34), IQ (F=.10, p=.76), age of first cannabis use (F=.98, p=.34), days of pretreatment cannabis use (F=.72, p=.41), pretreatment Addiction Severity Index (ASI) drug composite scores (F=.56, p=.46), current or lifetime major depression (χ2=1.96, p=.16; χ2=.22, p=.64), current or lifetime alcohol use disorder (χ2=2.42, p=.30; χ2=1.32, p=.25) or lifetime anxiety disorders (χ2= 57, p= 45); none met criteria for current anxiety disorders.
As expected, abstinent participants spent more days in treatment (F=13.72, p=.002), had lower post-treatment ASI cannabis scores (F=7.04, p=.02), had more cannabis-negative urines during treatment (F=8.74, p=.008) and reported more total days of abstinence at one-year follow-up (F=5.45, p=.03; abstinent: 70.16% days abstinent; non-abstinent: 32.68% days abstinent) in comparison to non-abstinent participants.
Ten of the 20 cannabis-dependent participants received either CM (n=3) or CBT (n=7) alone, and the remaining 10 participants received combined CBT and CM. Abstinent versus non-abstinent cannabis participants did not differ in the types of therapy received (χ2=.30, p=.86).
2.3. Monetary Incentive Delay (MID) task design
The version of the MID task used in this study has been described in detail previously (Andrews et al., 2011). The task design is available as Supplemental Material 1. During task performance, participants were presented with one of six cues (WIN $0, WIN $1, WIN $5, LOSE $0, LOSE $1, LOSE $5) for 1000 milliseconds (prospect of reward or loss phase; A1), followed by a fixation cross for a variable delay. Participants were then presented with a target stimulus. In order to win (or avoid losing) the amount of money indicated by the cue, participants had to respond with a single button press while the target was on the screen. Following the target stimulus, a fixation cross was again presented (anticipation of reward or loss phase; A2). Finally, participants were given feedback on the outcome of the trial (e.g., WON $1; DID NOT WIN $1; LOST $1; DID NOT LOSE $1).
Prior to MID task performance, participants were informed that they would receive a total payment based on their performance. Subsequently, all participants received a fixed amount of compensation for their participation along with a possible bonus payment based on their performance. This payment methodology is consistent with our previous studies using the MID task to study reward-processing among individuals with substance use disorders, in comparison to HC subjects (e.g., Jia, Worhunsky et al. 2011). Total run time was 12 minutes (22 win trials, 22 loss trials, 11 neutral trials), and each participant performed two runs. Details of structural and functional data acquisition are provided as Supplementary Material2.
2.5. Analyses
2.5.1. Functional data
Spatial pre-processing was conducted using SPM8 (Wellcome Functional Imaging Laboratory, London, United Kingdom). All functional scans were realigned separately prior to normalization to Montreal Neurological Institute (MNI) standard space (voxel size=3×3×3mm3). Scans with participant motion in excess of one voxel were excluded. Data were smoothed with a 6mm full-width-half-maximum (FWHM) Gaussian kernel.
Functional ROIs centering on the left and right VS were selected as a priori regions of interest (ROIs) and ROI coordinates were defined using meta-analytic data of published MID task fMRI studies (Knutson and Greer, 2008). Anatomical ROIs of the caudate were defined independently using the automated labeling atlas (Tzourio-Mazoyer et al., 2002) within the WFU pickatlas toolbox for SPM8 (Maldjian et al., 2003). Group-level random-effects models were conducted using the small volume correction (SVC) tool in SPM8 with a spherical ROI (9mm radius) centered on the VS coordinates described above, and p-values were thresholded using family-wise error correction (pFWE<.05). Specifically, two-tailed t-tests were used to explore any significant between-group differences in BOLD response during the different temporal phases of the MID task (prospect of wins/losses; anticipation of wins/losses; outcome of wins/losses). Consistent with previous published methods on this task (Knutson and Greer, 2008), all events of interest (e.g., ‘WIN $5’) were contrasted with their neutral comparison event (e.g., ‘WIN $0’).
Between-group comparisons of whole-brain task-related activations were conducted to confirm localization of ROI findings and to guide subsequent analyses exploring differences in BOLD responses within the striatum among individuals with cannabis-dependence based on their later achievement of three or more weeks of abstinence during treatment, in comparison to healthy comparison participants. Data were compared using group-level random-effects models. Images were cluster-level corrected at pFWE<.05.
2.5.2. Structural data
Structural data were analyzed using FSL (Oxford, United Kingdom): Individual participant T1-weighted images were brain extracted and affine-registered to the MNI152 1mm template prior to undergoing automated segmentation with FSL’s default parameters (‘run_first_all’) and with boundary correction to produce mesh and volumetric outputs (Patenaude et al., 2011). The caudate and putamen were defined using standard labels from the Center for Morphometric Analysis (CMA, MGH, Boston). Volumetric information (total number of voxels) for the caudate and putamen were calculated from individual participants’ segmented images using ‘fslstats’ and these values were entered into SPSS for between-group comparisons. Between-group comparisons of bilateral caudate and putamen volumes3 were conducted using a GLM with group (cannabis dependent, HC) as the independent variable, structural volume as the dependent variable and total tissue volume as a covariate. In order to explore the relationship between pretreatment brain structure and the subsequent achievement of 21-days of abstinence, data were further analyzed using GLMs including group (abstinent, not-abstinent, HC) as the independent variable, structural volume as the dependent variables and total tissue volume as a covariate.
As reported above, cannabis-dependent and HC participants were not matched on IQ. Thus, post-hoc comparisons co-varying for IQ were conducted in order to explore possible effects of IQ on brain structure or function. Specifically, a multivariate GLM including group (cannabis-dependent, HC) as a fixed-factor, bilateral caudate and putamen volumes as the dependent factors and IQ and total tissue volume as covariates was used to examine the influence of IQ on pretreatment brain structure within the striatum. In order to explore the influence of IQ on BOLD signal responses within the caudate, a multivariate GLM including group (cannabis-dependent, HC) as a fixed-factor, BOLD responses within the caudate as a dependent factor and IQ as a covariate was conducted. In order to explore the effects of abstinence, the same GLMs were also conducted using the three-group variable of abstinent/not-abstinent/HC as a fixed factor.
3. RESULTS
3.1. fMRI MID
3.1.1. Behavioral performance
There were no significant between-group differences in reaction times for win or loss trials (p’s>.05). Mean (±standard deviation) hit rates (number of successful trials/total possible trials) did not differ across groups for loss (HCs=.71±.16; abstinent=.76±.10; non-abstinent=.71±.08) or win (HCs=.71±.13; abstinent=.80±.10; non-abstinent=.74±.08) trials (p’s>.05).
3.1.2. HC versus cannabis-dependent participants
3.1.2.2. fMRI MID ventral striatal region-of-interest analyses
There were no significant between-group differences in VS activity during the prospect (A1) or anticipation (A2) phases for wins or losses (pFWE’s>.05), or during the outcome phase for wins (pFWE’s>.1). Cannabis-dependent participants had significantly increased activity within the right VS when presented with losing outcomes (t=3.93, pFWE=.02), but did not differ in left VS activity (t=3.1, pFWE=.09), in comparison to HC participants. Inspection of whole-brain statistical maps (cluster-level-corrected, pFWE<.05) confirmed this finding and also revealed a pattern of increased activation that encompassed not only the VS but also the caudate bilaterally (with the identified cluster extending into the thalamus, brainstem and putamen). Findings from whole-brain, between-group comparisons across all phases of the MID are shown in the Supplemental Materials4 (pFWE<.05).
Given the absence of between-group differences in VS activity during the A1 and A2 phases of the MID task, exploratory analyses looking at BOLD responses within the VS during the anticipation of $1 (in comparison to $0) and $5 (in comparison to $0) gains and losses separately were further conducted. These analyses revealed significantly greater activation among cannabis-dependent participants (versus controls) within the left (t=4.26, p=.009), but not the right (t=3.45, p=.06), VS during the anticipation (A2) phase for losses. Cannabis-dependent participants also had significantly increased activity within the right VS when presented with losing outcomes (t=3.63, pFWE=.04), but did not differ in left VS activity (t=1.94, pFWE=.47), in comparison to HC participants.
3.1.2.3. HC versus cannabis-dependent participants by abstinence status
Given the diffuse nature of the detected activation (whole-brain findings, above), an anatomical ROI of the caudate was used to extract individual-participant BOLD signal (details in section 2.5.1.) and the resulting data were used to explore the effects of subsequent abstinence. Statistical comparisons revealed significantly increased activation within the caudate among non-abstinent, cannabis-dependent participants in comparison to HC participants (t=3.33, p=.003). However, there were no significant between-group differences in caudate activity between abstinent, cannabis-dependent participants and HC participants (t=1.27, p=.21), or between non-abstinent and abstinent cannabis-dependent participants (t=1.21, p=.24).
3.2. Structure
3.2.1. HC versus cannabis-dependent participants
In comparison to HC participants, cannabis-dependent participants had significantly decreased left and right putamen volumes (F=6.82, p=.01; F=4.69, p=.04). No significant between-group differences in caudate volume were found between HC and cannabis-dependent participants (p’s>.1).
3.2.2. HC versus cannabis-dependent participants by abstinence status
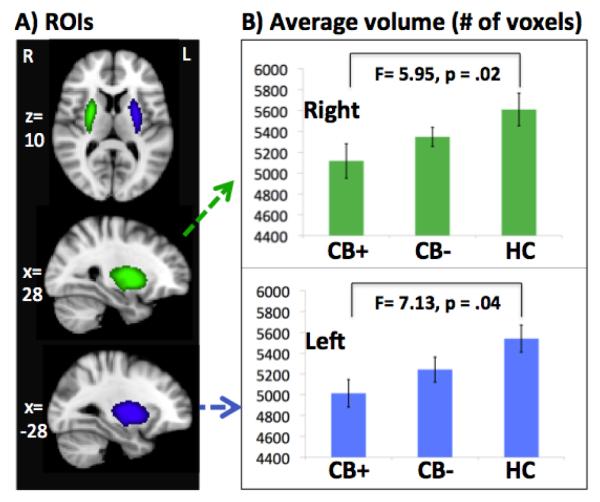
There was a significant main effect of group (abstinent, non-abstinent, HC) on left (F=4.11, p=.03) but not right (F=3.12, p=.06) putamen volumes. Subsequent comparisons revealed significantly lower GM volumes within the left and right putamen among nonabstinent participants (F=7.13, p=.04; F=5.95, p=.02), in comparison to HC participants. By contrast, there were no significant between-group differences in putamen volume between abstinent cannabis-dependent and HC participants (p’s>.1).
Post-hoc comparisons (detailed above in Section 2.5.2.) revealed no significant effect of IQ on striatal activations during MID task performance or on left or right putamen volumes (p’s>.1).
4. DISCUSSION
To our knowledge, this is the first study reporting on functional and structural MRI data from cannabis-dependent men prior to undergoing behavioral treatment, in comparison to a group of age-matched HC men. Overall, cannabis-dependent versus HC participants demonstrated greater VS activation during the processing of losing outcomes and less putamenal volume bilaterally. Although no differences in clinical or demographic factors were observed between abstinent versus non-abstinent cannabis-dependent individuals prior to treatment, the abstinent and non-abstinent individuals displayed different pretreatment functional and structural characteristics when compared to HC participants. Specifically, in comparison to HC participants, non-abstinent cannabis-dependent participants had higher activity within the caudate in response to losing outcomes (during the MID task) and smaller putamenal volumes prior to treatment. By contrast, subsequently abstinent cannabis-dependent participants did not significantly differ from HC participants on these measures. These initial findings indicate that specific aspects of striatal function and structure relate to treatment outcomes for cannabis dependence. However, further research using other fMRI tasks to probe striatal functioning is needed to determine whether the current functional findings are specific to MID task performance or reflective of a more general alteration in striatal responsivity among individuals with cannabis dependence.
4.1. Functional findings
Partially consistent with our primary hypothesis, cannabis-dependent (versus HC) participants had significantly higher VS activity during MID task performance, suggesting hyperactivation of reward-related neurocircuitry. However, this pattern of activation was observed during the receipt of losing outcomes and not during reward anticipation or during the receipt of winning outcomes as hypothesized.
Our finding is partially consistent with previous findings from studies of cannabis users which have reported increased striatal responses among frequent cannabis users during different stages of MID task performance including during both reward anticipation and during the receipt of losing outcomes (Nestor et al., 2010; Jager et al., 2013). However, to our knowledge, this is the first report of heightened reward responses among cannabis-dependent adults. In our primary analyses (which combined $1 and $5 trials for wins and losses), we did not observe blunted VS activations during reward anticipation as has been seen in individuals with other addictive disorders, e.g., cocaine abuse (Goldstein et al., 2007), alcohol dependence (Wrase et al., 2007; Beck et al., 2009), nicotine dependence (Peters et al., 2011), pathological gambling (Balodis et al., 2012; Donovan et al., 2012) – although not always consistently (Rogers and Robbins, 2001; van Holst and Schilt, 2011; Potenza, 2013). In contrast, our exploratory, post-hoc comparisons found increases in VS activity during reward and loss anticipation for $1 and $5, respectively, among cannabis-dependent individuals. However, these findings should be interpreted cautiously, as they are based on a smaller number of events (e.g., eleven $1 anticipation events versus 22 total win anticipation events) than is often used in analyses of MID task data (Balodis et al., 2012). Future studies using more of each specific event type are needed to explore possible effects of reward/loss magnitude on BOLD signal responses among cannabis-dependent individuals in a systematic manner.
Both higher (Nestor et al., 2010; Jager et al., 2013) and lower (van Hell et al., 2010) VS activity during reward anticipation has been previously reported among cannabis users; thus, the extent to which these findings reflect differences in cannabis dependence in comparison to other addictive disorders warrants additional investigation. However, multiple possible reasons for the differences in findings exist. Unlike previous studies (e.g., van Hell et al., 2010; Filbey et al., 2013), all but one of the cannabis-dependent participants in this study were referred to treatment by the criminal justice system, possibly suggesting a more severe or distinct clinical profile. Further, the version of the MID task used in this study differs from that used in previous fMRI studies of cannabis abuse/dependence (Nestoer et al., van Hell et al., Filbey et al., 2013) in two important aspects (Andrews et al., 2011). Firstly, the modified MID task was designed to separate reward prospect (A1) and reward anticipation (A2) phases which are combined in other versions of the MID (Knutson, 2001). Secondly, the version of the MID used here does not include a working memory component as is uses explicit monetary cues (e.g., ‘WIN $1’) rather than symbols (as in the original MID designed by Knutson and colleagues (2001). Thus, differences in both task designs and study populations may account for the differences between our findings and those reported in previous studies. Future studies could aim to compare differences in reward anticipation and prospect phases between cannabis-dependent individuals who are mandated to attend treatment versus those who are not.
In comparison to HC participants, non-abstinent cannabis-dependent participants demonstrated greater activation within the caudate while processing losing outcomes. By contrast, no differences in caudate activity were observed between HC participants and subsequently abstinent individuals with cannabis dependence, suggesting that differences in striatal reactivity may relate to treatment outcomes. This finding is also reminiscent of our previous report of a positive association between pretreatment activity in VS and duration of abstinence among individuals with cocaine dependence (Brewer et al., 2008), suggesting that alterations within the striatum may contribute to the success/failure of abstinence attainment across multiple addictions. However, given the small samples and differences in tasks employed across studies, additional research is needed to examine these possibilities.
In contrast to the increased VS activity observed among cannabis-dependent individuals during the receipt of losing outcomes, we did not observe differences in VS activation in response to winning outcomes. This finding is partially consistent with recent data suggesting differential responses to rewards versus losses among individuals with cannabis dependence (Filbey et al., 2013). Thus, future research should further explore neural responses to positive versus negative incentives in cannabis dependence and their relationship to treatment outcomes.
The striatum is involved in motivational control processes related to addictions, in the encoding of rewarding and aversive stimuli and in affiliative processes such as trust, social cooperation and empathy (Montague et al., 2004; Bora et al., 2009; Everitt and Robbins, 2013). Thus, it is possible that the altered engagement of the caudate seen prior to treatment among participants who did not subsequently achieved longer durations (3+ weeks) of abstinence during treatment (in comparison to HC participants) may relate to an altered ability to appropriately process negatively valenced stimuli and/or engage in emotionally salient aspects of behavioral therapies, as has been suggested previously (Forbes et al., 2010). An alternative, related hypothesis is that individuals with increased caudate activity during loss outcome processing may be more sensitive to negatively valenced stimuli, and therefore have greater difficulty in coping with other aversive states – such as withdrawal from cannabis – and thus may be less likely to achieve sustained abstinence. However, both of these possibilities remain speculative and warrant additional investigation.
The observed differences in BOLD responses during the processing of losing outcomes were found in the right (pFWE<.003) but not left VS (pFWE=.09). This lateralization may relate to individual differences in handedness (data on which was not available for all participants), and clarification of this issue will be important for future studies.
4.2. Structural findings
Findings from previous structural MRI studies of chronic cannabis use have been mixed, as both higher, lower and no differences in volumes within multiple GM structures have been reported across comparisons with HC samples (Batalla et al., 2013). However, to our knowledge, this is the first GM volumetric study conducted among individuals meeting DSM-IV criteria for cannabis dependence. Given its central role in reward processing and in experiences of marijuana craving (Cousijn et al., 2013; Goldman et al., 2013), we focused our analyses on the striatum (i.e., caudate and putamen). Partially consistent with our secondary hypothesis, individuals with cannabis dependence (relative to HC participants) demonstrated significantly smaller putamenal volumes bilaterally. By contrast, there were no differences in caudate volumes among individuals with cannabis dependence when compared to HCs. We believe that this is the first report of striatal structural differences among individuals with cannabis dependence or with heavy cannabis use.
Although the etiologies of the currently observed structural and functional differences are unknown, multiple possibilities exist. Administration of exogenous cannabinoids such as Δ9-tetrahydrocannabinol (THC) – the primary psychoactive component of cannabis – increases dopamine release within the striatum via the activation of cannabinoid CB1 receptors (Bossong et al., 2008). In chronic substance abuse (i.e., long-term, non-medical use), such repeated drug-induced increases in dopamine may trigger neuroadaptations (e.g., in dopamine transporters (DAT) (Chang et al., 2007) or brain glucose metabolism (Volkow et al., 1993)) that persist beyond the acute effects of the drug have abated and contribute to the maintenance of addictive behaviors (Koob and Volkow, 2009; Volkow et al., 2009). Thus, it is possible that the reduced putamenal volumes or VS activations observed in this study may relate to neurochemical alterations in glucose metabolism and/or DAT availability within these regions (Schlageter et al., 1987; Kim et al., 2006; van Wingen et al., 2013). This interpretation is consistent with reductions in glucose metabolism within the putamen among young men with cannabis dependence (Sevy et al., 2008) and findings of decreased striatal DAT availability among individuals with both cannabis and tobacco use (Leroy et al., 2012). However, further studies explicitly examining the relationship between these factors and GM structures among individuals with cannabis dependence are needed to test these hypotheses.
There was a main effect of group (abstinent, non-abstinent, HC) on putamenal volume, such that non-abstinent – but not abstinent – cannabis-dependent participants exhibited lower putamenal volumes compared to HC participants. Interestingly, this finding is consistent with a recent study reporting lower pretreatment putamenal volumes among non-abstinent versus abstinent tobacco smokers (Froeliger et al., 2010), suggesting that differences in putamenal structure might relate to treatment responses across different addictive disorders.
Preclinical data have demonstrated that the putamen is critically involved in the learning of action-outcome contingencies, e.g., damage to the dorsolateral striatum/putamen disrupts habit formation in rats (Yin et al., 2004; Pierce and Vanderschuren, 2010). Thus, it is possible that individuals with diminished putamenal volumes might be less responsive to behavioral therapies such as CM due to a decreased ability to acquire new action-outcome contingencies, although this possibility currently remains very speculative. While further research is needed to test this latter hypothesis, our findings – along with complimentary data from a study of nicotine dependence (Froeliger et al., 2010) – nonetheless suggest that pretreatment putamenal volumes may relate importantly to treatment responses among individuals with addictions. Future studies should examine the efficacy of medications influencing neural structure and function within the striatum (e.g., varenicline; Crunelle et al., 2011) as an adjunct to behavioral therapy for cannabis- and other substance-use disorders (Crunelle et al., 2011; van Wingen et al., 2013).
4.3. Strengths and limitations
This study has several strengths, including its combination of structural and functional imaging data analyzed using well-validated techniques (Jia et al., 2011; Patenaude et al., 2011) and the careful characterization of a group of cannabis-dependent individuals on multiple clinical variables. However, the cannabis-dependent and HC groups were not matched for daily tobacco use. Previous studies suggest significant effects of tobacco use on brain function and structure (e.g., Brody et al., 2004; Froeliger et al., 2013); thus, our findings may have been confounded by tobacco smoking in the cannabis-dependent group. However, the rate of tobacco use (75%) among the cannabis-dependent participants included in this study was equivalent to that found in the parent RCT (74%; Carroll et al. 2012). Tobacco use is common among cannabis-dependent individuals (Stinson et al. 2006). As such, excluding individuals with tobacco use might have limited the generalizability of our findings (Peters et al., 2012). Future studies of cannabis dependence should seek to examine possible influences of tobacco use on brain structure and function.
The study is further limited by the absence of female participants and the relatively modest sample size, which prevented us from exploring influences of treatment type (CBT, CM or combined CBT and CM) on abstinence. Given the relatively small number of cannabis-dependent participants who did not achieve 21 days of consecutive abstinence (n=7), the study had limited power to detect between-group differences within cannabis-dependent participants (abstinent versus nonabstinent). Thus, an important future direction will be to replicate and extend these findings in larger samples.
The potential of using pretreatment neurobiological characteristics to ‘predict’ longer-term outcomes remains controversial, thus our findings should be interpreted cautiously. Additionally, future studies should obtain MRI measures following treatment in order to examine the extent to which treatment might alter brain structure and function. Such research might further clarify the construct validity of studying pretreatment neural characteristics. The current findings nonetheless provide important preliminary evidence suggesting that individual differences in brain function and structure prior to treatment relate to individual variability in response to behavioral therapies for cannabis dependence.
4.4. Conclusions
The effectiveness of current behavioral treatment options for cannabis dependence remains suboptimal (Denis et al., 2006; Kadden et al., 2007; Carroll et al., 2012; Danovitch and Gorelick, 2012), and it is unclear why some individuals respond preferentially to treatment. The current findings suggest that pretreatment brain structure and function within the striatum relate to outcomes following behavioral treatments for cannabis dependence. These findings are similar to those previously reported among individuals with other substance-related disorders (Froeliger et al., 2010; Brewer et al. 2008). The striatum is involved in affiliative processes which may be important for successful engagement in therapy, e.g., social cooperation and empathy (Montague et al., 2004; Bora et al., 2009; Everitt and Robbins, 2013). Moreover, given the role of the striatum in associative reward-learning (Schönberg et al., 2007), one hypothesis is that heightened functional responsivity to negative outcomes accompanied by decreased structural volume may relate to faulty stimulus-response learning which could influence the acquisition of new adaptive skills during behavioral treatments for addictions. However, this possibility is speculative and warrants further investigation. Further research is needed to determine the extent to which behavioral treatments for cannabis dependence might impact directly on brain function and structure (e.g., post-treatment changes in neurobiology), whether pretreatment function and structure themselves directly predispose to treatment responses, or both.
Supplementary Material
Figure 1.
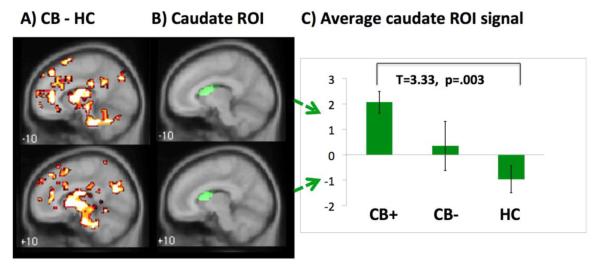
A shows cluster-level corrected whole-brain comparisons of cannabis dependent (CB) participants versus healthy comparison (HC) participants (pFWE<.05) during the presentation of losing outcomes (versus neutral outcomes).
B shows the anatomical caudate ROI which was used to extract individual participants’ signals (plotted in Figure 1C).
C shows mean (± standard error) signal change within caudate ROI for abstinent individuals with cannabis dependence (CB+), non-abstinent individuals with cannabis dependence (CB−) and healthy comparison (HC) participants. R=right, L=left.
Figure 2.
ROI = region of interest; CB+ = non-abstinent cannabis dependence; CB− = abstinent cannabis dependence; HC = healthy comparison
A shows bilateral structural putamen ROIs (Harvard-Oxford Subortical Structural Atlas).
B shows mean (± standard error) putamen volumes for the right and left putamen among non-abstinent cannabis-dependent participants (CB+), abstinent cannabis-dependent participants (CB−) and healthy comparison (HC) participants.
In comparison to healthy comparison participants, non-abstinent cannabis dependent participants had significantly reduced left and right putamen volumes (F= 7.13, p= .04; F= 5.95, p= .02). After controlling for total tissue volume, these reductions remained significant (F=6.82, p=.01, F=4.69, p=.04). There were no significant differences in left or right putamen volume between abstinent cannabis dependent participants and healthy comparison participants (p’s > .1).
Table 1B.
Demographic and clinical characteristics of cannabis-dependent participants who achieved 21-days of consecutive abstinence
| Abstinent (n=13) | Not Abstinent (n=7) |
|||||
|---|---|---|---|---|---|---|
| Mean | St. Error | Mean | St. Error | F | p | |
| Age | 28.31 | 3.12 | 23.57 | 5.8 | 1.07 | 0.32 |
| Shipley IQ | 93.77 | 3.77 | 91.86 | 4.57 | 0.10 | 0.76 |
| n | % | n | % | χ 2 | p | |
| Gender (male) | 13 | 100.00 | 7 | 100.00 | - | - |
| Ethnicity | 1 | 7.70 | 0 | 0.00 | 0.57 | 0.45 |
| Race/Ethnicity | ||||||
| African American | 9 | 69.20 | 3 | 42.90 | 2.64 | 0.45 |
| Caucasian | 2 | 15.40 | 3 | 42.90 | ||
| Hispanic* | 1 | 7.70 | 0 | 0.00 | ||
| Biracial** | 1 | 7.70 | 1 | 14.30 | ||
| Married/Serious Relationship | 1 | 7.70 | 0 | 0.00 | 0.57 | 0.45 |
| Employed | 7 | 53.80 | 3 | 42.90 | 0.22 | 0.64 |
| Tobacco user | 8 | 61.50 | 7 | 100.00 | 3.59 | 0.06 |
| Co-occurring disorders *** | ||||||
| Current major depression | 0 | 0.00 | 1 | 14.30 | 1.96 | 0.16 |
| Lifetime major depression | 1 | 7.70 | 1 | 14.30 | 0.22 | 0.64 |
| Current anxiety disorder | 0 | 0.00 | 0 | 0.00 | - | - |
| Lifetime anxiety disorder | 1 | 7.70 | 0 | 0.00 | 0.57 | 0.45 |
| Current alcohol abuse | 1 | 7.70 | 0 | 0.00 | 0.57 | 0.45 |
| Lifetime alcohol use disorder | 4 | 30.80 | 4 | 57.10 | 1.32 | 0.25 |
| Substance use pretreatment | Mean | St. Error | Mean | St. Error | F | p |
| Years of cannabis use | 14.38 | 3.33 | 8.72 | 1.89 | 1.4 | 0.25 |
| Age of first cannabis use | 13.38 | 0.46 | 14.14 | 0.59 | 0.98 | 0.34 |
| Days pretreatment cannabis use |
16.15 | 2.69 | 20.14 | 4.05 | 0.72 | 0.41 |
| Days pretreatment alcohol use | 4.15 | 1.49 | 3.43 | 1.38 | 0.10 | 0.75 |
| Composite ASI cannabis score | 0.24 | 0.04 | 0.33 | 0.13 | 0.56 | 0.46 |
| Composite ASI alcohol score | 0.04 | 0.01 | 0.05 | 0.01 | 0.04 | 0.85 |
| Composite ASI other drug score | 0.02 | 0.01 | 0.00 | 0.00 | 0.89 | 0.36 |
| n | % | n | % | χ 2 | p | |
| Treatment-seeking | 1 | 7.70 | 0 | 0.00 | 0.57 | 0.45 |
One cannabis-dependent participant reported their race as Hispanic
Indicates half African American and half Caucasian
Healthy comparison participants were excluded for co-occurring disorders
ASI= Addiction Severity Index
Acknowledgments
Role of Funding: This research was funded in part by NIH grants from NIDA (R01 DA020908, R01 DA035058, P50 DA09241, K12 DA00167), the Connecticut State Department of Mental Health and Addictions Services, and the Connecticut Mental Health Center. EED was funded by K12 DA031050 from NIDA, NIAAA, ORWH and OD. The funding agencies did not provide input or comment on the content of the manuscript, and the content of the manuscript reflects the contributions and thoughts of the authors and not necessarily reflect the views of the funding agencies.
Footnotes
Author Disclosures Contributors: Drs. Potenza and Carroll designed the protocol and study. Dr. Yip conducted statistical analyses and wrote the first draft of the manuscript. Dr. DeVito assisted in compiling and coordinating clinical and demographic data. Dr. Kober contributed to statistical analyses. All authors consulted on the interpretation of the analyses and data and have provided critical feedback on the manuscript.
Disclosures: The authors report no financial conflicts of interest with respect to the content of this manuscript. Dr. Carroll is a member of CBT4CBT LLC, the company which makes CBT4CBT available to clinical providers. She has nothing else to disclose. Dr. Potenza has received financial support or compensation for the following: Dr. Potenza has consulted for and advised Boehringer Ingelheim, Lundbeck and Ironwood; has consulted for and has financial interests in Somaxon; has received research support from the National Institutes of Health, Veteran’s Administration, Mohegan Sun Casino, the National Center for Responsible Gaming, and Forest Laboratories, Ortho-McNeil, Oy-Control/Biotie, Glaxo-SmithKline, and Psyadon pharmaceuticals; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse control disorders or other health topics; has consulted for gambling entities, law offices and the federal public defender’s office in issues related to impulse control disorders; provides clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; has performed grant reviews for the National Institutes of Health and other agencies; has guest-edited journal sections; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
See Supplemental Figure 1 by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
See ‘MRI data acquisition’ in the Supplemental Materials by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
See Supplemental Figure 5 for structural ROIs with functional coordinates of the ventral striatum overlaid by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
See Supplemental Table 1 and Supplemental Figures 2-4 by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
REFERENCES
- Andrade LF, Carroll KM, Petry NM. Marijuana use is associated with risky sexual behaviors in treatment-seeking polysubstance abusers. Am. J. Drug Alcohol Abuse. 2013;39:266–271. doi: 10.3109/00952990.2013.803112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews MM, Meda SA, Thomas AD, Potenza MN, Krystal JH, Worhunsky P, Stevens MC, O’Malley S, Book GA, Reynolds B, Pearlson GD. Individuals family history positive for alcoholism show functional magnetic resonance imaging differences in reward sensitivity that are related to impulsivity factors. Biol. Psychiatry. 2011;69:675–683. doi: 10.1016/j.biopsych.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis IM, Kober H, Worhunsky PD, Stevens MC, Pearlson GD, Potenza MN. Diminished frontostriatal activity during processing of monetary rewards and losses in pathological gambling. Biol. Psychiatry. 2012;71:749–757. doi: 10.1016/j.biopsych.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batalla A, Bhattacharyya S, Yücel M, Fusar-Poli P, Crippa JA, Nogué S, Torrens M, Pujol J, Farré M, Martin-Santos R. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS ONE. 2013;8:e55821. doi: 10.1371/journal.pone.0055821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Schlagenhauf F, Wustenberg T, Hein J, Kienast T, Kahnt T, Schmack K, Hagele C, Knutson B, Heinz A, Wrase J. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol. Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Bolla KL, Brown K, Eldreth DA, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurolology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Allen NB. Neurobiology of human affiliative behaviour: implications for psychiatric disorders. Curr. Opin. Psychiatry. 2009;22:320–325. doi: 10.1097/YCO.0b013e328329e970. [DOI] [PubMed] [Google Scholar]
- Bossong MG, van Berckel BNM, Boellaard R, Zuurman L, Schuit RC, Windhorst AD, van Gerven JMA, Ramsey NF, Lammertsma AA, Kahn RS. [Delta]9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacol. 2008;34:759–766. doi: 10.1038/npp.2008.138. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment brain activation during Stroop task is associated with outcomes in cocaine-dependent patients. Biol. Psychiatry. 2008;64:998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, Bota RG, Bartzokis G, London ED. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol. Psychiatry. 2004;55:77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Easton CJ, Nich C, Hunkele KA, Neavins TM, Sinha R, Ford HL, Vitolo SA, Doebrick CA, Rounsaville BJ. The use of contingency management and motivation/skills building therapy to treat young adults with marijuana dependence. J. Consult. Clin. Psychol. 2006;74:955–966. doi: 10.1037/0022-006X.74.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Nich C, LaPaglia DM, Peters EN, Easton CJ, Petry NM. Combining cognitive behavioral therapy and contingency management to enhance their effects in treating cannabis dependence: less can be more, more or less. Addiction. 2012;107:1650–1659. doi: 10.1111/j.1360-0443.2012.03877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102:16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Chung T, Pajtek S, Clark DB. White matter integrity as a link in the association between motivation to abstain and treatment outcome in adolescent substance users. Psychol. Addict. Behav. 2013;27:533–542. doi: 10.1037/a0026716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousijn J, Goudriaan AE, Ridderinkhof KR, van den Brink W, Veltman DJ, Wiers RW. Neural responses associated with cue-reactivity in frequent cannabis users. Addict. Biol. 2013;18:570–580. doi: 10.1111/j.1369-1600.2011.00417.x. [DOI] [PubMed] [Google Scholar]
- Crunelle CL, Schulz S, de Bruin K, Miller ML, van den Brink W, Booij J. Dose-dependent and sustained effects of varenicline on dopamine D2/3 receptor availability in rats. Eur. Neuropsychopharmacol. 2011;21:205–210. doi: 10.1016/j.euroneuro.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Danovitch I, Gorelick DA. State of the art treatments for cannabis dependence. Psychiatr. Clin. North Am. 2012;35:309–326. doi: 10.1016/j.psc.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Coffey C, Romaniuk H, Swift W, Carlin JB, Hall WD, Patton GC. The persistence of the association between adolescent cannabis use and common mental disorders into young adulthood. Addiction. 2013;108:124–133. doi: 10.1111/j.1360-0443.2012.04015.x. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet. 2012;379:55–70. doi: 10.1016/S0140-6736(11)61138-0. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Hall WD, Lynskey M, McGrath J, McLaren J, Calabria B, Whiteford H, Vos T. Should burden of disease estimates include cannabis use as a risk factor for psychosis? PLoS Med. 2009;6:e1000133. doi: 10.1371/journal.pmed.1000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis C, Lavie E, Fatseas M, Auriacombe M. Psychotherapeutic interventions for cannabis abuse and/or dependence in outpatient settings. Cochrane Database Syst. Rev. C. 2006:D005336. doi: 10.1002/14651858.CD005336.pub2. [DOI] [PubMed] [Google Scholar]
- Donovan DM, Bigelow GE, Brigham GS, Carroll KM, Cohen AJ, Gardin JG, Hamilton JA, Huestis MA, Hughes JR, Lindblad R, Marlatt GA, Preston KL, Selzer JA, Somoza EC, Wakim PG, Wells EA. Primary outcome indices in illicit drug dependence treatment research: systematic approach to selection and measurement of drug use end-points in clinical trials. Addiction. 2012;107:694–708. doi: 10.1111/j.1360-0443.2011.03473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci. Biobehav. Rev. 2013;37:1946–1954. doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Chung T. Neuroimaging mechanisms of change in psychotherapy for addictive behaviors: emerging translational approaches that bridge biology and behavior. Psychol. Addict. Behav. 2013;27:329–335. doi: 10.1037/a0031491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Dunlop J, Myers US. Neural effects of positive and negative incentives during marijuana withdrawal. PLoS ONE. 2013;8:e61470. doi: 10.1371/journal.pone.0061470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV. Patient Edition American Psychiatric Press, Inc; Washington, DC: 1995. [Google Scholar]
- Forbes E, Olino T, Ryan N, Birmaher B, Axelson D, Moyles D, Dahl R. Reward-related brain function as a predictor of treatment response in adolescents with major depressive disorder. Cogn. Affect. Behav. Neurosci. 2010;10:107–118. doi: 10.3758/CABN.10.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froeliger B, Kozink RV, Rose JE, Behm FM, Salley AN, McClernon FJ. Hippocampal and striatal gray matter volume are associated with a smoking cessation treatment outcome: results of an exploratory voxel-based morphometric analysis. Psychopharmacol (Berl.) 2010;210:577–583. doi: 10.1007/s00213-010-1862-3. [DOI] [PubMed] [Google Scholar]
- Froeliger B, Modlin LA, Kozink RV, Wang L, Garland EL, Addicott MA, McClernon FJ. Frontoparietal attentional network activation differs between smokers and nonsmokers during affective cognition. Psychiatry Res. 2013;211:57–63. doi: 10.1016/j.pscychresns.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman M, Szucs-Reed RP, Jagannathan K, Ehrman RN, Wang Z, Li Y, Suh JJ, Kampman K, O’Brien CP, Childress AR, Franklin TR. Reward-related brain response and craving correlates of marijuana cue exposure: a preliminary study in treatment-seeking marijuana-dependent subjects. J. Addict. Med. 2013;7:8–16. doi: 10.1097/ADM.0b013e318273863a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, Telang F, Caparelli EC, Chang L, Ernst T, Samaras D, Squires NK, Volkow ND. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? Am. J. Psychiatry. 2007;164:43–51. doi: 10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Sagar KA, Dahlgren MK, Racine M, Lukas SE. Age of onset of marijuana use and executive function. Psychol. Addict. Behav. 2012;26:496–506. doi: 10.1037/a0026269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374:1383–1391. doi: 10.1016/S0140-6736(09)61037-0. [DOI] [PubMed] [Google Scholar]
- Jager G, Block RI, Luijten M, Ramsey NF. Tentative evidence for striatal hyperactivity in adolescent cannabis-using boys: a cross-sectional multicenter fMRI study. J. Psychoactive Drugs. 2013;45:156–167. doi: 10.1080/02791072.2013.785837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Worhunsky PD, Carroll KM, Rounsaville BJ, Stevens MC, Pearlson GD, Potenza MN. An initial study of neural responses to monetary incentives as related to treatment outcome in cocaine dependence. Biol. Psychiatry. 2011;70:553–560. doi: 10.1016/j.biopsych.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadden RM, Litt MD, Kabela-Cormier E, Petry NM. Abstinence rates following behavioral treatments for marijuana dependence. Addict. Behav. 2007;32:1220–1236. doi: 10.1016/j.addbeh.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Lyoo IK, Hwang J, Chung A, Hoon Sung Y, Kim J, Kwon D-H, Chang KH, Renshaw PF. Prefrontal grey-matter changes in short-term and long-term abstinent methamphetamine abusers. Int. J. Neuropsychopharmacol. 2006;9:221–228. doi: 10.1017/S1461145705005699. [DOI] [PubMed] [Google Scholar]
- Kingree JB, Betz H. Risky sexual behavior in relation to marijuana and alcohol use among African-American, male adolescent detainees and their female partners. Drug Alcohol Depend. 2003;72:197–203. doi: 10.1016/s0376-8716(03)00196-0. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J. Neurosci. 2001;21:151–155. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Greer SM. Anticipatory affect: neural correlates and consequences for choice. Philosoph. Trans. R. Soc. B. 2008;363:3771–3786. doi: 10.1098/rstb.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacol. 2009;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy C, Karila L, Martinot J-L, Lukasiewicz M, Duchesnay E, Comtat C, Dollé F, Benyamina A, Artiges E, Ribeiro M-J, Reynaud M, Trichard C. Striatal and extrastriatal dopamine transporter in cannabis and tobacco addiction: a high-resolution PET study. Addict. Biol. 2012;17:981–990. doi: 10.1111/j.1369-1600.2011.00356.x. [DOI] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 2011;35:1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mathews RR, Hall WD, Gartner CE. Depression and psychological distress in tobacco smokers and people with cannabis dependence in the National Survey of Mental Health and Wellbeing. Med. J. Aust. 2011;195:S12–15. doi: 10.5694/j.1326-5377.2011.tb03259.x. [DOI] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RSE, McDonald K, Ward A, Poulton R, Moffitt TE. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc. Nat. Acad. Sci. 2012;109:2657–2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Hyman SE, Cohen JD. Computational roles for dopamine in behavioural control. Nature. 2004;431:760–767. doi: 10.1038/nature03015. [DOI] [PubMed] [Google Scholar]
- Moore THM, Zammit S, Lingford-Hughes A, Barnes TRE, Jones PB, Burke M, Lewis G. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- Nestor L, Hester R, Garavan H. Increased ventral striatal BOLD activity during non-drug reward anticipation in cannabis users. NeuroImage. 2010;49:1133–1143. doi: 10.1016/j.neuroimage.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters EN, Budney AJ, Carroll KM. Clinical correlates of co-occurring cannabis and tobacco use: a systematic review. Addict. 2012;107:1404–1417. doi: 10.1111/j.1360-0443.2012.03843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Bromberg U, Schneider S, Brassen S, Menz M, Banaschewski T, Conrod PJ, Flor H, Gallinat J, Garavan H, Heinz A, Itterman B, Lathrop M, Martinot J-L, Paus T, Poline J-B, Robbins TW, Rietschel M, Smolka M, Ströhle A, Struve M, Loth E, Schumann G, Büchel C. Lower ventral striatal activation during reward anticipation in adolescent smokers. Am. J. Psychiatry. 2011;168:540–549. doi: 10.1176/appi.ajp.2010.10071024. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Vanderschuren LJMJ. Kicking the habit: the neural basis of ingrained behaviors in cocaine addiction. Neurosci. Biobehav. Rev. 2010;35:212–219. doi: 10.1016/j.neubiorev.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza M. How central is dopamine to pathological gambling or gambling disorder? Front. Behav. Neurosci. 2013 doi: 10.3389/fnbeh.2013.00206. doi: 10.3389/fnbeh.2013.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza Marc N., Sofuoglu M, Carroll Kathleen M., Rounsaville Bruce J. Neuroscience of behavioral and pharmacological treatments for addictions. Neuron. 2011;69:695–712. doi: 10.1016/j.neuron.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Curr. Opin. Neurobiol. 2001;11:250–257. doi: 10.1016/s0959-4388(00)00204-x. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45:587–597. doi: 10.1016/j.neuron.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Schlageter NL, Horwitz B, Creasey H, Carson R, Duara R, Berg GW, Rapoport SI. Relation of measured brain glucose utilisation and cerebral atrophy in man. J. Neurol. Neurosurg. Psychiatry. 1987;50:779–785. doi: 10.1136/jnnp.50.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönberg T, Daw ND, Joel D, O’Doherty JP. Reinforcement learning signals in the human striatum distinguish learners from nonlearners during reward-based decision making. J. Neurosci. 2007;27:12860–12867. doi: 10.1523/JNEUROSCI.2496-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevy S, Smith G, Ma Y, Dhawan V, Chaly T, Kingsley P, Kumra S, Abdelmessih S, Eidelberg D. Cerebral glucose metabolism and D2/D3 receptor availability in young adults with cannabis dependence measured with positron emission tomography. Psychopharmacol. 2008;197:549–556. doi: 10.1007/s00213-008-1075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer RE, Hutchison KE. Neuroimaging in clinical studies of craving: importance of reward and control networks. Psychol. Addict. Behav. 2013;27:543–546. doi: 10.1037/a0030275. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B.Joliot, M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van Hell HH, Vink M, Ossewaarde L, Jager G, Kahn RS, Ramsey NF. Chronic effects of cannabis use on the human reward system: an fMRI study. Eur. Neuropsychopharmacol. 2010;20:153–163. doi: 10.1016/j.euroneuro.2009.11.010. [DOI] [PubMed] [Google Scholar]
- van Holst RJ, Schilt T. Drug-related decrease in neuropsychological functions of abstinent drug users. Curr. Drug Abuse Rev. 2011;4:42–56. doi: 10.2174/1874473711104010042. [DOI] [PubMed] [Google Scholar]
- van Wingen GA, van den Brink W, Veltman DJ, Schmaal L, Dom G, Booij J, Crunelle CL. Reduced striatal brain volumes in non-medicated adult ADHD patients with comorbid cocaine dependence. Drug Alcohol Depend. 2013;131:198–203. doi: 10.1016/j.drugalcdep.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacol. 2009;56(Suppl. 1):3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DL, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, Beck A, Strohle A, Juckel G, Knutson B, Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Xu J, DeVito EE, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. White matter integrity is associated with treatment outcome measures in cocaine dependence. Neuropsychopharmacol. 2010;35:1541–1549. doi: 10.1038/npp.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur. J. Neurosci. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.