Abstract
Purpose
To determine the effect of docosahexaenoic acid (DHA) on the growth of human melanoma in vitro and in vivo and to better understand the potential role of the G-protein coupled receptors in mediating this effect.
Materials and Methods
For in vitro studies, human melanoma and control fibroblast cells were treated with DHA and TAK-875 (selective GPR40 agonist) and a cell viability assay was performed to determine cell counts.
A murine subcutaneous xenograft model of human melanoma was used to test the effect of dietary treatment with an omega-3 fatty acid (FA) rich diet compared to an omega-6 FA rich diet on the growth of human melanoma in vivo. A similar animal model was used to test the effect of oral TAK-875 on the growth of established melanoma tumors in vivo.
Results
DHA has an inhibitory effect on the growth of human melanoma both in vitro and in vivo. Tumors from animals on the omega-3 FA rich diet were 69% smaller in weight (P=0.005) and 76% smaller in volume compared to tumors from animals on the omega-6 FA rich diet.
TAK-875 has an inhibitory effect on the growth of human melanoma both in vitro and in vivo. Tumors from animals treated with TAK-875 were 46% smaller in weight (P=0.07), 62% smaller in volume (P=0.03) and grew 77% slower (P=0.04) compared to the placebo group.
Conclusion
DHA and TAK-875 have a profound and selective inhibitory effect on the growth of human melanoma both in vitro and in vivo.
Keywords: melanoma, omega-3 fatty acid, omega-3 polyunsaturated fatty acid, docosahexaenoic acid, G-protein coupled receptor, GPR40, GPR120
1. INTRODUCTION
Melanoma is the most deadly form of skin cancer (1). The annual incidence has been steadily increasing, with a greater than 60% increase over the last 30 years. This rate equates with the fastest rate of rise in the incidence of any cancer worldwide, and can not be explained by currently known risk factors such as sun exposure (2, 3). Current treatment strategies for advanced melanoma are limited to high-dose chemotherapy and recent antibody mediated treatments, both unfortunately associated with very significant, and oftentimes treatment-limiting, side effects (4-6). The partial clinical successes with these treatments, observed responses in only a subset of patients, and eventual development of drug resistance and disease progression emphasizes the ongoing need for effective and safe treatment strategies for this highly aggressive disease.
Changes in the dietary patterns of humans over time may provide insight into novel approaches to the treatment of human melanoma. Anthropological and nutritional studies document a remarkable change in the human diet over the last 100 years, most notably with regard to the type and amount of fat consumed (7-12). These changes are manifested by both an absolute and a relative change in the omega-6 and omega-3 fatty acid (FA) consumption. Today, the Western diet provides an omega-6 to omega-3 FA ratio of as high as 25:1, which is in stark contrast to the 1:1 ratio historically consumed by humans (10), creating a nutritional environment that is very different from our ancestors. This change is particularly relevant in light of the growing body of data indicating that dietary fats influence the development and progression of many malignancies, suggesting that the increase in omega-6 FA consumption over the last 100 years may be partially responsible to the upward trend in incidence of melanoma (2, 3).
The beneficial effects of omega-3 FAs were first elucidated by studies that showed that Greenland Eskimos, who consume a high seafood diet with a 1:1 ratio of omega-6:omega-3 FAs, have very low rates of coronary artery disease, asthma and diabetes mellitus (9). Since these early observations, the beneficial health effects of omega-3 FAs have been extended to include several malignancies (9, 13-18). The mechanism via which omega-3 FAs exert their potential effects in melanoma, and in general, remain largely unknown, although a recent report by Oh and colleagues did for the first time suggest that these fats exert many of their metabolic effects through specific G-protein coupled receptors (19).
The primary purpose of this study is to determine the effect of the omega-3 FA, docosahexaenoic acid (DHA; 22:6 n-3), on the growth of human melanoma both in vitro and in vivo. Herein, we demonstrate that DHA has a profound inhibitory effect on human melanoma, both in vitro and in an in vivo subcutaneous murine model of human melanoma. With this knowledge, we then sought to better understand the mechanism via which DHA inhibits the growth of human melanoma. Given the recent data suggesting that omega-3 fatty acids may mediate their effects through specific G-protein coupled receptors, we focused our effects on these receptors. We were able to identify the G-protein coupled receptor 40 (GPR40) as a cell membrane protein that may mediate the inhibitory effects of DHA on human melanoma. Our data implicates GPR 40 as a potential relevant target for the design of new nutritional and therapeutic strategies for human melanoma.
2. MATERIALS AND METHODS
2.1 Cell Lines and Cultures
Human foreskin fibroblast (Hs27) and human melanoma (A2058, A375, SK-Mel 3) cell lines were purchased (ATCC, Manassas VA). Cells were plated in 48-well plates at a concentration of 7.5×103 cells/well in either DMEM (Hs27, A2058, A375; Invitrogen/GIBCO, Grand Island NY) or McCoy’s 5a Medium (SK-Mel 3; Invitrogen/GIBCO) supplemented with 10% FBS (Invitrogen/GIBCO) and incubated at 37°C in a humid ified atmosphere with 5% CO2. After 24 hours the medium was replaced with either DMEM (Hs27, A2058, A375) or McCoy’s 5a Medium (SK-Mel 3) containing 1% heat inactivated-FBS (Invitrogen/GIBCO) and 0.1% FA-free BSA (Invitrogen/GIBCO) with various concentrations of either DHA (Martek, Columbia MD), GW9508 (Cayman Chemical, Ann Arbor MI) or TAK-875 (Selleck Chemicals, Houston TX) prepared in 4μL of ETOH. DHA was provided at 25-250μM, GW9508 at 25-150μM and TAK-875 at 0-0.4μM. Controls were treated with 4μL of ETOH. All concentrations were tested in quadruplicate.
Following either 144 (DHA) or 72 hours of treatment (GW9508, TAK-875) cells were trypsinized (Trypsin-EDTA; Invitrogen/GIBCO) and viable cells were counted using an absorbance-based cell viability assay (Cell Titer Blue Cell Viability Assay; Promega, Madison WI). During the treatment, medium was changed at 72 hours for the DHA studies. Cell counts are reported as a percent of the control cell count.
2.2 Real-time Quantitative Polymerase Chain Reaction (RT-qPCR)
Total RNA was extracted from purified human fibroblast (Hs27), human melanoma (A2058, A237, SK-Mel3), human neuroblastoma (SKNSH, IMR32, SKNAS; ATCC) and human breast cancer (MCF7; ATCC) cells, suspended in PBS (Invitrogen/GIBCO) using a commercially available kit (Allprep DNA/RNA/Protein Mini Kit; QIAGEN, Valencia CA). RNA concentration and quality was analyzed in a Nanodrop ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington DE). Real-time quantitative PCR was performed on an Applied Biosystems 7000 Real-Time PCR System using RNA at 10ng/mL, a Taqman probe for human GPR40 (Hs03045166_s1; Applied Biosystems, Grand Island NY), human GPR120 (Hs00699184_m1; Applied Biosystems) and human GAPDH (Hs02758991_g1; Applied Biosystems) as an endogenous control and Taqman reagents under default conditions. All assays were performed in duplicate.
2.3 Animals and Housing
All animal husbandry and experimental procedures were reviewed and approved by the institutional animal care and use committee of Boston Children’s Hospital. Six-week old SCID mice (Massachusetts General Hospital, Boston MA) were housed on paper chip bedding in a barrier room with regulated temperature (21°C±1.6°C), humidity (45%±10%), and an alternating 12-hour light and dark cycle with ad libitum access to water and diet. All animals were allowed to acclimate to their environment for 72 hours prior to initiation of experimental treatments.
2.4 Diets
For dietary treatment studies, animals were randomized to one of three different diet groups (N=19/group) each containing 10% of total calories as fat provided as: soybean oil (SOY; #110990, Dyets Inc., Bethlehem PA, USA), hydrogenated coconut oil (HCO; #102328, Dyets Inc.) or a 20:1 ratio of DHA:AA (DHA; #102536, Dyets Inc.) (Table 1). The diets were provided for three weeks prior to tumor cell inoculation and continued throughout the study period. Drug treatment animals were fed a standard rodent chow.
Table 1.
Composition of Experimental Diets
| HCO | SOY | DHA | |
|---|---|---|---|
| Casein | 501.2 | 501.2 | 501.2 |
| L-Cystine | 7.2 | 7.2 | 7.2 |
| Sucrose | 400 | 400 | 400 |
| Cornstarch | 1676.5 | 1676.5 | 1676.3 |
| Dyetrose | 589 | 589 | 589 |
| Mineral Mix #210050 | 29.4 | 29.4 | 29.4 |
| Vitamin Mix #310025 | 38.7 | 38.7 | 38.7 |
| Hydrogenated Coconut Oil | 360 | 0 | 284.4 |
| Soybean Oil | 0 | 360 | 0 |
| Docosahexaenoic Acid (DHA) | 0 | 0 | 72 |
| Arachidonic Acid (AA) | 0 | 0 | 3.6 |
| Total | 3602.0 | 3602.0 | 3601.8 |
All values reported as kcal/kg diet.
2.5 Drug (TAK-875)
Animals with established tumors were randomized to treatment with once daily oral TAK-875 at 100mg/kg (in 0.2mL of 50% DMSO) or vehicle (0.2mL of 50% DMSO) via oral gavage (N=7/group). Treatment was initiated once tumors reached ~200mm3 and was continued for 14 days.
2.6 Tumor implantation and Experimental Treatment
For tumor implantation, 1×106 human melanoma tumor cells (A2058) in 0.2μL of PBS (Invitrogen/GIBCO) were subcutaneously injected into the left flank. Animal weights and tumor dimensions were measured every 48 hours. Tumor volume was calculated (volume=length*width2*0.52).
Animals in the dietary treatment arm of the study were pre-treated with their respective diet for three weeks prior to tumor cell inoculation. The diets were continued for four weeks following tumor implantation.
Animals in the drug treatment arm of the study were fed a standard rodent chow and were started on drug treatment (TAK-875 versus vehicle) once the tumors reached ~200mm3. Drug treatment was continued for 14 days.
2.7 Euthanasia
All animals were euthanized with Avertin (Sigma-Aldrich, St. Louis MO). At euthanasia, animal weight, tumor volume and tumor weight were measured and serum and tumor samples were collected and stored at -80°C for FA analysis.
2.8 FA Analysis
Total FA were extracted per the modified Folch method (20). FA analysis was performed on a Hewlett-Packard 6890N gas chromatograph (GMI Inc., Ramsey MN) coupled to an HP-5975B mass spectrometer equipped with Supelcowax SP-10 capillary column (GMI Inc.). FA concentrations were calculated by proportional comparison of peak areas to the area of the 17:0 internal standard.
2.9 Statistical Analysis
All continuous variables presented as mean ± standard deviation (SD) except for animal weight, tumor weight/volume and change in tumor volume which are presented as mean ± standard error of the mean (SEM). Continuous variables were analyzed with the Student’s t-test or the Mann-Whitney U test as appropriate. Continuous variables from more than three independent groups were analyzed with the Kruskal-Wallis ANOVA. Significance was assessed using a two-sided 5% alpha level. All statistical analysis was performed with GraphPad Prism software (version 5.0; San Diego CA).
3. RESULTS
3.1 DHA Treatment
DHA had a profound, selective inhibitory effect on the growth of all three human melanoma cell lines tested in culture but was not toxic to control fibroblast cells (Figure 1A).
Figure 1. The effect of DHA on the growth of human melanoma.
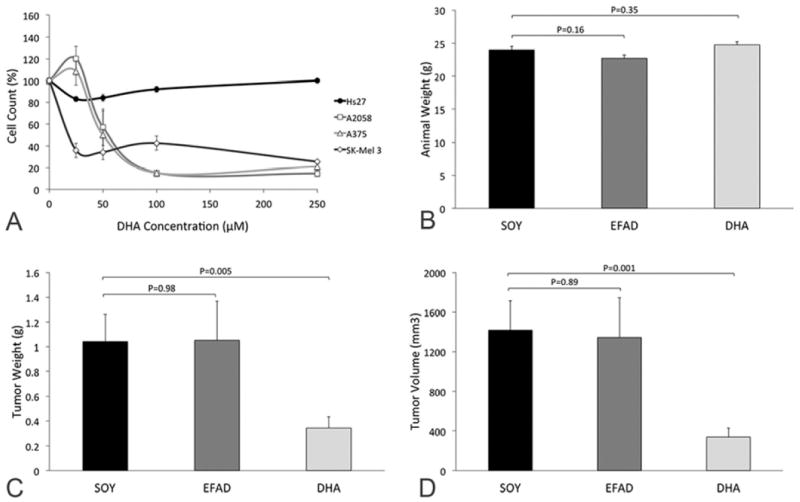
(A) DHA has an inhibitory effect on the growth of all three different human melanoma cell lines (A2058, A375, SK-Mel 3) while there is no toxic effect on the growth of the control fibroblast cell line even at very high concentrations of DHA. (B-D) A murine subcutaneous xenograft model of human melanoma (A2058) was used to evaluate the effect of a diet rich in DHA on the growth of human melanoma (n=19/group). Animal weights did not differ between the three different diet groups (B) however tumor weights (C) and volumes (D) were significantly lower in the DHA group as compared to the SOY group.
To test the effect of dietary treatment with DHA on the growth of human melanoma in vivo, a murine subcutaneous xenograft model of human melanoma was used. An omega-3 FA rich diet (DHA) was designed to mimic the FA composition of cold water fish (21, 22), with an omega-3 to omega-6 FA ratio of 20:1 provided as DHA and arachidonic acid (AA; 20:4n-6; omega-6 FA). In contrast an omega-6 FA rich diet (SOY) was designed to mimic the standard western diet, with fat provided as soybean oil and thus containing an omega-6 to omega-3 FA ratio of ~8:1 provided as linoleic acid (LA; 18:2n-6; omega-6 FA) and alpha-linolenic acid (ALA; 18:3n-3; omega-3 FA). A third diet in which all fat was provided as hydrogenated coconut oil (EFAD), which is deficient in essential FAs, was used as a control for essential fatty acid deficiency (EFAD). Although animals in each of the dietary treatment groups (N=19/group) had similar body weights (Figure 1B), tumors were 69% smaller in weight (P=0.005; Figure 1C) and 76% smaller in volume (P=0.001; Figure 1D) in the DHA compared to the SOY group. Animals in the EFAD group had tumors comparable to the SOY group.
No animals in either the SOY or the DHA diet groups had any evidence of biochemical EFAD (serum triene:tetraene ratio >0.2). In contrast, all animals on the EFAD diet had evidence of biochemical EFAD (Figure 2A; Table 2). The dietary treatments did significantly change the serum FA profiles resulting in a lower omega-6/omega-3 ratio in the DHA compared to the SOY and EFAD groups (Figure 2B-D). Interestingly, the serum omega-6/omega-3 ratio in the SOY group (4.64 ± 1.00) was very similar to the ratio reported for humans consuming a typical Western diet (4.72 ± 0.19) (23). The tumor FA profiles reflected the serum FA profiles (Figure 2E-H, Table 3).
Figure 2. Serum and Tumor Fatty acid Profiles.
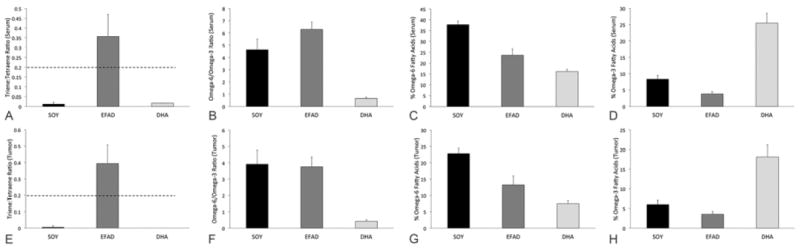
Serum FA profiles (A-D) following dietary treatment demonstrate biochemical EFAD, defined as a triene:tetraene ratio >0.2, in the EFAD group only (A) although omega-6 FA content was significantly lower and omega-3 FA content was significantly higher in the DHA group compared to the SOY group (B-D). Tumor FA profiles (E-H) following dietary treatment also demonstrated EFAD only in tumors from animals on the EFAD diet (E). The tumors from animals on the DHA diet had omega-6 and omega-3 FA contents very similar to those seen in the serum (F-H).
Table 2.
Serum fatty acid profiles
| Fatty acid | SOY (N=5) | EFAD (N=5) | DHA (N=5) | PANOVA | PSOY vs DHA |
|---|---|---|---|---|---|
| Saturated Fats | |||||
| Tetradecanoic (myristic) acid, 14:0 | 0.769±0.332 | 3.042±0.816 | 2.197±0.841 | 0.006 | 0.017 |
| Hexadecanoic (palmitic) acid, 16:0 | 19.576±1.674 | 19.341±0.632 | 20.585±0.715 | 0.145 | 0.270 |
| Octadecanoic (stearic) acid, 18:0 | 12.289±2.611 | 12.049±1.107 | 10.890±0.382 | 0.395 | 0.691 |
|
| |||||
| Monounsaturated Fats | |||||
| Hexadecenoic (palmitoleic) acid, 16:1ω7 | 3.122±0.918 | 5.495±1.003 | 4.245±0.627 | 0.006 | 0.058 |
| Octadecenoic (vaccenic) acid, 18:1ω7 | 1.891±0.733 | 3.158±0.520 | 1.795±0.386 | 0.019 | 0.805 |
| Octadecenoic (oleic) acid, 18:1ω9 | 14.041±1.968 | 18.521±0.811 | 13.825±0.997 | 0.009 | 0.835 |
|
| |||||
| Polyunsaturated Fats | |||||
| Octadecatrienoic (alpha-linolenic) acid, 18:3ω3 | 1.366±0.584 | 0.098±0.042 | 0.081±0.031 | 0.009 | 0.008 |
| Eicosapentaenoic (trimnodonic)acid, 20:5ω3 | 0.553±0.104 | 0.161±0.046 | 5.049±1.545 | 0.002 | 0.008 |
| Docosapentaenoic (clupanodonic) acid, 22:5ω3 | 0.489±0.159 | 0.093±0.028 | 1.044±0.150 | 0.002 | 0.001 |
| Docosahexaenoic acid, 22:6ω3 | 5.911±0.730 | 3.430±0.339 | 19.284±3.101 | 0.002 | 0.008 |
| Octadecadienoic (linoleic) acid, 18:2ω6 | 20.754±2.426 | 10.432±0.755 | 8.028±1.569 | 0.003 | <0.001 |
| Octadecatrienoic (gamma-linolenic) acid, 18:3ω6 | 0.309±0.130 | 0.181±0.044 | 0.041±0.017 | 0.005 | 0.008 |
| Eicosatrienoic (dihomo-gamma-linolenic) acid, 20:3ω6 | 1.540±0.259 | 1.613±0.266 | 0.460±0.159 | 0.009 | <0.001 |
| Eicosatetraenoic (arachidonic) acid, 20:4ω6 | 14.313±2.248 | 10.902±2.288 | 7.477±0.436 | 0.005 | 0.008 |
| Eicosatrienoic (mead) acid, 20:3ω9 | 1.164±0.066 | 3.732±0.919 | 1.129±0.037 | 0.008 | 0.354 |
|
| |||||
| Totals | |||||
| Total ω3 | 8.318±1.214 | 3.782±0.314 | 25.459±4.486 | 0.002 | 0.008 |
| Total ω6 | 37.742±4.109 | 23.727±2.909 | 16.133±1.778 | 0.002 | <0.001 |
| Total ω9 | 14.916±2.028 | 23.274±0.938 | 14.534±1.103 | 0.009 | 0.724 |
| Total ω-7 | 2.234±0.819 | 3.333±0.531 | 1.884±0.417 | 0.032 | 0.433 |
| Total saturated FA | 33.385±1.780 | 38.715±2.519 | 37.722±2.306 | 0.012 | 0.013 |
| Total monounsaturated FA | 20.108±3.260 | 28.371±1.502 | 20.541±1.808 | 0.009 | 0.804 |
| Total polyunsaturated FA | 46.507±3.860 | 32.915±3.186 | 41.736±3.373 | 0.005 | 0.076 |
|
| |||||
| Ratios | |||||
| Triene:Tetraene ratio | 0.012±0.006 | 0.357±0.130 | 0.017±0.004 | 0.005 | 0.135 |
| ω6/ω3 ratio | 4.636±0.999 | 6.289±0.711 | 0.653±0.151 | 0.003 | 0.008 |
All values represent percent ± SD.
Table 3.
Tumor fatty acid profiles
| Fatty acid | SOY (N=4) | EFAD (N=5) | DHA (N=5) | PANOVA | PSOY vs DHA |
|---|---|---|---|---|---|
| Saturated Fats | |||||
| Tetradecanoic (myristic) acid, 14:0 | 2.391±0.729 | 3.557±0.582 | 2.858±0.470 | 0.040 | 0.330 |
| Hexadecanoic (palmitic) acid, 16:0 | 22.064±3.647 | 22.428±0.855 | 23.862±1.500 | 0.243 | 0.423 |
| Octadecanoic (stearic) acid, 18:0 | 13.860±1.522 | 12.979±0.780 | 13.772±0.818 | 0.325 | 0.922 |
|
| |||||
| Monounsaturated Fats | |||||
| Hexadecenoic (palmitoleic) acid, 16:1ω7 | 4.820±2.189 | 8.663±1.508 | 6.853±1.209 | 0.050 | 0.171 |
| Octadecenoic (vaccenic) acid, 18:1ω7 | 4.298±1.559 | 6.654±0.776 | 5.233±0.585 | 0.018 | 0.338 |
| Octadecenoic (oleic) acid, 18:1ω9 | 20.888±2.708 | 22.425±2.600 | 19.470±2.497 | 0.221 | 0.450 |
|
| |||||
| Polyunsaturated Fats | |||||
| Octadecatrienoic (alpha-linolenic) acid, 18:3ω3 | 0.468±0.376 | 0 | 0 | 0.002 | NA |
| Eicosapentaenoic (trimnodonic)acid, 20:5ω3 | 0.009±0.0123 | 0 | 1.305±0.316 | 0.004 | 0.020 |
| Docosapentaenoic (clupanodonic) acid, 22:5ω3 | 0.529±0.071 | 0.094±0.086 | 2.172±0.415 | 0.003 | 0.016 |
| Docosahexaenoic acid, 22:6ω3 | 4.894±1.037 | 3.455±0.666 | 14.646±2.350 | 0.003 | <0.001 |
| Octadecadienoic (linoleic) acid, 18:2ω6 | 13.850±3.206 | 5.289±0.750 | 4.177±0.693 | 0.008 | 0.0016 |
| Octadecatrienoic (gamma-linolenic) acid, 18:3ω6 | 0 | 0 | 0 | NA | NA |
| Eicosatrienoic (dihomo-gamma-linolenic) acid, 20:3ω6 | 0.351±0.237 | 1.583±2.276 | 0.126±0.082 | 0.022 | 0.166 |
| Eicosatetraenoic (arachidonic) acid, 20:4ω6 | 5.289±0.995 | 4.763±0.543 | 2.718±0.490 | 0.010 | 0.009 |
| Eicosatrienoic (mead) acid, 20:3ω9 | 0.020±0.039 | 1.880±0.602 | 0 | 0.004 | NA |
|
| |||||
| Totals | |||||
| Total ω3 | 6.008±1.105 | 3.548±0.688 | 18.124±3.050 | 0.003 | <0.001 |
| Total ω6 | 22.836±1.626 | 13.215±2.776 | 7.511±0.915 | 0.003 | <0.001 |
| Total ω9 | 22.378±2.939 | 26.250±2.195 | 20.603±2.553 | 0.026 | 0.377 |
| Total ω-7 | 4.554±1.557 | 7.325±0.974 | 5.557±0.613 | 0.020 | 0.311 |
| Total saturated FA | 38.984±4.270 | 40.175±1.854 | 41.325±2.007 | 0.566 | 0.369 |
| Total monounsaturated FA | 31.732±2.860 | 40.349±2.960 | 33.040±3.794 | 0.015 | 0.577 |
| Total polyunsaturated FA | 29.283±1.486 | 19.466±3.787 | 25.634±3.290 | 0.014 | 0.078 |
|
| |||||
| Ratios | |||||
| Triene:Tetraene ratio | 0.005±0.010 | 0.394±0.113 | 0 | 0.004 | NA |
| ratio ω6/ω3 ratio | 3.916±0.863 | 3.756±0.606 | 0.422±0.080 | 0.011 | 0.016 |
All values represent percent ± SD.
3.2 G-protein Coupled Receptor Agonist Treatment
The effect of two different G-protein coupled receptor agonists was tested on the growth of human melanoma cells in vitro. GW9508, the non-selective G-protein coupled receptor agonist that stimulates GPR120 and GPR40 (24), had a profound inhibitory effect on the growth of all three human melanoma cell lines but was not toxic to the growth of the control fibroblast cells (Figure 3A). The expression of GPR120 by human melanoma and control fibroblast cells was negligible (not shown). In contrast, the expression of GPR40 was significantly higher in all three human melanoma cell lines while being negligible in the control fibroblast cell line (Figure 3B). The expression of GPR40 in other malignancies was evaluated and was found to be overall lower in both neuroblastoma and breast cancer cells as compared to human melanoma. Given the high expression of GPR40 and the negligible expression of GPR120 by human melanoma cells we focused our attention on GPR 40 by using the selective GPR40 agonist, TAK-875. TAK-875 had a profound selective inhibitory effect on the growth of all three human melanoma cell lines while again not being toxic to the control fibroblast or human breast cancer cells (Figure 3C).
Figure 3. The effect of GPR agonists on the growth of human melanoma.
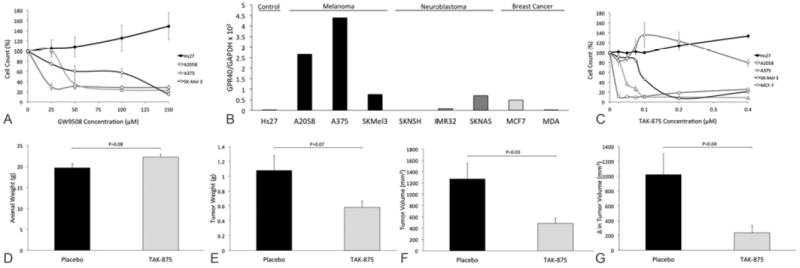
(A) The non-selective GPR120 and GPR40 agonist GW9508 has an inhibitory effect on the growth of all three different human melanoma cells lines (A2058, A375, SK-Mel 3) while there is no toxic effect on the growth of the control fibroblast cell line. (B) GPR40 expression determined by RT-qPCR is higher in human melanoma cells compared to the control fibroblast cell line and also compared to the human neuroblastoma and breast cancer cells tested. (C) The selective GPR40 agonist TAK-875 also has a selective inhibitory effect on the growth of all three human melanoma cell lines tested without negatively effecting the growth of the control fibroblast or human breast cancer cell lines even at very high concentrations. (D-G) TAK-875 administered at 100mg/kg via once daily orogastric gavage inhibits the growth of established human melanoma tumors in a murine subcutaneous xenograft model of human melanoma. Animal weights were not significantly different between placebo and treatment groups (N=7/group) (D) although tumors weights (E), tumors volumes (F) and change in tumor volume over the 14 day treatment period (G) were lower with TAK-875 treatment compared to placebo.
A murine subcutaneous xenograft model of human melanoma was used to test the effect of once daily oral TAK-875 on the growth of established melanoma tumors in vivo. Animal weights were not significantly different between the placebo and TAK-875 groups (N=7/group; Figure 3D), however, tumors were 46% smaller in weight (P=0.07, Figure 3E) and 62% smaller in volume (P=0.03, Figure 3F) in the TAK-875 group. Most importantly the change in tumor volume from start of drug treatment to completion of 14 days of treatment, was 77% lower in the TAK-875 group compared to placebo (P=0.04, Figure 3G).
4. DISCUSSION
Armed with the knowledge that the shift in human dietary habits over the last 100 years toward a very high omega-6 to omega-3 FA ratio is accompanied by a rising incidence of malignant melanoma, we sought to determine the impact of a diet rich in omega-3 FAs on the growth of human melanoma. We demonstrate that DHA has a profound inhibitory effect on the growth of human melanoma cells in vitro and for the first time demonstrate that the growth of human melanoma in vivo is also inhibited by a diet rich in DHA. Given recent data suggesting that omega-3 fatty acids may exert their effects through specific G-protein coupled receptors (19), we chose to study the potential role of G-protein coupled receptors in mediating the inhibitory effect of DHA on human melanoma. We found that a specific G-protein coupled receptor, GPR40, is expressed at high levels in several human melanoma cell lines while being expressed at very low to negligible levels in a control fibroblast cell line in addition to several human neuroblastoma and breast cancer cell lines. Moreover, we found that stimulating GPR40 has a profound inhibitory effect on the growth of human melanoma cells both in vitro and on established human melanoma tumors in vivo. Thus, we propose that the GPR40 as a new therapeutic target for the treatment of human melanoma.
The field of omega-3 FA research may be in its infancy, but it is becoming increasingly clear that these fats have certain beneficial effects on human health. It is unfortunate that parallel with our improved understanding of the beneficial health effects of omega-3 FAs there has been an increasing trend towards the consumption of a diet rich in omega-6 FAs and relatively deplete in omega-3 FAs (10). This change is particularly relevant to melanoma given that the shift in dietary habits over the last 100 years is accompanied by an upward trend in incidence of melanoma, with this rate now equating to the fastest rate of rise in the incidence of any cancer worldwide (2, 3).
The majority of the data associating omega-3 FAs with beneficial effects of malignancies relates to favorable in vitro and in vivo results that have been obtained for breast cancer (7-9), colon cancer (5,10), liver tumors (4) and leukemia (11). With regards to melanoma, there have been some studies primarily in vitro that have indicated that omega-3 FAs may inhibit the growth of this malignancy. Albino et al, demonstrated for the first time that long-chain omega-3 FAs inhibit the proliferation of a majority of cell lines derived from human metastatic melanoma and that this inhibition involves the pRb pathway (25). Given the mounting evidence at the time that COX-2 may play a role in the development and progression of malignant epithelial tumors (26-29), several researchers sought to further investigate the relationship between COX-2, omega-3 FAs and malignant melanoma. Ciu and Ooi demonstrated that NSAIDS and DHA have synergistic effects on the growth of the human melanoma cell line A-375 (30) and, Denkins et al demonstrated that incubation of human melanoma cells with omega-3 FAs downregulated COX-2 expression indicating that these FAs regulate COX-2-mediated invasion in brain metastatic melanoma (31). A few in vivo studies have also been performed, but only with murine melanoma cell lines, and these studies do suggest that dietary omega-3 FAs slow the growth and progression of metastatic murine melanoma (32-34). To the best of our knowledge this is the first study to evaluate the effect of dietary omega-3 FAs on the growth of a human melanoma cell line in vivo.
Although the data on the beneficial health effects of omega-3 FAs is mounting, our understanding of the mechanisms via which these molecules exert their effects remains relatively undeveloped. Various molecular mechanisms have been proposed with different levels of evidence, including alterations in AA oxidative metabolism and metabolic conversion of omega-3 FAs to bioreactive derivatives, modification of oxidative stress and changes in membrane fluidity and structure (35). The recent implication of GPR120 for the first time described a receptor via which these effects may be mediated (19). Herein, we have shown that GPR40 is expressed at a higher level by human melanoma cells compared to control fibroblast cells and other malignancies. Most powerful is the data demonstrating that just once daily treatment with the orally bioavailable GPR40 receptor specific agonist, TAK-875, significantly inhibits the growth of established human melanoma tumors in vivo.
The fact that TAK-875 has been shown to be safe and well tolerated at very high doses by rats and healthy human subjects without significant side-effects makes the potential of using this drug in the treatment of metastatic melanoma especially exciting. Previous reports demonstrate that this receptor is not expressed in the vast majority of normal tissues (36) thus, stimulating this receptor, would in theory, not have any detrimental effects on these normal tissues. In addition, two Phase II clinical trials for type II diabetes and demonstrate that the drug is well tolerated even at high doses with a very favorable safety profile (37-39). The dose used in the current study is the mouse equivalent of the dose previously used and reported to be safe in rat studies (40).
In summary, we demonstrate that DHA has a profound and selective inhibitory effect on the growth of human melanoma cells in vitro and for the first time use a murine model of human melanoma to demonstrate that dietary DHA inhibits the growth of human melanoma in vivo. In addition, we identify the GPR40 as being expressed at relatively high levels in human melanoma and demonstrate that stimulating this receptor with the selective agonist, TAK-875, inhibits the growth of human melanoma cells in vitro and also established tumors in vivo. Given the oral bioavailability and the already confirmed favorable safety profile of TAK-875, we identify GPR40 as a novel, safe and potentially effective therapeutic target for the treatment of human melanoma.
Acknowledgments
This research was supported by the Boston Children’s Hospital Surgical Foundation, the Boston Children’s Hospital Vascular Biology Program and by a generous donation by the Haberman family. Docosahexaenoic acid was provided as a gift by Martek. D.N. was supported by grant T32DK007754-13. E.M.F. was supported by the Joshua Ryan Rappaport Fellowship.
Abbreviations
- AA
arachidonic acid
- DHA
docosahexaenoic acid
- EFAD
essential fatty acid deficiency
- FA
fatty acids
- GPR40
G-protein coupled receptor 40
- GRP120
G-protein coupled receptor 120
- RT-qPCR
real time quantitative polymerase chain reaction
Footnotes
Author Contributions:
Deepika Nehra: conception and design, analysis and interpretation, data collection, writing the article, critical revision of the article and obtaining funding.
Amy H. Pan: conception and design, analysis and interpretation, data collection, critical revision of the article.
Hau D. Le: conception and design, analysis and interpretation, data collection, critical revision of the article.
Erica M. Fallon: conception and design, analysis and interpretation, data collection, critical revision of the article.
Sarah J. Carlson: conception and design, analysis and interpretation, data collection, critical revision of the article.
Brian T. Kalish: conception and design, analysis and interpretation, data collection, critical revision of the article.
Mark Puder: conception and design, analysis and interpretation, data collection, critical revision of the article and obtaining funding.
CONFLICT OF INTEREST DISCLOSURE:
A provisional patent application has been submitted on behalf of Dr. Deepika Nehra, Dr. Brian Kalish and Dr. Mark Puder on the use of omega-3 fatty acids and TAK-875 in the treatment of human melanoma.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–781. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Foundation AM. Skin Cancer Fact Sheet. 2009 [Google Scholar]
- 3.Lens MB, Dawes M. Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. Br J Dermatol. 2004;150:179–185. doi: 10.1111/j.1365-2133.2004.05708.x. [DOI] [PubMed] [Google Scholar]
- 4.Seetharamu N, Ott PA, Pavlick AC. Novel therapeutics for melanoma. Expert Rev Anticancer Ther. 2009;9:839–849. doi: 10.1586/era.09.40. [DOI] [PubMed] [Google Scholar]
- 5.Nicholas C, Lesinski GB. Immunomodulatory cytokines as therapeutic agents for melanoma. Immunotherapy. 2011;3:673–690. doi: 10.2217/imt.11.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eggermont AM. Advances in systemic treatment of melanoma. Ann Oncol. 2010;21(Suppl 7):vii339–344. doi: 10.1093/annonc/mdq364. [DOI] [PubMed] [Google Scholar]
- 7.Eaton SB, Konner M. Paleolithic nutrition. A consideration of its nature and current implications. N Engl J Med. 1985;312:283–289. doi: 10.1056/NEJM198501313120505. [DOI] [PubMed] [Google Scholar]
- 8.Simopoulos AP. Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr. 1991;54:438–463. doi: 10.1093/ajcn/54.3.438. [DOI] [PubMed] [Google Scholar]
- 9.Simopoulos AP. Importance of the ratio of omega-6/omega-3 essential fatty acids: evolutionary aspects. World Rev Nutr Diet. 2003;92:1–22. doi: 10.1159/000073788. [DOI] [PubMed] [Google Scholar]
- 10.Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother. 2006;60:502–507. doi: 10.1016/j.biopha.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 11.Simopoulos AP. Evolutionary aspects of the dietary omega-6:omega-3 fatty acid ratio: medical implications. World Rev Nutr Diet. 2009;100:1–21. doi: 10.1159/000235706. [DOI] [PubMed] [Google Scholar]
- 12.Simopoulos AP. Importance of the omega-6/omega-3 balance in health and disease: evolutionary aspects of diet. World Rev Nutr Diet. 2011;102:10–21. doi: 10.1159/000327785. [DOI] [PubMed] [Google Scholar]
- 13.Litin L, Sacks F. Trans-fatty-acid content of common foods. N Engl J Med. 1993;329:1969–1970. doi: 10.1056/NEJM199312233292621. [DOI] [PubMed] [Google Scholar]
- 14.Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21:495–505. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- 15.Wagner RF, Jr, DiSorbo DM, Nathanson L. Nutrition and melanoma. Int J Dermatol. 1984;23:453–457. doi: 10.1111/ijd.1984.23.7.453. [DOI] [PubMed] [Google Scholar]
- 16.Mackie BS, Mackie LE, Curtin LD, Bourne DJ. Melanoma and dietary lipids. Nutr Cancer. 1987;9:219–226. doi: 10.1080/01635588709513930. [DOI] [PubMed] [Google Scholar]
- 17.Kirkpatrick CS, White E, Lee JA. Case-control study of malignant melanoma in Washington State. II. Diet, alcohol, and obesity. Am J Epidemiol. 1994;139:869–880. doi: 10.1093/oxfordjournals.aje.a117093. [DOI] [PubMed] [Google Scholar]
- 18.Veierod MB, Thelle DS, Laake P. Diet and risk of cutaneous malignant melanoma: a prospective study of 50,757 Norwegian men and women. Int J Cancer. 1997;71:600–604. doi: 10.1002/(sici)1097-0215(19970516)71:4<600::aid-ijc15>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 19.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 21.Le HD, Meisel JA, de Meijer VE, Fallon EM, Gura KM, Nose V, Bistrian BR, Puder M. Docosahexaenoic Acid and Arachidonic Acid Prevent Essential Fatty Acid Deficiency and Hepatic Steatosis. JPEN J Parenter Enteral Nutr. 2011 doi: 10.1177/0148607111414580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le HD, Meisel JA, de Meijer VE, Fallon EM, Gura KM, Nose V, Bistrian BR, Puder M. Docosahexaenoic acid and arachidonic acid prevent essential fatty acid deficiency and hepatic steatosis. JPEN J Parenter Enteral Nutr. 2012;36:431–441. doi: 10.1177/0148607111414580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ambring A, Johansson M, Axelsen M, Gan L, Strandvik B, Friberg P. Mediterranean-inspired diet lowers the ratio of serum phospholipid n-6 to n-3 fatty acids, the number of leukocytes and platelets, and vascular endothelial growth factor in healthy subjects. Am J Clin Nutr. 2006;83:575–581. doi: 10.1093/ajcn.83.3.575. [DOI] [PubMed] [Google Scholar]
- 24.Briscoe CP, Peat AJ, McKeown SC, Corbett DF, Goetz AS, Littleton TR, McCoy DC, Kenakin TP, Andrews JL, Ammala C, Fornwald JA, Ignar DM, Jenkinson S. Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40: identification of agonist and antagonist small molecules. Br J Pharmacol. 2006;148:619–628. doi: 10.1038/sj.bjp.0706770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albino AP, Juan G, Traganos F, Reinhart L, Connolly J, Rose DP, Darzynkiewicz Z. Cell cycle arrest and apoptosis of melanoma cells by docosahexaenoic acid: association with decreased pRb phosphorylation. Cancer Res. 2000;60:4139–4145. [PubMed] [Google Scholar]
- 26.Friedman ES, LaNatra N, Stiller MJ. NSAIDs in dermatologic therapy: review and preview. J Cutan Med Surg. 2002;6:449–459. doi: 10.1007/s10227-001-0137-3. [DOI] [PubMed] [Google Scholar]
- 27.Denkert C, Kobel M, Berger S, Siegert A, Leclere A, Trefzer U, Hauptmann S. Expression of cyclooxygenase 2 in human malignant melanoma. Cancer Res. 2001;61:303–308. [PubMed] [Google Scholar]
- 28.Pentland AP, Schoggins JW, Scott GA, Khan KN, Han R. Reduction of UV-induced skin tumors in hairless mice by selective COX-2 inhibition. Carcinogenesis. 1999;20:1939–1944. doi: 10.1093/carcin/20.10.1939. [DOI] [PubMed] [Google Scholar]
- 29.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T, Su LK, Levin B, Godio L, Patterson S, Rodriguez-Bigas MA, Jester SL, King KL, Schumacher M, Abbruzzese J, DuBois RN, Hittelman WN, Zimmerman S, Sherman JW, Kelloff G. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 30.Chiu LC, Tong KF, Ooi VE. Cytostatic and cytotoxic effects of cyclooxygenase inhibitors and their synergy with docosahexaenoic acid on the growth of human skin melanoma A-375 cells. Biomed Pharmacother. 2005;59(Suppl 2):S293–297. doi: 10.1016/s0753-3322(05)80049-6. [DOI] [PubMed] [Google Scholar]
- 31.Denkins Y, Kempf D, Ferniz M, Nileshwar S, Marchetti D. Role of omega-3 polyunsaturated fatty acids on cyclooxygenase-2 metabolism in brain-metastatic melanoma. J Lipid Res. 2005;46:1278–1284. doi: 10.1194/jlr.M400474-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Salem ML, Kishihara K, Abe K, Matsuzaki G, Nomoto K. N-3 polyunsaturated fatty acids accentuate B16 melanoma growth and metastasis through suppression of tumoricidal function of T cells and macrophages. Anticancer Res. 2000;20:3195–3203. [PubMed] [Google Scholar]
- 33.Xia S, Lu Y, Wang J, He C, Hong S, Serhan CN, Kang JX. Melanoma growth is reduced in fat-1 transgenic mice: impact of omega-6/omega-3 essential fatty acids. Proc Natl Acad Sci U S A. 2006;103:12499–12504. doi: 10.1073/pnas.0605394103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mannini A, Kerstin N, Calorini L, Mugnai G, Ruggieri S. An enhanced apoptosis and a reduced angiogenesis are associated with the inhibition of lung colonisation in animals fed an n-3 polyunsaturated fatty acid-rich diet injected with a highly metastatic murine melanoma line. Br J Nutr. 2009;101:688–693. doi: 10.1017/S0007114508043791. [DOI] [PubMed] [Google Scholar]
- 35.Calviello G, Serini S, Piccioni E, Pessina G. Antineoplastic effects of n-3 polyunsaturated fatty acids in combination with drugs and radiotherapy: preventive and therapeutic strategies. Nutr Cancer. 2009;61:287–301. doi: 10.1080/01635580802582777. [DOI] [PubMed] [Google Scholar]
- 36.Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422:173–176. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- 37.Kaku K, Araki T, Yoshinaka R. Randomized, double-blind, dose-ranging study of TAK-875, a novel GPR40 agonist, in Japanese patients with inadequately controlled type 2 diabetes. Diabetes Care. 2013;36:245–250. doi: 10.2337/dc12-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leifke E, Naik H, Wu J, Viswanathan P, Demanno D, Kipnes M, Vakilynejad M. A multiple-ascending-dose study to evaluate safety, pharmacokinetics, and pharmacodynamics of a novel GPR40 agonist, TAK-875, in subjects with type 2 diabetes. Clin Pharmacol Ther. 2012;92:29–39. doi: 10.1038/clpt.2012.43. [DOI] [PubMed] [Google Scholar]
- 39.Burant CF, Viswanathan P, Marcinak J, Cao C, Vakilynejad M, Xie B, Leifke E. TAK-875 versus placebo or glimepiride in type 2 diabetes mellitus: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2012;379:1403–1411. doi: 10.1016/S0140-6736(11)61879-5. [DOI] [PubMed] [Google Scholar]
- 40.Tsujihata Y, Ito R, Suzuki M, Harada A, Negoro N, Yasuma T, Momose Y, Takeuchi K. TAK-875, an orally available G protein-coupled receptor 40/free fatty acid receptor 1 agonist, enhances glucose-dependent insulin secretion and improves both postprandial and fasting hyperglycemia in type 2 diabetic rats. J Pharmacol Exp Ther. 2011;339:228–237. doi: 10.1124/jpet.111.183772. [DOI] [PubMed] [Google Scholar]


