Abstract
Rationale
Our previous studies in rats have shown that the adipocyte-derived hormone leptin induces antidepressant-like effects with a behavioral profile similar to selective serotonin reuptake inhibitor (SSRI) antidepressants. Acute SSRI treatment causes paradoxical anxiogenic responses, although chronic treatment has therapeutic effects on anxiety. However, the role of leptin in anxiety remains to be established.
Objectives
The scope of this study was to investigate the acute effects of leptin on anxiety-related behaviors in comparison with the SSRI antidepressant fluoxetine.
Materials and methods
Adult male C57BL/6J mice received intraperitoneal injection of leptin or fluoxetine. Thirty minutes after injection, mice were subjected to the tail suspension test (TST) and forced swim test (FST) for evaluating antidepressant activity. Anxiety-like behavior was assessed in the elevated plus maze (EPM), social interaction, and open field tests 30 min following drug treatment.
Results
While leptin and fluoxetine showed similar antidepressant-like behavioral effects in the TST and FST, they differed in the behavioral assays for anxiety. Open arm exploration in the EPM was increased by leptin but decreased by fluoxetine. Analysis of social interaction revealed that distinct social behavioral components were modulated by leptin and fluoxetine. The total time of active social behaviors was increased by leptin but reduced by fluoxetine. In addition, self-grooming, a non-social behavior, was suppressed by leptin treatment. Neither leptin nor fluoxetine produced significant effects in the open field test.
Conclusions
In contrast to anxiogenic-like effects induced by acute fluoxetine, leptin elicits anxiolytic-like effects after acute administration. These results suggest that leptin has both antidepressant-like and anxiolytic-like properties.
Keywords: Leptin, Fluoxetine, Anxiety, Depression, Tail suspension, Forced swim, Elevated plus maze, Social interaction
Introduction
Leptin, a 16-kDa protein, is encoded by the obese (ob) gene and secreted by adipocytes (Zhang et al. 1994). It can be transported across the blood–brain barrier to enter the brain, where it binds to its receptors to influence a wide spectrum of functions. It is well documented that leptin acts as a negative feedback signal in the regulation of food intake and body weight gain via interaction with receptors localized in the hypothalamus. However, leptin receptors are also distributed in other brain areas, including several limbic structures implicated in the control of mood and emotion (Leshan et al. 2006; Scott et al. 2009). Our previous pharmacological studies have shown that leptin has antidepressant-like properties (Lu et al. 2006). Circulating leptin levels are low in rat models of depression (Lu et al. 2006), and some patients with major depression also have low levels of leptin in serum or cerebrospinal fluid (Atmaca et al. 2002; Jow et al. 2006; Kraus et al. 2001; Westling et al. 2004). Depression is often comorbid with anxiety. However, whether leptin plays a role in the modulation of anxiety behaviors remains to be characterized.
Current antidepressants, especially selective serotonin reuptake inhibitors (SSRIs) are clinically effective for the treatment of anxiety disorders after chronic administration. However, these SSRIs can worsen anxiety in the initial phase of the treatment (Den Boer and Westenberg 1990; den Boer et al. 1987; Gorman et al. 1987; Grillon et al. 2007; Jick et al. 2004). The anxiogenic effect of acute SSRIs, especially fluoxetine, has been demonstrated in animal models (Belzung et al. 2001; Burghardt et al. 2004; Drapier et al. 2007; File et al. 1999; Griebel et al. 1994; Kurt et al. 2000). Leptin and fluoxetine exhibit similar antidepressant-like behavioral profiles in the rat forced swim test (Lu et al. 2006; Lucki 1997; Page et al. 1999). Evidence has suggested that leptin may functionally interact with the serotonergic system (Calapai et al. 1999; Charnay et al. 2000; Collin et al. 2000; Finn et al. 2001; Hay-Schmidt et al. 2001). The aim of the present study was to characterize the acute pharmacological effects of leptin on anxiety-like behaviors in comparison with the SSRI fluoxetine. We showed the antidepressant-like effects of leptin and fluoxetine in two mouse behavioral tests for screening antidepressants, i.e., tail suspension and forced swim tests. The effects of leptin and fluoxetine on anxiety-like behavior were evaluated using the elevated plus maze, social interaction, and open field tests. We found that acute administration of leptin produces anxiolytic effects, while acute fluoxetine elicits anxiogenic effects in the same anxiety tests.
Materials and methods
Animals
Adult male C57BL/6J mice (8 weeks old on arrival, The Jackson Laboratory) were housed in groups of five under a 12-h light–dark cycle (lights on at 0700 hours) with ad libitum access to food and water except during behavioral tests. Animals were allowed to acclimate for at least 1 week before beginning the experiments. All animal procedures were conducted in accordance with NIH guidelines and approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio.
Drugs
Recombinant rat leptin (R&D systems, Inc., Minneapolis, MN) and fluoxetine hydrochloride (Sigma-Aldrich, St. Louis, MO) were dissolved in saline before use. For all behavioral experiments, 0, 0.25, and 1.0 mg/kg leptin and 10 mg/kg fluoxetine were given intraperitoneally (i.p.) with an injection volume of 10 ml/kg. Saline was given i.p. as a vehicle control. The dose for fluoxetine was selected based upon previous reports showing that fluoxetine at this dose is effective in reducing depressive (Dhir and Kulkarni 2007; Perrault et al. 1992; Ukai et al. 1998) and enhancing anxiety-related behaviors after acute administration (Griebel et al. 1995; Holmes and Rodgers 2003; Kurt et al. 2000).
Behavioral testing
All behavioral tests were performed during the late light phase between 1400 and 1700 hours. Mice were handled and sham-injected for 2 days before drug administration and behavioral testing. On the test day, animals were weighed, counterbalanced into different groups, and singly housed in a new cage with some home cage bedding to avoid the stressful effect of sequential removal of the mice from the cage on remaining mice (Kask et al. 2001). Animals were transferred to the testing room and habituated for 3–4 h prior to the beginning of the experiments. The animals were used only once for behavioral testing. Each behavioral test was performed multiple times using different animals except for the locomotor activity measurement. The group size was derived from the sum of animal number for each treatment in individual behavioral test.
Tail suspension test
Tail suspension test (TST) is widely used for screening antidepressant properties of drugs (Steru et al. 1985). The apparatus was constructed of a wooden box (30×30×30 cm) with an open front. A horizontal bar was placed 1 cm from the top and a vertical 9 cm bar hanging down in the center. Fifty-six animals were weighed and counter-balanced into four different treatment groups that received i.p. injection of drugs or vehicle: saline (n=18), 0.25 mg/kg leptin (n=9), 1 mg/kg leptin (n=15), or 10 mg/kg fluoxetine (n=14). Thirty minutes after receiving i.p. injection, mice were individually suspended by the tail to the vertical bar with adhesive tape affixed 2 cm from the tip of the tail. A charge-coupled device (CCD) camera was positioned in front of the TST box. The animal’s behavior was recorded for 6 min. The immobility and escape-oriented behaviors were subsequently scored by a trained observer who was blind to the treatments. The apparatus was cleaned with 20% alcohol between each animal. Immobility is defined as the absence of any limb or body movements, except those caused by respiration.
Forced swim test
This test was performed using the original method described by Porsolt (Porsolt et al. 1977). Thirty-eight mice were weighed and counter-balanced into four different treatment groups that received i.p. injection of drugs or vehicle: saline (n=11), 0.25 mg/kg leptin (n=9), 1 mg/kg leptin (n=9), or 10 mg/kg fluoxetine (n=9). Thirty minutes after i.p. injection, mice were placed in a clear Plexiglas cylinder (25 cm high; 10 cm in diameter) filled to a depth of 15 cm with 24°C water. The tank was cleaned, and fresh water was used for each animal. A CCD camera positioned directly above the cylinder recorded the swim session. In a 6-min test session, the first 2 min were designated as a habituation period, and the duration of immobility was measured during last 4 min using Noldus EthoVision 3.0 system (Noldus Information Technology Inc., Leesburg, VA). The software acquired all pixel coordinates of the tracking object in 200-ms intervals and determined the changed pixels of this object between current sample and previous sample (referred to as changed area). Mobility was calculated using the following formula: Mobility = (CAn/(An−1 + An) × 100 (CAn=changed area for current sample; An=area for current sample; An−1=area for previous sample). If the mobility value was less than 20%, the animal was considered immobile. This threshold was chosen as it produced similar immobility scores as using manual scoring method.
Elevated plus maze
Forty-eight mice were weighed and counter-balanced into four treatment groups that received i. p. injection of either drug or vehicle: saline (n=12), 0.25 mg/kg leptin (n = 10), 1 mg/kg leptin (n = 16), or 10 mg/kg fluoxetine (n=10). Mice were tested in an elevated plus maze test 30 min after i.p. injection. The elevated plus maze was made of white acrylic, with four arms (30-cm long and 5-cm wide) arranged in the shape of a “plus” sign and elevated to a height of 70 cm from the floor. Two arms have no side or end walls (open arms). The other two arms have side and end walls (12-cm high) but are open on top (closed arms). The open and closed arms intersect, having a central 5×5 cm square platform giving access to all arms. The mice were placed in the central square facing the corner between a closed arm and an open arm and allowed to explore the elevated plus maze for 5 min. Their activity on the elevated plus maze was recorded. After each test, the maze was thoroughly cleaned with 20% alcohol to eliminate the odor and trace of the previously tested animal. The time spent on the open and closed arms and the numbers of entries made into each arm were measured. Entry was defined as all four paws being positioned within one arm. The degree of anxiety was assessed by calculating the percentage of open arm entries (entries into the open arms/total entries into all arms) and percentage of open arm time (time spent in the open arms/ total time spent in all arms).
Social interaction
Social interaction is another measure of anxiety (File and Seth 2003). The test apparatus consisted of a white box (40×40×40 cm) with an open top. The illumination in the test arena was adjusted to 250 lx. Animals were not habituated to the test box prior to testing. Seventy mice (35 pairs) were assigned into four treatment groups receiving i.p. injection: saline (n=9 pairs), 0.25 mg/kg leptin (n=8 pairs), 1 mg/kg leptin (n=10 pairs), or 10 mg/kg fluoxetine (n=8 pairs). Thirty minutes after i.p. injection, mice were tested for social interaction with an unknown test partner from different cages but with the same treatment and approximately the same body weight (<0.6±0.1 g difference in body weight). Two mice were placed simultaneously in the opposite corners of arena. Their social activity was recorded for 10 min using a CCD camera that was mounted directly over the test arena. The apparatus was thoroughly cleaned and dried after each test session with 20% alcohol. The behaviors of animals were scored by two trained observers, who were blind to the experimental conditions. The active social behaviors were scored, including nosing, following, and non-aggressive physical contacts (body sniffing, anogenital sniffing, and body crossing; Murcia et al. 2005; Stemmelin et al. 2008; To et al. 1999; Yamada et al. 2000). In addition, self-grooming and locomotor activity in the test area were quantified. Self-grooming was characterized by forepaw licking, face washing, scratching, and sniffing themselves. Locomotor activity was assessed by placing a grid (4×4) over the test arena on the computer screen and counting the number of squares crossing.
Open-field exploration
The apparatus was made of wood and consisted of a 60×60 cm open arena with 40-cm-high walls. The open field arena was divided into nine equal squares. The center square was defined as the central zone, in which animal’s activity is usually regarded as a measure of anxiety (Simon et al. 1994; Treit and Fundytus 1988). The four corners of the test arena were adjusted to even illumination. Thirty-one mice were assigned into four treatment groups: saline (n=9), 0.25 mg/kg leptin (n=6), 1 mg/kg leptin (n=9), or 10 mg/kg fluoxetine (n=7). Thirty minutes after i.p. injection, mice were placed in the center of the arena. Their activity in the arena was recorded for 5 min using a CCD camera. The open field apparatus was cleaned after each testing session to prevent subsequent mice from being influenced by odors deposited by previous animals. Activities in the central zone including the number of entries, the distance traveled and total time spent in the central zone were measured using the Noldus EthoVision 3.0 system. The percent distance mice traveled in the central zone over total distance traveled in the open arena was also quantified. The overall motor activity during the open field test was assessed as the total distance traveled (horizontal movement) and the number of rearing events (vertical movement).
Locomotor activity
Twenty-four mice were studied in this experiment: saline (n=6), 0.25 mg/kg leptin (n=6), 1 mg/kg leptin (n=6), or 10 mg/kg fluoxetine (n=6). After i.p. injection, mice were immediately placed in an open field arena (40×40×40 cm) and allowed to freely explore for total 60 min. The apparatus was cleaned after each test session. The total distance traveled in a 5-min interval was measured using a Noldus EthoVision 3.0 system.
Statistical analysis
Results are expressed as mean±standard error of the mean (SEM). One-way analysis of variance (ANOVA) was used for the analysis of behavioral data collected from the depression and anxiety behavioral tests. One-way ANOVA with repeated measures was used to analyze the time course of locomotor activity. Post hoc comparisons were performed using Bonferroni/Dunn. P<0.05 was considered statistically significant.
Results
Effect of leptin in comparison with fluoxetine on depression-like behaviors
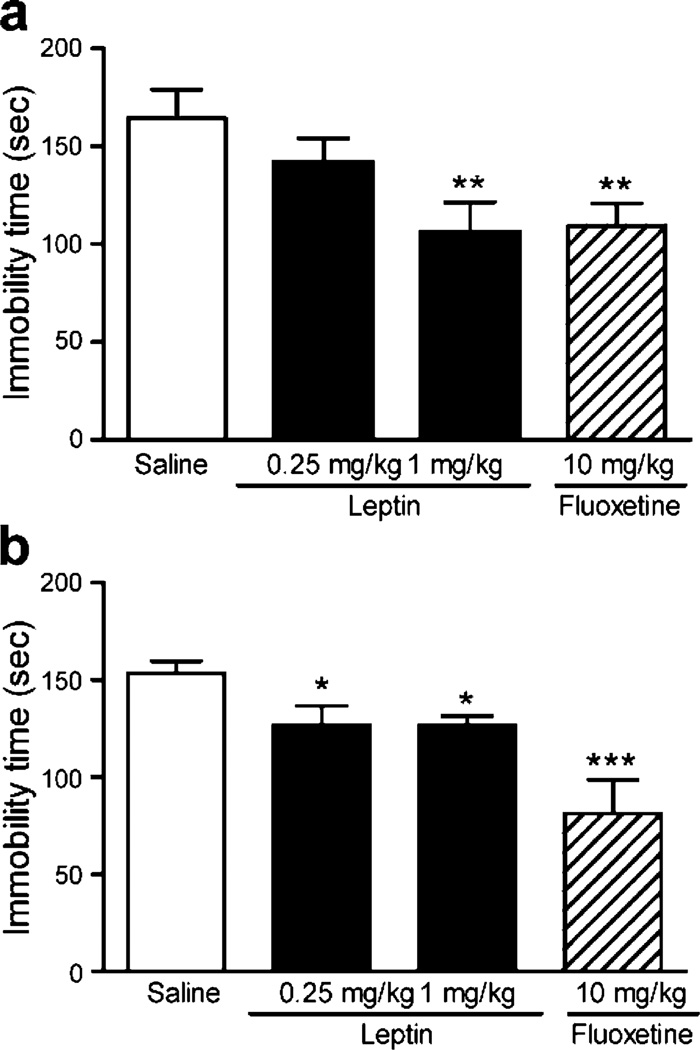
Antidepressant effects of leptin as well as fluoxetine were assessed in mice with the TST and forced swim test (FST). In both tests, animals are subjected to an inescapable stressful situation and develop a characteristic immobile posture. The duration of immobility in TST and FST has been inferred as an index of “behavioral despair” (Porsolt et al. 1977; Steru et al. 1985). In mice, it has been shown that a single injection of antidepressant drugs is capable of acutely reducing immobility time (Cesana et al. 1993; Cryan et al. 2005). Thus, the effects of leptin and fluoxetine on immobility were examined 30 min after a single i.p. injection. ANOVA analysis indicated that leptin had a significant effect on immobility time in the TST [F(2,39)= 4.239, P<0.05]. Post hoc comparisons revealed that leptin at 1 mg/kg significantly decreased the immobility time by 35% compared to vehicle-treated controls (P<0.01; Fig. 1a). Leptin at a lower dose of 0.25 mg/kg failed to show a significant effect. Immobility time was also significantly reduced by fluoxetine (10 mg/kg) compared to the vehicle treatment (P<0.01; Fig. 1a). This is consistent with previous findings on fluoxetine at this dose in the same test (Jain et al. 2003; Perrault et al. 1992; Ukai et al. 1998). Such antidepressant behavioral effects of leptin and fluoxetine were confirmed in the FST. Acute administration of leptin also manifested a shorter time of immobility in FST [F(2,26)=4.815, P<0.05]. Post hoc analysis indicated significant decreases in immobility in response to leptin at doses of 0.25 mg/kg (P<0.05) and 1.0 mg/kg leptin (P<0.05; Fig. 1b). The immobility time was also significantly decreased by fluoxetine as compared to the vehicle treatment (P<0.001; Fig. 1b). These results confirmed the antidepressant properties of leptin and fluoxetine.
Fig. 1.
Antidepressant-like effect of acute administration of leptin and fluoxetine. a The tail suspension test (TST) was performed 30 min after i.p. injections of saline (n=18), 0.25 mg/kg leptin (n=9), 1 mg/kg leptin (n=15), or 10 mg/kg fluoxetine (n=14). The animal’s behavior was recorded for 6 min, and immobility time was assessed for the entire period of 6 min. b The forced swim test (FST) was performed 30 min after i.p. administration with saline (n=11), 0.25 mg/kg leptin (n=9), 1 mg/kg leptin (n=9), or 10 mg/kg fluoxetine (n=9). The animal’s behavior was recorded for 6 min, and immobility time was scored during the last 4 min. Data are expressed as the mean±SEM. *P<0.05, **P<0.01, and ***P<0.001 compared with the vehicle-treated control group
Effect of leptin in comparison with fluoxetine on anxiety-related behaviors
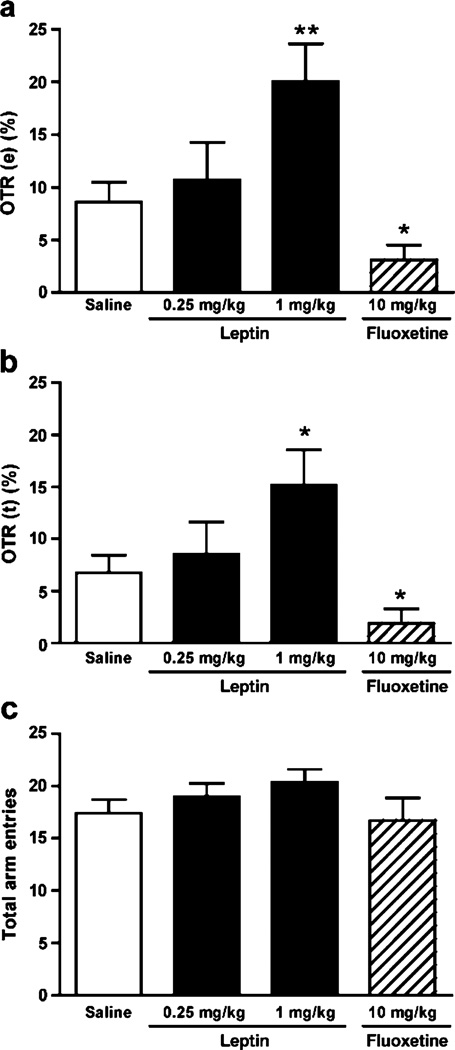
We performed three tests to assess the effect of leptin on anxiety-related behaviors in comparison with that of fluoxetine. First, we examined the effects of leptin and fluoxetine on elevated plus maze behaviors. The elevated plus maze is a widely used anxiety paradigm, which is based upon the animal’s conflict between an innate fear of exposed spaces and a tendency to explore new environments. This test has high predictive validity for expressing anxious behavior, and anxiolytic drugs such as diazepam and alprazolam consistently exhibit positive effects when administered shortly before testing (Griebel et al. 1996). The percentage of open arm entries and time spent in the open arms has been validated as a measure of anxiety (Rodgers and Dalvi 1997). Thirty minutes after administration of leptin, fluoxetine, or vehicle, mice were allowed to explore on the elevated plus maze for 5 min. We found that leptin evoked a significant effect on the percentage of open arm entries [F(2, 35)=4.792, P<0.05] and the percentage of time spent in the open arms [F(2, 35)=3.194, P<0.05]. Post hoc comparisons revealed that leptin at the dose of 1 mg/kg but not at the lower dose, 0.25 mg/kg, significantly increased the percentage of time spent in the open arms (P<0.05) and the percentage of entries into the open arms (P<0.01; Fig. 2a, b), suggesting a dose-dependent effect. In contrast, acute injection of fluoxetine at the dose eliciting antidepressant effects (10 mg/kg) decreased the percentage of open arm entries (P<0.05) and time spent in the open arm (P<0.05; Fig. 2a, b). This finding is in agreement with previous reports on the effect of fluoxetine on the elevated plus maze behaviors (Kurt et al. 2000; Silva and Brandao 2000). Neither leptin nor fluoxetine significantly changed the number of total arm entries [ANOVA, F(3,44)=2.161, P=0.1061] (Fig. 2c), indicating no effect on locomotor activity. Together, these data suggest that leptin is anxiolytic and fluoxetine is anxiogenic in the elevated plus maze test.
Fig. 2.
Effect of acute administration of leptin and fluoxetine on the elevated plus maze behavior. Mice were injected i.p. with saline (n= 12), 0.25 mg/kg leptin (n=10), 1 mg/kg leptin (n=16), or 10 mg/kg fluoxetine (n=10) 30 min before the 5 min elevated plus maze test. The percentage of entries made into the open arms/total entries made into all arms [OTR(e)] (a) and the percentage of time spent in the open arms/total time spent [OTR(t)] in all arms (b), as well as the number of total arm entries (c) were calculated. Data are expressed as mean± SEM. *P<0.05 and **P<0.01 compared with the vehicle-treated control group
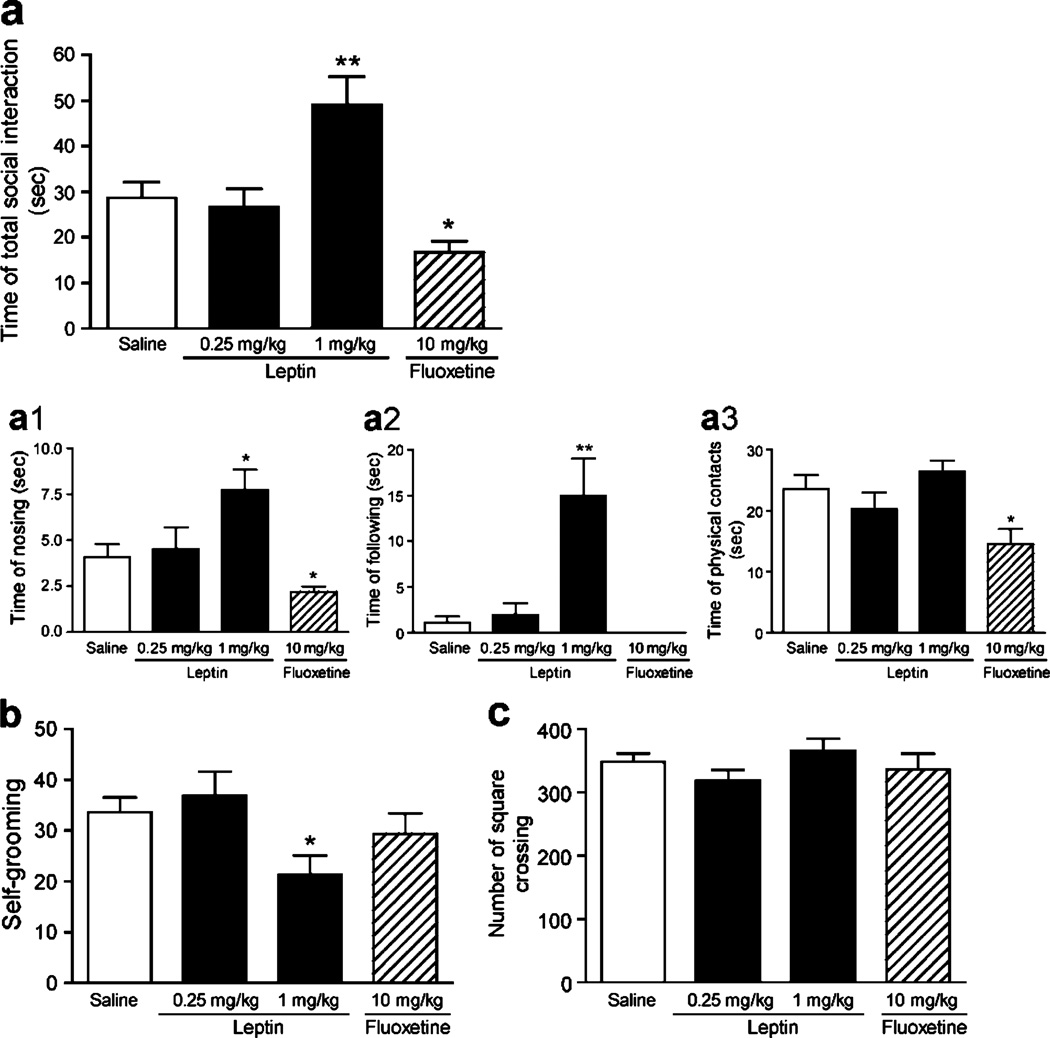
The effects of leptin and fluoxetine on anxiety levels were also examined in the social interaction test. In this test, two mice from the same treatment group that were unfamiliar to each other were simultaneously placed in a novel test arena. Following acute administration of saline, leptin, or fluoxetine, active social behavior was scored. ANOVA analyses revealed a significant effect of leptin treatment on the total social interaction time [F(2, 24)= 6.341.042, P<0.01]. Leptin at the dose of 1 mg/kg significantly increased the total social interaction time as compared to vehicle-treated controls (P<0.01; Fig. 3a). In contrast, fluoxetine (10 mg/kg) decreased total social interaction time relative to vehicle-treated controls (P<0.05; Fig. 3a). These data confirmed the anxiolytic and anxiogenic effects of leptin and fluoxetine, respectively. Further dissection of active social behavior into nosing, following and non-aggressive physical contacts (body sniffing, anogenital sniffing, and body crossing), demonstrated the significant effects of drug treatment on these social behaviors [F(3, 31)=6.476, P<0.01 for nosing; F(3, 31)=8.732, P<0.001 for following; F(3, 31)=4.445, P<0.01 for non-aggressive physical contacts]. Leptin at 1 mg/kg significantly increased nosing (P<0.05) and following (P<0.01) social behaviors, and fluoxetine decreased nosing (P<0.05) and non-aggressive physical contacts (P<0.05) (Fig. 3a1–3). The fluoxetine-treated mice showed no following behavior. These results indicate that leptin and fluoxetine affect distinct social behavioral components. Aggressive behaviors such as biting attacks, mounting, and wrestling were rarely observed in all pairs regardless of their treatment. In addition, leptin at the dose of 1 mg/kg significantly decreased the time spent self-grooming, a response to novelty stress (P<0.05), whereas fluoxetine treatment did not show any effect on self-grooming behavior (P=0.313; Fig. 3b). As a control for nonspecific changes in locomotor activity, square crossing was also measured. No differences in locomotion were observed after either leptin or fluoxetine treatment [F(3,31)= 0.999, P=0.4061] (Fig. 3c). Together, these results indicate that leptin promotes active social interaction and suppresses stress responses, whereas fluoxetine inhibits active social behaviors
Fig. 3.
Effect of leptin and fluoxetine on social interaction. Mice were i.p. injected with saline (n=9 pairs), 0.25 mg/kg leptin (n=8 pairs), 1 mg/kg leptin (n=10 pairs), or 10 mg/kg fluoxetine (n=8 pairs) 30 min before the social interaction test. a The total time spent in active social interaction: a1 nosing, a2 following. Fluoxetine-treated mice showed no following behavior. a3 Non-aggressive physical contacts. b Self-grooming. c The locomotor activity, which was assessed by quantifying the number of square crossing on the base of the arena. Data are expressed as mean±SEM. *P<0.05, **P< 0.01 compared with the vehicle-treated control group
Additionally, we performed the open field test, which is a standard neophobic test of anxiety based upon the same conflict situation as in the elevated plus maze test. In this test, mice naturally tend to avoid open spaces. Thus, the time spent in the central zone of the open field arena is a measure of anxiety state (Prut and Belzung 2003). Mice were tested for 5 min in the open field after receiving i.p. injection of saline, leptin, or fluoxetine. Neither leptin nor fluoxetine showed significant effects on time spent in the central zone [F(3, 27)=0.663, P=0.582], numbers of entries into the central zone [F(3, 27)=0.514, P=0.676], and locomotor activity in the central zone [F(3, 27)=0.710, P=0.554] (Table 1). Furthermore, the percentage of distance traveled in the central zone (calculated as distance traveled/ total distance traveled) was not affected by either compound [F(3, 27)=0.955, P=0.428] (Table 1). The horizontal locomotor activity (total distance traveled) and vertical activity (rearing) during the open field test was quantified and showed no difference between treatment groups [F(3, 27)=0.147, P=0.931 for total distance traveled; F(3, 27)= 1.046, P=0.388 for rearing]. These results suggest that this test under the conditions set for this study is not sensitive to either leptin or fluoxetine.
Table 1.
Effect of acute administration of leptin and fluoxetine on the open-field activity
| Saline (n= 9) |
leptin |
Fluoxetine (10mg/kg) (n= 7) |
|||
|---|---|---|---|---|---|
| 0.25mg/kg (n= 6) |
1.0mg/kg (n= 9) |
||||
| Central zone Activity |
Time spent in central zone (s) | 30.1 ±3.15 | 25.3±2.74 | 31.8±4.07 | 30.1 ±3.15 |
| Number of entries into central zone | 9.2±1.25 | 11.7± 1.76 | 10.7±1.26 | 11.1 ± 1.97 | |
| Locomotion (cm) | 215±29.3 | 184±40.8 | 254±31.0 | 215±41.8 | |
| Central zone/total distance traveled (%)a |
6.2±1.09 | 5.0±0.96 | 7.1±0.76 | 5.6±0.74 | |
| Total distance traveled (cm) | 3,589±201 | 3,523±199 | 3,646±228 | 3,761±352 | |
| Number of rearing | 25.1±2.53 | 25.3±3.19 | 22.8±1.98 | 18.7±4.07 | |
Mice were i.p. injected with saline, 0.25 mg/kg leptin, 1.0 mg/kg leptin, or 10 mg/kg fluoxetine 30 min before the 5-min open field test. Data are expressed as means±SEM
Percentage of distance traveled in the central zone/total distance traveled in the whole arena
Effect of leptin in comparison with fluoxetine on locomotor activity
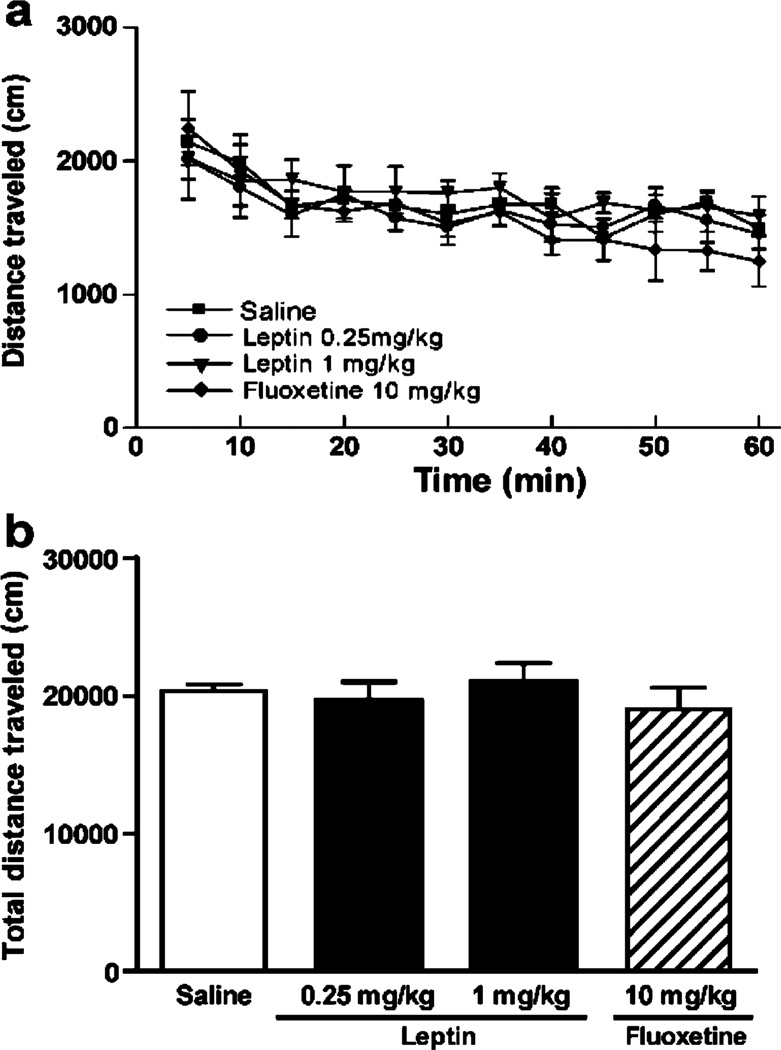
Locomotor activity was measured to ensure that the effects of leptin and fluoxetine in the anxiety and depression behavioral tests were not due to nonspecific changes in locomotor activity. Sixty-minute locomotor activity was analyzed in 5-min intervals. The locomotor activity for 60 min was analyzed and pooled over 5-min bins. The locomotor activity decreased significantly over time in all treatment groups [F(11, 220)=11.585, P<0.0001]; however, there was no difference between the treatment groups [F(33, 220)=0.813, P=0.757] (Fig. 4a). No significant difference in total distance traveled between groups was noted [F(3, 11)=0.430, P=0.734] (Fig. 4b). These results confirm that effects of leptin and fluoxetine on depression-and anxiety-like behaviors are not due to changes in nonspecific locomotor activity.
Fig. 4.
Effect of leptin and fluoxetine on locomotor activity. Mice were injected i.p. with saline (n=6), 0.25 mg/kg leptin (n=6), 1 mg/kg leptin (n=6), or 10 mg/kg fluoxetine (n=6) and immediately placed in an open field arena. Animals were allowed to freely explore for a total of 60 min. a The distance traveled in a 5 min interval. b Total distance traveled within the entire 60 min. Data are expressed as mean±SEM
Discussion
The main finding of the present study was that acute leptin treatment in mice produced anxiolytic-like effects as indicated by increased novelty exploration, enhanced active social behavior, and reduced self-grooming. In contrast, acute treatment with the SSRI antidepressant fluoxetine, an antidepressant clinically used for the treatment of anxiety after chronic administration (Schoevers et al. 2008), elicited anxiogenic-like effects in the same behavioral tests. In addition, this study showed that leptin, similar to fluoxetine, reduced “behavioral despair” in the TST and FST, which confirmed the antidepressant-like efficacy in rats reported previously (Lu et al. 2006). These results suggest that leptin could represent a novel therapeutic target for the treatment of both depression and anxiety.
The observations that acute administration of leptin in mice can decrease immobility in the TST and FST, two tests for screening novel antidepressants, confirmed the antidepressant-like potential as reported previously in rats (Lu et al. 2006). However, a study by Hirano et al. showed that leptin had antidepressant-like effects in the TST in diabetic mice but not in non-diabetic mice (Hirano et al. 2007). This discrepancy may be due to differences in age and strain of mice used in these studies. Antidepressant response, as measured by the TST and FST, varies with age and strain of animals (Crowley et al. 2005; Mason et al. 2009). Hirano et al. used the juvenile ICR mice (4-week-old), whereas we used adult C57/ BL mice (9–10-week-old at the time for behavioral tests) in the present study. It has been demonstrated that juvenile mice have higher immobility in both the TST and FST and are less sensitive to antidepressant drugs in comparison with adult mice (Mason et al. 2009). This could provide an explanation for the discrepancies between our findings and the report by Hirano et al.
The SSRI antidepressants have been used successfully in the treatment of several anxiety disorders including generalized anxiety disorders (Ball et al. 2005; Sramek et al. 2002), panic disorders (Bruce et al. 2003), social anxiety disorder (Blanco et al. 2003), obsessive–compulsive, and post-traumatic stress disorders (Berlant 2003; den Boer et al. 1995). However, the initial effect of acute administration of SSRIs in humans is anxiogenic (Den Boer and Westenberg 1990; den Boer et al. 1987; Gorman et al. 1987; Grillon et al. 2007; Jick et al. 2004). This acute anxiogenic behavioral effect was observed in the present study in behavioral assays for anxiety. In the elevated plus maze test, a widely used behavioral paradigm to assess anxiety levels (Lister 1987; Pellow et al. 1985), mice treated acutely with fluoxetine spent shorter times in the open arms and made fewer entries into the open arms without changing closed arm entries, indicating enhanced anxiety behavior. The anxiogenic activity of fluoxetine was also observed in another behavioral test for anxiety, i.e. the social interaction test. In this test, increases in active social interaction are indicative of an anxiolytic effect, and decreases indicate an anxiogenic response. A single injection of fluoxetine reduced the total time spent by a pair of male mice in active social interaction. Our findings of anxiogenic effects of acute fluoxetine treatment are in general agreement with previous reports in rats or mice (Bagdy et al. 2001; Drapier et al. 2007; File et al. 1999; Holmes and Rodgers 2003; Silva and Brandao 2000). In contrast, we found that acute administration of leptin displays opposite effects on anxiety-related behaviors in the same tests under the same testing conditions. In the elevated plus maze test, acute leptin treatment increased the frequency and time spent on open arms, indicative of an anxiolytic effect. An earlier study reported that repeated administration of leptin for 5 days in leptin-deficient (ob/ob) obese mice increased open arm time and entries (Asakawa et al. 2003). However, this study cannot rule out the possibility that the increase in open arm entries is attributable to nonspecific locomotor elevation because of lack of wild-type controls and increased total arm entries induced by leptin treatment in these ob/ob mice (Asakawa et al. 2003). It has been previously shown that leptin deficiency or leptin receptor deficiency results in decreased locomotor activity (Dauncey 1986; Kudo et al. 2004; Laposky et al. 2006), and leptin replacement in leptin-deficient mice restores locomotor activity (Pelleymounter et al. 1995). However, leptin treatment in wild-type mice exhibited no stimulatory effect on locomotion, as indicated by the measures of total arm entries in the elevated plus maze. Thus, our findings in wild-type mice support a specific anxiolytic-like effect of leptin in the elevated plus maze test.
The anxiolytic effect of leptin was confirmed in the social interaction test. A marked increase in total time spent in active social behavior was observed after acute administration of leptin at 1 mg/kg. Further dissection of active social behavior indicated that leptin significantly increased nosing and following behaviors without affecting non-aggressive physical contacts. To our knowledge, leptin’s effects on social interaction have not been previously reported. In addition, we found that self-grooming, a non-social behavior, was suppressed by leptin treatment. When animals are exposed to mild threat or a stressful environment, self-grooming behavior can occur (Gispen and Isaacson 1981; Spruijt et al. 1992; van Erp et al. 1994). Anxiogenic stimuli can increase self-grooming, whereas anxiolytic drugs decrease self-grooming, which has been considered as an index of anxiety (Lazosky and Britton 1991; Moody et al. 1988). In our study, animals were tested in an unfamiliar open field arena; the novelty stress could contribute to the observed self-grooming behavior. The inhibitory effect of leptin on self-grooming therefore is indicative of anxiolytic-like activity. We also measured anxiety levels in the open field test following acute administration of leptin and fluoxetine. However, this test appears to be insensitive to either compound. Mice receiving leptin or fluoxetine failed to show any difference in expression of anxiety-related behaviors including the measures of entries, time, and distance traveled in the central zone of the open field arena. This is somewhat surprising given the positive effects of leptin and fluoxetine identified in the elevated plus maze and social interaction test. It is possible that our testing conditions were not optimal for detecting anxiogenic and anxiolytic effects. Alternatively, the open field paradigm may be insensitive to acute effects of leptin and fluoxetine. In line with this possibility, it has been suggested that the open field test is not sensitive to non-benzodiazepine agents (Prut and Belzung 2003).
Our data suggest that leptin has putative anxiolytic and antidepressant properties with rapid onset of action. While the neural substrates and molecular mechanisms underlying the rapid behavioral action of leptin remain to be determined, a growing body of evidence support that neuroplasticity is involved in the therapeutic action of mood-alleviating drugs. For instance, adult hippocampal neurogenesis has been demonstrated to play an important role in antidepressant and anxiolytic action (David et al. 2009; Jiang et al. 2005; Santarelli et al. 2003). Adult hippocampal neurongenesis can be promoted by leptin both in vitro and in vivo (Garza et al. 2008). However, the regulation of hippocampal neurogenic activity is unlikely to be a mechanism underlying the acute response to leptin because neurogenesis is a relatively slow process with multi-steps including proliferation of progenitors, differentiation, maturation, and integration of newborn neurons into hippocampal circuitry (Ming and Song 2005). After cell division, it takes about 4 weeks for newly generated neurons in the dentate gyrus to develop functional properties similar to those of mature granule cells (van Praag et al. 2002). Thus, changes in neurogenesis would not be expected to occur during acute leptin treatment. On the other hand, emerging evidence suggests that changes in synaptic plasticity are involved in the response to anti-depressants. Particularly, glutamatergic neurotransmission has been recently implicated in a possible mechanism for rapid onset antidepressants (Berman et al. 2000; Zarate et al. 2006; Preskorn et al. 2008). In contrast to monoamine-based classical antidepressants requiring weeks or months of treatment before a therapeutic effect is observed, a single injection of ketamine, a glutamate-NMDA antagonist, induces a rapid (within 2 h) and sustained (up to 2 weeks) antidepressant effect in treatment-refractory patients with depression (Berman et al. 2000; Zarate et al. 2006). Preclinical studies also suggest that drugs targeting various components of glutamate neurotransmission have anxiolytic effects (Bergink et al. 2004). Several lines of evidence suggest a role of leptin in glutamatergic transmission and plasticity. Electrophysiological recording on brain slices reveals that leptin treatment can modulate glutamate neurotransmission both presynaptically and postsynaptically (Harvey et al. 2006; Oomura et al. 2006; Xu et al. 2008). Moreover, chronic elevation of leptin regulates expression of glutamate receptors (Walker et al. 2007). Whether and how glutamate activity is involved in the acute behavioral responses to leptin await for further investigation.
In summary, the results of this study support a role of leptin in both anxiety- and depression-related behaviors. It is noteworthy that its anxiolytic effects after acute administration is in contrast to SSRI antidepressants, which require chronic treatment to reduce anxiety in rodents and humans (Burghardt et al. 2004; Den Boer and Westenberg 1990; den Boer et al. 1987; Gorman et al. 1987; Griebel et al. 1994; Kurt et al. 2000). Given that a large proportion of depressed patients also have anxiety disorders (Pollack 2005), the antidepressant- and anxiolytic-like properties of leptin have clinical significance especially for the treatment of comorbid conditions. However, questions remain on how long the acute behavioral effects of a single systemic injection of leptin can last and whether these effects will be sustained after chronic administration. In a rat chronic unpredictable stress model of depression, we observed that chronic injection (2 weeks) of leptin to rats subjected to daily stress reversed the stress-induced depression-like behavior (unpublished data). Future studies will investigate the effect of chronically administered leptin on anxiety-like behavior and test whether leptin is effective in animal models with genetic manipulations or environmental exposures predisposing to anxiety.
Acknowledgements
The authors thank Tung-Yi Huang for his contribution to this study. This work was supported by American Heart Association AHA0530345N (X.Y.L.) and National Institute of Health grants MH073844 and MH076929 (X.Y.L.).
References
- Asakawa A, Inui A, Inui T, Katsuura G, Fujino MA, Kasuga M. Leptin treatment ameliorates anxiety in ob/ob obese mice. J Diabetes Complicat. 2003;17:105–107. doi: 10.1016/s1056-8727(02)00185-x. [DOI] [PubMed] [Google Scholar]
- Atmaca M, Kuloglu M, Tezcan E, Ustundag B, Bayik Y. Serum leptin and cholesterol levels in patients with bipolar disorder. Neuropsychobiology. 2002;46:176–179. doi: 10.1159/000067809. [DOI] [PubMed] [Google Scholar]
- Bagdy G, Graf M, Anheuer ZE, Modos EA, Kantor S. Anxiety-like effects induced by acute fluoxetine, sertraline or m-CPP treatment are reversed by pretreatment with the 5-HT2C receptor antagonist SB-242084 but not the 5-HT1A receptor antagonist WAY-100635. Int J Neuropsychopharmacol. 2001;4:399–408. doi: 10.1017/S1461145701002632. [DOI] [PubMed] [Google Scholar]
- Ball SG, Kuhn A, Wall D, Shekhar A, Goddard AW. Selective serotonin reuptake inhibitor treatment for generalized anxiety disorder: a double-blind, prospective comparison between paroxetine and sertraline. J Clin Psychiatry. 2005;66:94–99. doi: 10.4088/jcp.v66n0113. [DOI] [PubMed] [Google Scholar]
- Belzung C, Le Guisquet AM, Barreau S, Calatayud F. An investigation of the mechanisms responsible for acute fluoxetine-induced anxiogenic-like effects in mice. Behav Pharmacol. 2001;12:151–162. doi: 10.1097/00008877-200105000-00001. [DOI] [PubMed] [Google Scholar]
- Bergink V, van Megen HJ, Westenberg HG. Glutamate and anxiety. Eur Neuropsychopharmacol. 2004;14:175–183. doi: 10.1016/S0924-977X(03)00100-7. [DOI] [PubMed] [Google Scholar]
- Berlant J. New drug development for post-traumatic stress disorder. Curr Opin Investig Drugs. 2003;4:37–41. [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Blanco C, Schneier FR, Schmidt A, Blanco-Jerez CR, Marshall RD, Sanchez-Lacay A, Liebowitz MR. Pharmacological treatment of social anxiety disorder: a meta-analysis. Depress Anxiety. 2003;18:29–40. doi: 10.1002/da.10096. [DOI] [PubMed] [Google Scholar]
- Bruce SE, Vasile RG, Goisman RM, Salzman C, Spencer M, Machan JT, Keller MB. Are benzodiazepines still the medication of choice for patients with panic disorder with or without agoraphobia? Am J Psychiatry. 2003;160:1432–1438. doi: 10.1176/appi.ajp.160.8.1432. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Sullivan GM, McEwen BS, Gorman JM, LeDoux JE. The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biol Psychiatry. 2004;55:1171–1178. doi: 10.1016/j.biopsych.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Calapai G, Corica F, Corsonello A, Sautebin L, Di Rosa M, Campo GM, Buemi M, Mauro VN, Caputi AP. Leptin increases serotonin turnover by inhibition of brain nitric oxide synthesis. J Clin Invest. 1999;104:975–982. doi: 10.1172/JCI5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesana R, Ceci A, Ciprandi C, Borsini F. Mesulergine antagonism towards the fluoxetine anti-immobility effect in the forced swimming test in mice. J Pharm Pharmacol. 1993;45:473–475. doi: 10.1111/j.2042-7158.1993.tb05578.x. [DOI] [PubMed] [Google Scholar]
- Charnay Y, Cusin I, Vallet PG, Muzzin P, Rohner-Jeanrenaud F, Bouras C. Intracerebroventricular infusion of leptin decreases serotonin transporter binding sites in the frontal cortex of the rat. Neurosci Lett. 2000;283:89–92. doi: 10.1016/s0304-3940(00)00951-4. [DOI] [PubMed] [Google Scholar]
- Collin M, Hakansson-Ovesjo ML, Misane I, Ogren SO, Meister B. Decreased 5-HT transporter mRNA in neurons of the dorsal raphe nucleus and behavioral depression in the obese leptin-deficient ob/ob mouse. Brain Res Mol Brain Res. 2000;81:51–61. doi: 10.1016/s0169-328x(00)00167-4. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Blendy JA, Lucki I. Strain-dependent antidepressant-like effects of citalopram in the mouse tail suspension test. Psychopharmacology (Berl) 2005;183:257–264. doi: 10.1007/s00213-005-0166-5. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Dauncey MJ. Activity-induced thermogenesis in lean and genetically obese (ob/ob) mice. Experientia. 1986;42:547–549. doi: 10.1007/BF01946696. [DOI] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, Hen R. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Boer JA, Westenberg HG. Serotonin function in panic disorder: a double blind placebo controlled study with fluvox-amine and ritanserin. Psychopharmacology (Berl) 1990;102:85–94. doi: 10.1007/BF02245749. [DOI] [PubMed] [Google Scholar]
- den Boer JA, Westenberg HG, Kamerbeek WD, Verhoeven WM, Kahn RS. Effect of serotonin uptake inhibitors in anxiety disorders; a double-blind comparison of clomipramine and fluvoxamine. Int Clin Psychopharmacol. 1987;2:21–32. doi: 10.1097/00004850-198701000-00002. [DOI] [PubMed] [Google Scholar]
- den Boer JA, Westenberg HG, De Leeuw AS, van Vliet IM. Biological dissection of anxiety disorders: the clinical role of selective serotonin reuptake inhibitors with particular reference to fluvoxamine. Int Clin Psychopharmacol. 1995;9(Suppl 4):47–52. [PubMed] [Google Scholar]
- Dhir A, Kulkarni SK. Effect of addition of yohimbine (alpha-2-receptor antagonist) to the antidepressant activity of fluoxetine or venlafaxine in the mouse forced swim test. Pharmacology. 2007;80:239–243. doi: 10.1159/000104877. [DOI] [PubMed] [Google Scholar]
- Drapier D, Bentue-Ferrer D, Laviolle B, Millet B, Allain H, Bourin M, Reymann JM. Effects of acute fluoxetine, paroxetine and desipramine on rats tested on the elevated plus-maze. Behav Brain Res. 2007;176:202–209. doi: 10.1016/j.bbr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- File SE, Ouagazzal AM, Gonzalez LE, Overstreet DH. Chronic fluoxetine in tests of anxiety in rat lines selectively bred for differential 5-HT1A receptor function. Pharmacol Biochem Behav. 1999;62:695–701. doi: 10.1016/s0091-3057(98)00208-1. [DOI] [PubMed] [Google Scholar]
- Finn PD, Cunningham MJ, Rickard DG, Clifton DK, Steiner RA. Serotonergic neurons are targets for leptin in the monkey. J Clin Endocrinol Metab. 2001;86:422–426. doi: 10.1210/jcem.86.1.7128. [DOI] [PubMed] [Google Scholar]
- Garza JC, Guo M, Zhang W, Lu XY. Leptin increases adult hippocampal neurogenesis in vivo and in vitro. J Biol Chem. 2008;283:18238–18247. doi: 10.1074/jbc.M800053200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gispen WH, Isaacson RL. ACTH-induced excessive grooming in the rat. Pharmacol Ther. 1981;12:209–246. doi: 10.1016/0163-7258(81)90081-4. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Liebowitz MR, Fyer AJ, Goetz D, Campeas RB, Fyer MR, Davies SO, Klein DF. An open trial of fluoxetine in the treatment of panic attacks. J Clin Psychopharmacol. 1987;7:329–332. [PubMed] [Google Scholar]
- Griebel G, Moreau JL, Jenck F, Misslin R, Martin JR. Acute and chronic treatment with 5-HT reuptake inhibitors differentially modulate emotional responses in anxiety models in rodents. Psychopharmacology (Berl) 1994;113:463–470. doi: 10.1007/BF02245224. [DOI] [PubMed] [Google Scholar]
- Griebel G, Blanchard DC, Agnes RS, Blanchard RJ. Differential modulation of antipredator defensive behavior in Swiss-Webster mice following acute or chronic administration of imipramine and fluoxetine. Psychopharmacology (Berl) 1995;120:57–66. doi: 10.1007/BF02246145. [DOI] [PubMed] [Google Scholar]
- Griebel G, Sanger DJ, Perrault G. The use of the rat elevated plus-maze to discriminate between non-selective and BZ-1 (omega 1) selective, benzodiazepine receptor ligands. Psychopharmacology (Berl) 1996;124:245–254. doi: 10.1007/BF02246664. [DOI] [PubMed] [Google Scholar]
- Grillon C, Levenson J, Pine DS. A single dose of the selective serotonin reuptake inhibitor citalopram exacerbates anxiety in humans: a fear-potentiated startle study. Neuropsychopharmacology. 2007;32:225–231. doi: 10.1038/sj.npp.1301204. [DOI] [PubMed] [Google Scholar]
- Harvey J, Solovyova N, Irving A. Leptin and its role in hippocampal synaptic plasticity. Prog Lipid Res. 2006;45:369–378. doi: 10.1016/j.plipres.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay-Schmidt A, Helboe L, Larsen PJ. Leptin receptor immunoreactivity is present in ascending serotonergic and catecholaminergic neurons of the rat. Neuroendocrinology. 2001;73:215–226. doi: 10.1159/000054638. [DOI] [PubMed] [Google Scholar]
- Hirano S, Miyata S, Kamei J. Antidepressant-like effect of leptin in streptozotocin-induced diabetic mice. Pharmacol Biochem Behav. 2007;86:27–31. doi: 10.1016/j.pbb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Holmes A, Rodgers RJ. Prior exposure to the elevated plus-maze sensitizes mice to the acute behavioral effects of fluoxetine and phenelzine. Eur J Pharmacol. 2003;459:221–230. doi: 10.1016/s0014-2999(02)02874-1. [DOI] [PubMed] [Google Scholar]
- Jain NN, Ohal CC, Shroff SK, Bhutada RH, Somani RS, Kasture VS, Kasture SB. Clitoria ternatea and the CNS. Pharmacol Biochem Behav. 2003;75:529–536. doi: 10.1016/s0091-3057(03)00130-8. [DOI] [PubMed] [Google Scholar]
- Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji SP, Bai G, Zhang X. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effects. J Clin Invest. 2005;115:3104–3116. doi: 10.1172/JCI25509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. JAMA. 2004;292:338–343. doi: 10.1001/jama.292.3.338. [DOI] [PubMed] [Google Scholar]
- Jow GM, Yang TT, Chen CL. Leptin and cholesterol levels are low in major depressive disorder, but high in schizophrenia. J Affect Disord. 2006;90:21–27. doi: 10.1016/j.jad.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Kask A, Nguyen HP, Pabst R, von Horsten S. Factors influencing behavior of group-housed male rats in the social interaction test: focus on cohort removal. Physiol Behav. 2001;74:277–282. doi: 10.1016/s0031-9384(01)00587-x. [DOI] [PubMed] [Google Scholar]
- Kraus T, Haack M, Schuld A, Hinze-Selch D, Pollmacher T. Low leptin levels but normal body mass indices in patients with depression or schizophrenia. Neuroendocrinology. 2001;73:243–247. doi: 10.1159/000054641. [DOI] [PubMed] [Google Scholar]
- Kudo T, Akiyama M, Kuriyama K, Sudo M, Moriya T, Shibata S. Night-time restricted feeding normalises clock genes and Pai-1 gene expression in the db/db mouse liver. Diabetologia. 2004;47:1425–1436. doi: 10.1007/s00125-004-1461-0. [DOI] [PubMed] [Google Scholar]
- Kurt M, Arik AC, Celik S. The effects of sertraline and fluoxetine on anxiety in the elevated plus-maze test in mice. J Basic Clin Physiol Pharmacol. 2000;11:173–180. doi: 10.1515/jbcpp.2000.11.2.173. [DOI] [PubMed] [Google Scholar]
- Laposky AD, Shelton J, Bass J, Dugovic C, Perrino N, Turek FW. Altered sleep regulation in leptin-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2006;290:R894–R903. doi: 10.1152/ajpregu.00304.2005. [DOI] [PubMed] [Google Scholar]
- Lazosky AJ, Britton DR. Effects of 5-HT-1A receptor agonists on CRF-induced behavior. Psychopharmacology (Berl) 1991;104:132–136. doi: 10.1007/BF02244567. [DOI] [PubMed] [Google Scholar]
- Leshan RL, Bjornholm M, Munzberg H, Myers MG., Jr. Leptin receptor signaling and action in the central nervous system. Obesity (Silver Spring) 2006;14(Suppl 5):208S–212S. doi: 10.1038/oby.2006.310. [DOI] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Lu XY, Kim CS, Frazer A, Zhang W. Leptin: a potential novel antidepressant. Proc Natl Acad Sci U S A. 2006;103:1593–1598. doi: 10.1073/pnas.0508901103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Mason SS, Baker KB, Davis KW, Pogorelov VM, Malbari MM, Ritter R, Wray SP, Gerhardt B, Lanthorn TH, Savelieva KV. Differential sensitivity to SSRI and tricyclic antidepressants in juvenile and adult mice of three strains. Eur J Pharmacol. 2009;602:306–315. doi: 10.1016/j.ejphar.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Moody TW, Merali Z, Crawley JN. The effects of anxiolytics and other agents on rat grooming behavior. Ann N Y Acad Sci. 1988;525:281–290. doi: 10.1111/j.1749-6632.1988.tb38613.x. [DOI] [PubMed] [Google Scholar]
- Murcia CL, Gulden F, Herrup K. A question of balance: a proposal for new mouse models of autism. Int J Dev Neurosci. 2005;23:265–275. doi: 10.1016/j.ijdevneu.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Oomura Y, Hori N, Shiraishi T, Fukunaga K, Takeda H, Tsuji M, Matsumiya T, Ishibashi M, Aou S, Li XL, Kohno D, Uramura K, Sougawa H, Yada T, Wayner MJ, Sasaki K. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phos-phorylation in rats. Peptides. 2006;27:2738–2749. doi: 10.1016/j.peptides.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Page ME, Detke MJ, Dalvi A, Kirby LG, Lucki I. Serotonergic mediation of the effects of fluoxetine, but not desipramine, in the rat forced swimming test. Psychopharmacology (Berl) 1999;147:162–167. doi: 10.1007/s002130051156. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Perrault G, Morel E, Zivkovic B, Sanger DJ. Activity of litoxetine and other serotonin uptake inhibitors in the tail suspension test in mice. Pharmacol Biochem Behav. 1992;42:45–47. doi: 10.1016/0091-3057(92)90444-k. [DOI] [PubMed] [Google Scholar]
- Pollack MH. Comorbid anxiety and depression. J Clin Psychiatry. 2005;66(Suppl 8):22–29. [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP , 606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28:631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev. 1997;21:801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Schoevers RA, Van HL, Koppelmans V, Kool S, Dekker JJ. Managing the patient with co-morbid depression and an anxiety disorder. Drugs. 2008;68:1621–1634. doi: 10.2165/00003495-200868120-00002. [DOI] [PubMed] [Google Scholar]
- Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. Leptin targets in the mouse brain. J Comp Neurol. 2009;514:518–532. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva RC, Brandao ML. Acute and chronic effects of gepirone and fluoxetine in rats tested in the elevated plus-maze: an ethological analysis. Pharmacol Biochem Behav. 2000;65:209–216. doi: 10.1016/s0091-3057(99)00193-8. [DOI] [PubMed] [Google Scholar]
- Simon P, Dupuis R, Costentin J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav Brain Res. 1994;61:59–64. doi: 10.1016/0166-4328(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Spruijt BM, van Hooff JA, Gispen WH. Ethology and neurobiology of grooming behavior. Physiol Rev. 1992;72:825–852. doi: 10.1152/physrev.1992.72.3.825. [DOI] [PubMed] [Google Scholar]
- Sramek JJ, Zarotsky V, Cutler NR. Generalised anxiety disorder: treatment options. Drugs. 2002;62:1635–1648. doi: 10.2165/00003495-200262110-00005. [DOI] [PubMed] [Google Scholar]
- Stemmelin J, Cohen C, Terranova JP, Lopez-Grancha M, Pichat P, Bergis O, Decobert M, Santucci V, Francon D, Alonso R, Stahl SM, Keane P, Avenet P, Scatton B, le Fur G, Griebel G. Stimulation of the beta(3)-adrenoceptor as a novel treatment strategy for anxiety and depressive disorders. Neuropsychopharmacology. 2008;33:574–587. doi: 10.1038/sj.npp.1301424. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- To CT, Anheuer ZE, Bagdy G. Effects of acute and chronic fluoxetine treatment of CRH-induced anxiety. Neuroreport. 1999;10:553–555. doi: 10.1097/00001756-199902250-00020. [DOI] [PubMed] [Google Scholar]
- Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav. 1988;31:959–962. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- Ukai M, Maeda H, Nanya Y, Kameyama T, Matsuno K. Beneficial effects of acute and repeated administrations of sigma receptor agonists on behavioral despair in mice exposed to tail suspension. Pharmacol Biochem Behav. 1998;61:247–252. doi: 10.1016/s0091-3057(98)00093-8. [DOI] [PubMed] [Google Scholar]
- van Erp AM, Kruk MR, Meelis W, Willekens-Bramer DC. Effect of environmental stressors on time course, variability and form of self-grooming in the rat: handling, social contact, defeat, novelty, restraint and fur moistening. Behav Brain Res. 1994;65:47–55. doi: 10.1016/0166-4328(94)90072-8. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CD, Long H, Williams S, Richard D. Long-lasting effects of elevated neonatal leptin on rat hippocampal function, synaptic proteins and NMDA receptor subunits. J Neurosci Res. 2007;85:816–828. doi: 10.1002/jnr.21173. [DOI] [PubMed] [Google Scholar]
- Westling S, Ahren B, Traskman-Bendz L, Westrin A. Low CSF leptin in female suicide attempters with major depression. J Affect Disord. 2004;81:41–48. doi: 10.1016/j.jad.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Xu L, Rensing N, Yang XF, Zhang HX, Thio LL, Rothman SM, Weisenfeld AE, Wong M, Yamada KA. Leptin inhibits 4-aminopyridine- and pentylenetetrazole-induced seizures and AMPAR-mediated synaptic transmission in rodents. J Clin Invest. 2008;118:272–280. doi: 10.1172/JCI33009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Wada E, Wada K. Male mice lacking the gastrinreleasing peptide receptor (GRP-R) display elevated preference for conspecific odors and increased social investigatory behaviors. Brain Res. 2000;870:20–26. doi: 10.1016/s0006-8993(00)02395-7. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]