Abstract
BACKGROUND
HIV-infected individuals are at increased risk for pulmonary hypertension and cardiomyopathy, portending a poor prognosis. Right ventricular (RV) dysfunction is associated with worse outcomes in these conditions, yet its prevalence is poorly defined in HIV. We sought to determine the prevalence of RV dysfunction in an outpatient HIV cohort.
METHODS
Echocardiograms were evaluated in 104 HIV-infected adults. Measurements included estimated pulmonary arterial systolic pressure (PAP) and several measures of RV function including tricuspid annular plane systolic excursion (TAPSE), RV longitudinal myocardial strain (RV LMS), RV fractional area change (RVFAC), and myocardial performance index (MPI).
RESULTS
Sixteen subjects (15%) had PAP>35 mm Hg, yet RV function did not differ significantly from those with normal estimated PAP. RV dysfunction defined by RVFAC < 35% occurred in 11%. RV LMS had a median value of −27.3% and individuals below the median had lower TAPSE, but no differences in LVEF, PAP, or other measures. Dyspnea was associated with the lowest quintile of RV LMS (≥−21.05%). There were 6 subjects with LVEF<50% and these individuals had lower TAPSE, but no difference PAP or other RV functional measures.
CONCLUSIONS
RV dysfunction was as common as estimated PAP>35 mm Hg and LV dysfunction, but these findings did not co-segregate. RV dysfunction in HIV-infected individuals may be a separate entity from LV/global cardiomyopathy or pulmonary hypertension and deserves further study.
Keywords: Pulmonary hypertension, HIV, Right ventricle, Cardiomyopathy
Introduction
Human immunodeficiency virus (HIV) infection has been associated with many cardiac abnormalities including cardiomyopathy, pulmonary hypertension (PH), and coronary artery disease (1, 2, 3, 4). In contrast, the presence and nature of right ventricular (RV) abnormalities have not been extensively studied. While isolated RV dysfunction in HIV has been reported in two studies from twenty years ago, the severity of RV dysfunction has not been documented in the current era except for one report utilizing radionuclide ventriculography (3, 4, 5). This report found isolated RV dysfunction in 5% of HIV patients. In addition, echocardiographic technology has advanced considerably, and several new measures of RV function have since been validated. Because RV dysfunction is linked to increased mortality in PH (6, 7) and most cardiac diseases (8, 9, 10, 11), understanding of RV function in HIV is important prognostically and, potentially, therapeutically.
Assessment of ventricular function is critical in the evaluation of PH because left ventricular (LV) dysfunction may cause PH, and RV function is at risk in PH (12). Assessment of ventricular function is particularly important in HIV-associated PH due to the wide range of cardiac abnormalities associated with HIV (1-4). While it is possible to use echocardiography to screen for PH, diagnosis requires invasive measurement of hemodynamics, as recent studies have found that echocardiographic measures in HIV may under- or overdiagnose PH in this population (13). Additionally, diagnosis is typically delayed up to several years, frequently not until the disease has reached later stages including RV dilation and dysfunction (14, 15). Whether echocardiographic examination of the RV could add to diagnosis of PH in HIV-infected individuals is unknown.
The objectives of the present study were to define the prevalence of RV dysfunction in an outpatient HIV cohort utilizing current era echocardiographic technology and to determine the association of RV dysfunction with echocardiographic signs of PH and left-sided heart failure.
Methods
Study Setting
Participants in an ongoing prospective multicenter study of lung and cardiac function in HIV were prospectively enrolled in the current echocardiographic substudy at our center only. Details of this study have been previously published (16 ,17). Briefly, inclusion criteria were documented HIV infection and attendance at the University of Pittsburgh HIV/AIDS clinic. Individuals were excluded if they were experiencing new or increasing respiratory symptoms or fevers within the previous four weeks. Participants in this substudy underwent echocardiography between September 16, 2009 and May 31, 2011. The protocol was approved by the University of Pittsburgh Institutional Review Board, and all participants signed written informed consent. Demographic and clinical data were collected by participant interview and medical record review. Laboratory studies were obtained from the medical record and included the most recent CD4+ T-lymphocyte cell count and plasma HIV ribonucleic acid (RNA) level within three months. The lower limit of detection for the HIV RNA polymerase chain reaction assay was 50 copies/mL. Antiretroviral therapy (ART) use was defined as use of at least three antiretroviral agents from at least two classes of medications in the past 3 months.
Transthoracic echocardiography
Echocardiography was performed with a GE-Vingmed Vivid 7 system (GE Vingmed Ultrasound, Horten, Norway). From standard 2-dimensional views, pulsed and continuous wave Doppler measurements were obtained as per the American Echocardiography Association recommendations (18, 19). The examination was recorded digitally. All studies were read by one investigator (CDL) and reviewed by an experienced cardiologist (MAS); any disagreements were reviewed by both readers and measures jointly agreed upon. Right ventricular end-diastolic (RVEDA) and end-systolic areas (RVESA) were measured from the apical 4-chamber view to calculate right ventricular fractional area change [RVFAC = (RVEDA – RVESA) / RVEDA) × 100] (20). Peak PA systolic pressures were estimated by calculating the systolic pressure gradient between the RV and RA by the maximum velocity of the tricuspid regurgitant jet using the modified Bernoulli equation, and then adding to this gradient an estimated right atrial pressure based on the size of the inferior vena cava and its variation with respiration (21). LV ejection fraction was calculated via the biplane Simpson method. Tricuspid annular systolic plane excursion (TAPSE) was measured by M-mode of the lateral tricuspid valve annulus (22). Myocardial performance index (MPI) was calculated as the ratio of the time of the isovolumic phases of the cardiac cycle to ejection time as derived from Doppler imaging of the RV inflow and outflow in the parasternal short axis (23). Eccentricity index was calculated as the ratio of LV diameter parallel to the septal wall plane to LV diameter perpendicular to the septal wall plane (24).
Strain analysis by speckle tracking was performed by tracing the endocardial RV surface in the apical 4-chamber view (mean frame rate: 77 ± 17 FPS), using a point-and-click approach with special care taken to adjust tracking of all endocardial segments (EchoPAC, GE Vingmed Ultrasound, Horten, Norway). A second larger concentric border was then automatically generated and manually adjusted near the epicardium. Speckle tracking automatically analyzed frame-by-frame movement of the stable patterns of natural acoustic markers, or speckles, over the cardiac cycle. The location shift of these acoustic markers representing tissue movement provided spatial and temporal data used to calculate regional strain vectors as change in length/initial length, with myocardial thickening along the longitudinal axis considered positive. The image was then automatically divided into 6 standard segments with corresponding time-strain curves from each segment. Strain values were reported for the mid and basal regions of the RV free wall.
Statistical analyses
Variables of RV function that were evaluated included the following: RVFAC, TAPSE, MPI, eccentricity index, and longitudinal myocardial strain of the mid and basal RV free wall. To evaluate the effect of PH on RV function, individuals were stratified by an estimated systolic pulmonary arterial pressure (derived from the echocardiogram) of 35 mm Hg, as has been used to define mild PH (25). To evaluate the effect of speckle tracking-derived longitudinal myocardial strain of the RV on other measures of RV function, participants were stratified by a value of −27.3% which was the median of the cohort. To evaluate the effect of TAPSE on other measures of RV function, participants were stratified by a value of 2.2 cm, which was the lowest quartile of the cohort. To evaluate the effect of LV function on measures of RV function, we stratified by a LV ejection fraction value of 50%. Participants were also stratified by whether or not they were receiving antiretroviral therapy. Variables were evaluated for normal distribution. Between group differences were calculated using the Student’s t-test for independent samples or the Mann-Whitney test, for normally and non-normally distributed variables, respectively, or Pearson Chi-Square for categorical variables. Data are expressed as mean ± standard deviation. Statistical significance was defined as two-sided p value <0.05. All statistical calculations were made using SPSS for Windows (version 20, SPSS, Inc., Chicago, Illinois).
Results
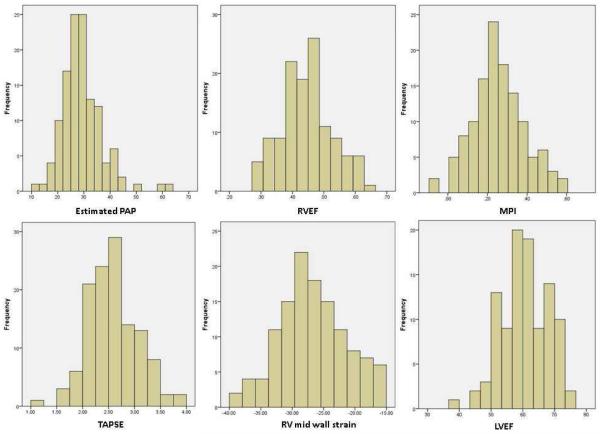
Echocardiography was performed in 131 individuals consecutively enrolled as part of a substudy of a larger project to assess lung and cardiac function in HIV (16). Of these, 104 were included in the present analysis after excluding 14 for missing studies and 13 for poor acoustic windows for imaging the RV free wall. The average age of the cohort was 47 years, and 29% were female (Table 1). The majority of participants had normal echocardiographic measures, displayed in figure 1 (LVEF 60 ± 7 %, RVFAC 45 ± 8%, TAPSE 2.56 ± 0.48 cm, MPI 0.26 ± 0.13, mid wall RV LMS −27.0 ± 5.5%). No individuals had known right-sided heart failure or pulmonary hypertension.
Table 1.
Clinical characteristics of the cohort.
| Demographics | |
| Age, mean years, (SD) | 47 (10) |
| Female gender, n (%) | 30 (29%) |
| CD4 cell count, median (range) | 591 (24 -1798) |
| Log HIV viral level, mean log copies/ml (SD) | 2.10 (0.96) |
| Antiretroviral therapy, n (%) | 93 (89%) |
| Smoking history, n (%) | 84 (81%) |
| Drug use, n (%) | 3 (3%) |
| Dyspnea, n (%) | 34 (33%) |
| Cough, n (%) | 26 (25%) |
| Wheezing, n (%) | 29 (28%) |
SD indicates standard deviation; CD4, cluster of differentiation 4; HIV, human immunodeficiency virus.
Figure 1.
Histograms of echocardiographic parameters. Estimated PAP indicates pulmonary arterial pressure (systolic, units are mm Hg) as calculated from the doppler echocardiographic tricuspid regurgitant jet velocity; RVFAC, right ventricular fractional area of change; MPI, myocardial performance index; TAPSE, tricuspid annular plane systolic excursion (units are cm); RV, right ventricular; LVEF, left ventricular ejection fraction.
Assessment of RV function
Several echocardiographic parameters were utilized to evaluate RV function, including TAPSE, RVFAC, MPI, eccentricity index and longitudinal myocardial strain of the RV free wall as assessed by speckle tracking (RV LMS). In general in the study cohort, RV function was relatively preserved. An example of speckle tracking strain analysis of the RV is shown in Figure 2. Stratifying subjects by the median RV LMS of −27.3%, there was no significant difference in other measures of RV function including RVFAC, MPI, and eccentricity index (Table 2). There was a small but significant difference in TAPSE, although TAPSE was still normal in subjects with low RV strain. To test repeatability in RV LMS measurements, interobserver variability testing was performed on the echocardiograms of 25 participants, yielding a Spearman correlation coefficient of 0.704 (P<0.001) and a κ statistic of 0.682 to classify RV LMS above or below the median value of −27.3%. Intraobserver variability was performed by having the same reader reassess RV LMS at least 3 months after the first reading, which yielded a Spearman correlation coefficient of 0.838 (P<0.001) and a a κ statistic of 0.667 (P=0.004).
Figure 2.
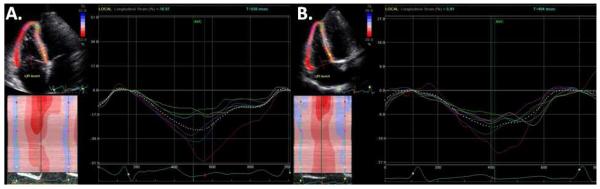
Example of speckle tracking in a subject with normal RV strain (Panel A, maximum longitudinal myocardial strain is −50% in the basal RV free wall [red strain plot] and −36% in the mid RV free wall [blue strain plot]) and diminished RV strain (Panel B, maximum longitudinal myocardial strain is −24% in the basal RV free wall [red strain plot] and −16% in the mid RV free wall [blue strain plot]).
Table 2.
Cohort characteristics stratified by median mid-RV longitudinal myocardial strain of - 27.3%.
| Mid-RVLMS ≥ −27.3 % Worse strain (n = 52) |
Mid-RVLMS < −27.3 % Better strain (n = 52) |
P | |
|---|---|---|---|
| Measures | |||
| Estimated PAP (mm Hg) | 29 (8) | 29 (8) | 0.81 |
| RVFAC (%) | 44 (9) | 46 (7) | 0.44 |
| TAPSE (cm) | 2.44 (0.49) | 2.69 (0.45) | 0.007 |
| MPI | 0.27 (0.12) | 0.24 (0.14) | 0.32 |
| Eccentricity Index, diastole | 1.31 (0.69) | 1.18 (0.70) | 0.35 |
| Eccentricity Index, systole | 1.17 (0.54) | 1.11 (0.57) | 0.61 |
| Mid-RV FW LMS (%) | −22.7 (3.6) | −31.3 (3.1) | <0.001 |
| Basal-RV FW LMS (%) | −26.4 (5.4) | −34.2 (7.1) | <0.001 |
PAP indicates pulmonary arterial pressure; RVFAC, right ventricular fractional area of change; TAPSE, tricuspid annular planar systolic excursion; MPI, myocardial performance index; RV, right ventricle; FW LMS, free wall longitudinal myocardial strain. Data shown as mean (standard deviation).
RV LMS did show some cosegregation with RVFAC when stratifying by RVFAC of 35%. Subjects with RVFAC <35% (n=11) had significantly worse RV LMS in the mid wall (−23.9±4.3% vs −27.3±5.5 in those with RVFAC ≥ 35%, P=0.047) as well as in the basal wall (−25.9±5.7% vs −30.8±7.4%, P=0.035). This subgroup also had lower TAPSE (2.23±0.53 cm vs 2.60±0.46 cm, P=0.045), but not MPI (0.28 ± 0.06 vs 0.25 ± 0.14, P=0.3).
There were 26 subjects with TAPSE < 2.2 cm (the lowest quartile cutoff) and 78 subjects with TAPSE ≥ 2.2 cm. Stratifying by TAPSE < 2.2 cm, there was no significant difference in RVFAC (46±10 vs 45 ± 8, for those with TAPSE < 2.2 cm vs TAPSE ≥ 2.2 cm, respectively, P=0.6), MPI (0.26±0.14 vs 0.25±0.13, P=0.7), eccentricity index (1.10±0.40 vs 1.30±0.76, P=0.1), or RV LMS (−25.5±6.2% vs −27.5±5.1%, P=0.1).
Relationship of echocardiographically derived estimated systolic PAP to RV and LV function
Sixteen subjects (15%) had an estimated PAP > 35 mm Hg. Their echocardiographic measures of RV function were not significantly different than measures in the other 88 subjects with estimated PAP ≤ 35 mm Hg (Table 3). Estimated systolic PAP was not significantly different when stratifying subjects by RV function as defined by RV LMS (Table 2), TAPSE (30±10 vs. 29±7 mm Hg, for 26 subjects with TAPSE < 2.2 cm and 78 subjects with TAPSE ≥ 2.2 cm, respectively, P = 0.7), or by RVFAC <35% (31±13% as compared to 29±7% in subjects with RVFAC ≥ 35%, p = 0.5). Estimated systolic PAP was not significantly different in those with LVEF < 50% (Table 4).
Table 3.
Subject characteristics stratified by estimated PAP of 35 mm Hg.
| Estimated PAP > 35 mm Hg (n = 16) |
Estimated PAP ≤ 35 mm Hg (n = 88) |
P | |
|---|---|---|---|
| Measures | |||
| Estimated PAP (mm Hg) | 42 (8) | 26 (5) | <0.001 |
| RVFAC (%) | 44 (9) | 45 (8) | 0.70 |
| TAPSE (cm) | 2.65 (0.73) | 2.54 (0.41) | 0.55 |
| MPI | 0.30 (0.12) | 0.25 (0.13) | 0.19 |
| Eccentricity Index, diastole |
1.31 (0.76) | 1.24 (0.68) | 0.70 |
| Eccentricity Index, systole |
1.32 (0.86) | 1.11 (0.47) | 0.35 |
| Mid-RV FW LMS (%) | −25.2 (5.5) | −27.1 (5.3) | 0.20 |
| Basal-RV FW LMS (%) | −28.7 (6.3) | −30.5 (7.5) | 0.38 |
PAP indicates pulmonary arterial pressure; RVFAC, right ventricular fractional area of change; TAPSE, tricuspid annular planar systolic excursion; MPI, myocardial performance index; RV, right ventricle; FW LMS, free wall longitudinal myocardial strain. Data shown as mean (standard deviation).
Table 4.
Subject characteristics stratified by LVEF of 50%.
| LVEF < 50% (n = 6) |
LVEF ≥ 50% (n = 96) |
P | |
|---|---|---|---|
| Measures | |||
| Estimated PAP (mm Hg) | 30 (11) | 29 (8) | 0.82 |
| RVFAC (%) | 40 (11) | 45 (8) | 0.15 |
| TAPSE (cm) | 2.15 (0.39) | 2.58 (0.47) | 0.02 |
| MPI | 0.24 (0.05) | 0.26 (0.13) | 0.64 |
| Eccentricity Index, diastole | 0.90 (0.10) | 1.27 (0.71) | 0.09 |
| Eccentricity Index, systole | 0.89 (0.17) | 1.16 (0.57) | 0.13 |
| Mid-RV FW LMS (%) | −24.4 (5.0) | −27.0 (5.4) | 0.27 |
| Basal-RV FW LMS (%) | −27.8 (4.7) | −30.3 (7.4) | 0.49 |
PAP indicates pulmonary arterial pressure; RVFAC, right ventricular fractional area of change; TAPSE, tricuspid annular planar systolic excursion; MPI, myocardial performance index; RV, right ventricle; FW LMS, free wall longitudinal myocardial strain. Data shown as mean (standard deviation).
Relationship of LV function to RV function
We evaluated the relationship of LV function to RV function. Overall, LV function was relatively preserved in the cohort. Participants were stratified by LVEF of 50% and showed a mild, but significant decrease in TAPSE with LVEF < 50%, but no differences in other measures (Table 4). Subjects with TAPSE <2.2 cm showed a trend towards decreased LVEF (56 ± 9% vs 61 ± 7% in those with TAPSE ≥ 2.2 cm, P=0.08). Lower LVEF was also seen in subjects with RVFAC<35% (55 ± 8% vs 61 ± 7 in those with RVFAC ≥ 35%, P = 0.01), but not when stratifying the cohort by RV LMS (Table 2).
Interaction between RV dysfunction, LV dysfunction, and estimated systolic PAP
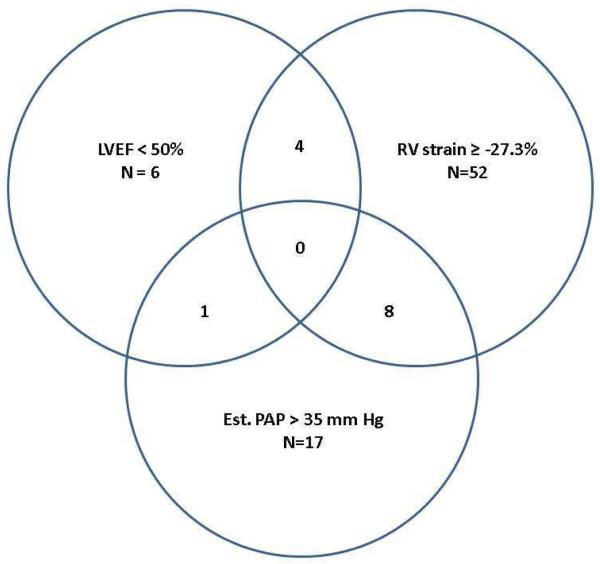
Utilizing RV LMS ≥ −27.3% to define RV dysfunction, LVEF < 50% to define LV dysfunction, and estimated systolic PA pressure > 35 mm Hg to define PH, we assessed the interaction of these processes. Overall, there was very little overlap (Figure 3). No subject had all 3 conditions, and 1-8 had a combination of any two. By chi square analysis, none of these conditions were significantly associated with each other.
Figure 3.
Relationship between LVEF, RV strain, and estimated PAP. LVEF indicates left ventricular ejection fraction; RV, right ventricular; Est. PAP, estimated pulmonary artery systolic pressure. The values within multiple circles indicate the number of patients exhibiting the traits shown by each overlapping circle.
Clinical characteristics associated with RV function, LV function, and estimated systolic PAP > 35 mm Hg
Subjects with dyspnea (defined as presence of dyspnea with hurrying on level ground or walking up a slight hill), were more likely to have diminished RV strain, when defined as the lowest quintile of >-22.05%, (11/21 (52%) vs. 23/83 (28%), P=0.03) as well as estimated systolic PA pressure > 35 mm Hg (12/17 (71%) vs 22/87 (25%), P=0.001). This association was not true for RV FAC < 35%, TAPSE < 2.2 cm, or LVEF < 50%. There was no difference in RV strain between smokers and nonsmokers, drug users, those with self-reported history of viral hepatitis, or those with cough or wheeze. Individuals with an overlap of two conditions (RV dysfunction, LV dysfunction, or estimated systolic PAP > 35 mm Hg) were more likely to be to have dyspnea (8/13 [62%] with >1 condition vs 16/48 [33%] with 1 condition vs 9/41 [22%] with none, P=0.03).
Length of HIV infection was also analyzed. Length of disease ranged from 537 – 11671 days (mean 5753 ± 2709 days). There was no significant difference in length of disease when stratifying patients by diminished RV strain ≥ −27.3% (5606 ± 2614 days vs 2898 ± 2816 days, P = 0.59), RV FAC < 35% (6219 ± 2548 days vs 5698 ± 2735 days, P = 0.55), TAPSE < 2.2 cm (5788 ± 2442 days vs 5742 ± 2804 days, P = 0.94), estimated PAP > 35 (5807 ± 2723 days vs 5484 ± 2699 days, P = 0.66) or LVEF<50% (4902 ± 2569 days vs 5814 ± 2739 days, P = 0.43). Length of disease did not correlate with PA pressure or any measure of RV or LV function (data not shown).
Viral loads in this cohort ranged from 49 – 522000 with a mean of 10,648 (Table 1). CD4 counts were relatively preserved (range 24 – 1798, median 591).
There were no significant differences in viral loads in patients with RV dysfunction, LV dysfunction, or estimated systolic PAP > 35 mm Hg.. There were 11 subjects not on antiretroviral therapy with higher viral loads (log viral load: 3.87 ± 1.31 vs 1.91 ± 0.69, for those not on antiretroviral therapy vs those on therapy, P < 0.001) but similar CD4 counts (584 ± 455 vs 638 ± 335, P = 0.62). There were no differences LV or RV function (LVEF: 58 ± 9% vs 61 ± 7%, P = 0.36; mid wall RV LMS: −29.0 ± 3.7% vs −26.7 ± 5.6%, P = 0.19; RV FAC: 45 ± 7% vs 45 ± 8%, P = 0.95; TAPSE: 2.65 ± 0.33 cm vs 2.55 ± 0.50 cm, P = 0.26) or estimated systolic PA pressure (30 ± 9 mm Hg vs 29 ± 8 mm Hg, P = 0.90). Review of HIV medications found that being currently on abacavir was associated with estimated systolic PAP > 35 mm Hg (4/16 patients (25%) with estimated systolic PAP > 35 mm Hg on medication vs 3/86 patients (4%) with estimated systolic PAP < 35 mm Hg on medication, P=0.008). Abacavir was also associated with being in the lowest quintile of RV LMS (4/21 patients (19%) with low strain on medication vs 3/83 patients [4%] with better strain on medication, P=0.037). Having ever taken efavirenz was associated with being below the median RV LMS of −27.3% (13/52 patients (25%) with low strain ever on medication vs 3/52 (6%) with high strain ever on medication, P=0.041).
Discussion
We found that 11-20% of HIV-infected participants screened by echocardiography had RV dysfunction, depending on the measure of RV function. A similar prevalence of elevated estimated systolic PA pressure (> 35 mm Hg) was also observed. However, not all individuals with elevated PA pressure had decreased RV function and, conversely, not all patients with decreased RV function had elevated PA pressure, suggesting a mixed effect of HIV on both pulmonary hemodynamics and RV myocardial function. Further, RV dysfunction was not explained by LV dysfunction, as participants with LVEF < 50% had only a mild decrease in TAPSE, but no difference in PAP or other measures of RV myocardial function.
Isolated RV dilation has been reported to occur in 4% of HIV-infected individuals in two studies from twenty years ago, with study sizes of 296 and 173 individuals (3, 4). These studies defined RV dilation as a qualitative observation of a larger RV than LV, and no measures of RV function were reported. More recently, Lebech et al. reported isolated RV dysfunction by radionuclide ventriculography in 5% (5 of 95 patients) of HIV-infected individuals, defined by study-specific control data as RVEF < 0.42 (5). In contrast, Karavidas et al. found RV systolic abnormalities by tissue Doppler imaging only in the setting of LV abnormalities (26). We have found a considerably higher prevalence of RV dysfunction in HIV (11% with RV FAC < 35%) as well as evaluated several newer echocardiographic parameters in this population, such as TAPSE and RV LMS by speckle tracking.
Assessing RV function by LMS via echocardiographic speckle tracking has been previously reported in patients with PH, but has not been performed in HIV-infected individuals (27, 28, 29). There is variability among studies in the wall segment studied, frame rate of recorded imaging, and system used. Optimal frame rates are reported to be 50–70 frames per second (FPS) (30). While the overall optimal methodology has yet to be agreed upon, a normal RV LMS has been shown to be in the range of −25% to −30% and to be decreased (generally around −15% to −20%) in PH (28, 29, 31, 32). Speckle-tracking seems to have advantages over other methods of strain imaging, such as tissue Doppler imaging, in that it is angle independent, can be analyzed from routine 2-dimensional images as opposed to specialized image acquisition, and is not as dependent on the selected region of interest. In the current study utilizing speckle tracking, we found a median strain of −27.3% to define RV dysfunction based upon the median value of the cohort. While −27.3% seems to fall within the normal range, the lowest quintile (>-22.05%) was significantly associated with dyspnea, suggesting that this value may be clinically useful in this population, similar to other patient populations (28, 29, 32, 33).
TAPSE was also found to be significantly lower in patients with decreased RV LMS (less than the median value of −27.3%), but interestingly, only by a very small amount (2.44 ± 0.49 vs 2.69 ± 0.45 cm) that is likely not clinically significant and still within the normal range. The lowest quartile of TAPSE was < 2.2 cm, but there was no significant difference in any other measure of RV function except a trend in decreased LVEF (56 ± 9 vs 61 ± 7, P=0.07). As TAPSE has been shown to be diminished with decreased LVEF (34), decreased left ventricular function may explain the altered TAPSE in this population. Unfortunately, we cannot comment on TAPSE ≤ 18 mm, a value which has been defined in the pulmonary arterial hypertension population to be associated with worse outcomes (22), due to the limited numbers in this cohort (only 4 participants).
RV dysfunction can result from PH, which has been associated with HIV infection (1, 2, 13,17). We therefore assessed the relationship of RV function to pulmonary artery pressures. Historically, the prevalence of right heart catheterization-documented PH associated with HIV is reported to be 0.5% (35). However, echocardiographic studies have reported much higher prevalence, ranging from approximately 5% to 57% (36, 37, 38). Some of this variability is likely due to different definitions of PH, but some may be due to limitations of echocardiography. We found estimated systolic PAP > 35 mm Hg to occur in this cohort with a prevalence of 15%. This cut-off value has been used by others for echocardiographic screening of HIV-infected individuals (36, 38) and is slightly more stringent than a value of > 30 mm Hg in other reports (1, 37). However, recent study found that echocardiography was inaccurate in 20% these patients (13). In the current study, not all patients with elevated PA pressure had decreased RV function while not all patients with decreased RV function had elevated PA pressure, suggesting that HIV has separate effects on both pulmonary hemodynamics and RV myocardial function. More specifically, this observation may likely indicate a primary RV myocardial pathological process that is independent from PH. There are several possible mechanisms of isolated RV dysfunction in HIV, which include a direct toxic effect from the virus, perhaps mitigated by genetic variability, opportunistic infections, adverse drug effects on the myocardium or mediated via oxidative stress, autoimmune response, autonomic dysfunction, nutritional deficiencies, and more (2, 39). It is also possible that RV dysfunction may be a more sensitive or specific indicator of true pulmonary hypertension, but right heart catheterization studies are needed to investigate this possibility.
Cardiomyopathy (defined as LV dysfunction) is known to occur in HIV (3, 4, 39). In some cases, the cardiomyopathy may be biventricular or may only affect one ventricle. LV dysfunction is a frequent cause of RV dysfunction, with estimated 20-40% of RV systolic function derived from the LV (40). Also, ventricular interactions can result in reducing ventricular filling via septal shifting from the pressure or volume overloaded ventricle into the other (41, 42). It seems likely that these processes are also at play in the phenotypic expression of HIV cardiomyopathy.
Importantly, we were able to relate symptoms to abnormalities found by echocardiography. Dyspnea was more frequently seen in those found to have RV dysfunction or elevated estimated PA pressure. Those with overlap of these findings were even more likely to be symptomatic. This finding underscores the importance of screening HIV patients with dyspnea via echocardiography. Interestingly, length of HIV infection could not be related to echocardiographic abnormalities, indicating that these abnormalities may occur early in the disease process.
Our study has limitations. Study size was moderate, and results should be confirmed in a larger population. This study was a substudy of a larger prospective trial examining predictors of pulmonary function in HIV and was not primarily designed to answer the question of RV dysfunction in HIV. The current data set did not include invasive hemodynamics to better ascertain RV function as well as pulmonary pressures which would confirm a diagnosis of PH. Ten percent of studies were excluded for poor acoustic windows for imaging the RV free wall. The current study did not evaluate, nor was powered, to evaluate the effect of these measures on clinical outcomes. Measures of RV dysfunction in the absence of clinical symptoms is still of uncertain significance and deserves further study.
In conclusion, the prevalence of global RV dysfunction in this HIV-infected outpatient cohort is 11% when defined echocardiographically by a RVFAC < 35%. The median value of regional RV function defined by RV LMS was −27.3% and the lowest quintile with a value ≥ −22.05% was significantly associated with dyspnea. LV dysfunction marked by LVEF < 50% co-segregated with a mild decrease in TAPSE, which is known to decrease with LV function, but not RV LMS. The prevalence of heightened PAP was 15% and similar to previous reports, but it was not associated with global or regional RV dysfunction. Thus, regional RV dysfunction in HIV may be a separate entity from elevated pulmonary pressures or LV/global cardiomyopathy.. Echocardiography is a useful screening tool for patients with HIV, who have increased risk of cardiac and pulmonary vascular disease.
Acknowledgements
The authors thank Kathy Edelman, research ultrasound sonographer, for performing the echocardiograms.
Sources of Funding This work supported in part by the NIH grants NIH P01HL103455 (MTG, HCC, AM), R01 HL083461 and HL090339 (AM), R01HL098032 (MTG), RO1HL096973 (MTG), Gilead Sciences (AM), the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania (MTG, HCC).
Footnotes
Disclosures Dr. Simon reports receiving research funding from Pfizer, receiving consulting fees or serving on paid advisory boards for United Therapeutics and Actelion. Dr. Morris received grant support from Gilead Sciences.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Speich R, Jenni R, Opravil M, Pfab M, Russi EW. Primary pulmonary hypertension in HIV infection. Chest. 1991;100(5):1268–1271. doi: 10.1378/chest.100.5.1268. [DOI] [PubMed] [Google Scholar]
- 2.Almodovar S, Cicalini S, Petrosillo N, Flores SC. Pulmonary hypertension associated with HIV infection: Pulmonary vascular disease: The global perspective. Chest. 2010;137(6 suppl):6S–12S. doi: 10.1378/chest.09-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Currie PF, Jacob AJ, Foreman AR, Elton RA, Brettle RP, Boon NA. Heart muscle disease related to HIV infection: Prognostic implications. BMJ. 1994;309:1605–7. doi: 10.1136/bmj.309.6969.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacob AJ, Sutherland GR, Bird AG, Brettle RP, Ludlam CA, McMillan A, et al. Myocardial dysfunction in patients infected with HIV: Prevalence and risk factors. Br Heart J. 1992;68:549–53. doi: 10.1136/hrt.68.12.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lebech AN, Gerstoft J, Hesse B, Peterse CL, Kjaer A. Right and left ventricular cardiac function in a developed world population with human immunodeficiency virus studied with radionuclide ventriculography. Am Heart J. 2004;147(3):482–488. doi: 10.1016/j.ahj.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 6.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Survival in patients with primary pulmonary hypertension: results from a national prospective registry. Ann Intern Med. 1991;115(5):343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin VV, Presberg KW, Doyle RL, Abman SH, McCrory DC, Fortin T, et al. Prognosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:78S–92S. doi: 10.1378/chest.126.1_suppl.78S. [DOI] [PubMed] [Google Scholar]
- 8.de Groote P, Millaire A, Foucher-Hossein C, Nugue O, Marchandise X, Ducloux G, et al. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J Am Coll Cardiol. 1998;32(4):948–54. doi: 10.1016/s0735-1097(98)00337-4. [DOI] [PubMed] [Google Scholar]
- 9.Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37(1):183–8. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 10.Sakata K, Yoshino H, Kurihara H, Iwamori K, Houshaku H, Yanagisawa A, et al. Prognostic significance of persistent right ventricular dysfunction as assessed by radionuclide angiocardiography in patients with inferior wall acute myocardial infarction. Am J Cardiol. 2000;85(8):939–44. doi: 10.1016/s0002-9149(99)00905-4. [DOI] [PubMed] [Google Scholar]
- 11.Bonow RO, Carabello B, de Leon AC, Jr, Edmunds LH, Jr, Fedderly BJ, Freed MD, et al. ACC/AHA guidelines for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients With Valvular Heart Disease) J Am Coll Cardiol. 1998;32:1486–588. doi: 10.1016/s0735-1097(98)00454-9. [DOI] [PubMed] [Google Scholar]
- 12.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol. 2009;53(17):1573–619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Selby Van N, Scherzer R, Barnett CF, MacGregor JS, Morelli J, Donovan C, et al. Doppler echocardiography does not accurately estimate pulmonary artery systolic pressure in HIV-infected patients. AIDS. 2012;26(15):1967–1969. doi: 10.1097/QAD.0b013e3283579653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badesch DB, Raskob GE, Elliott CG, Kirchman AM, Farber HW, Frost AE, et al. Pulmonary arterial hypertension: Baseline characteristics from the REVEAL Registry. Chest. 2010;137(2):376–387. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 15.Palevsky HI. The early diagnosis of pulmonary arterial hypertension: Can we do better? Chest. 2011;140(1):4–6. doi: 10.1378/chest.11-1149. [DOI] [PubMed] [Google Scholar]
- 16.Gingo MR, George MP, Kessinger CJ, Lucht L, Rissler B, Weinman R, et al. Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. Am J Respir Crit Care Med. 2010;182:790–796. doi: 10.1164/rccm.200912-1858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris A, Gingo MR, George MP, Lucht L, Kessinger C, Singh V, et al. Cardiopulmonary function in individuals with HIV infection in the antiretroviral therapy era. AIDS. 2012;26(6):731–40. doi: 10.1097/QAD.0b013e32835099ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry WL, DeMaria A, Gramiak R, King DL, Kisslo JA, Popp RL, et al. Report of the American Society of Echocardiography committee on nomenclature and standards in two-dimensional echocardiography. Circulation. 1980;62(2):212–217. doi: 10.1161/01.cir.62.2.212. [DOI] [PubMed] [Google Scholar]
- 19.Quiñones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: A report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15(2):167–184. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 20.Schenk P, Globits S, Koller J, Brunner C, Artemiou O, Klepetko W, et al. Accuracy of echocardiographic right ventricular parameters in patients with different end-stage lung diseases prior to lung transplantation. J Heart Lung Transplant. 2000;19(2):145–154. doi: 10.1016/s1053-2498(99)00121-7. [DOI] [PubMed] [Google Scholar]
- 21.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70(4):657–662. doi: 10.1161/01.cir.70.4.657. [DOI] [PubMed] [Google Scholar]
- 22.Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006;174(9):1034–41. doi: 10.1164/rccm.200604-547OC. [DOI] [PubMed] [Google Scholar]
- 23.Tei C, Dujardin KS, Hodge DO, Bailey KR, McGoon MD, Tajik AJ, et al. Doppler echocardiographic index for assessment of global right ventricular function. J Am Soc Echocardiogr. 1996;9(6):838–47. doi: 10.1016/s0894-7317(96)90476-9. [DOI] [PubMed] [Google Scholar]
- 24.Ryan T, Petrovic O, Dillon JC, Feigenbaum H, Conley MJ, Armstrong WF. An echocardiographic index for separation of right ventricular volume and pressure overload. J Am Coll Cardiol. 1985;5(4):918–27. doi: 10.1016/s0735-1097(85)80433-2. [DOI] [PubMed] [Google Scholar]
- 25.Bushuev VI, Miasnikova GY, Sergueeva AI, Polyakova LA, Okhotin D, Gaskin PR, et al. Endothelin-1, vascular endothelial growth factor and systolic pulmonary artery pressure in patients with Chuvash polycythemia. Haematologica. 2006;91(6):744–9. [PubMed] [Google Scholar]
- 26.Karavidas A, Tsiachris D, Lazaros G, Xylomenos G, Arapi S, Potamitis N, et al. Doppler tissue imaging unmasks right ventricular function abnormalities in HIV-infected patients. Cardiol J. 2010;17(6):587–93. [PubMed] [Google Scholar]
- 27.Sachdev A, Villarraga HR, Frantz RP, McGoon MD, Hsiao JF, Maalouf JF, et al. Right ventricular strain for prediction of survival in patients with pulmonary arterial hypertension. Chest. 2011;139(6):1299–309. doi: 10.1378/chest.10-2015. [DOI] [PubMed] [Google Scholar]
- 28.Fukuda Y, Tanaka H, Sugiyama D, Ryo K, Onishi T, Fukuya H, et al. Utility of right ventricular free wall speckle-tracking strain for evaluation of right ventricular performance in patients with pulmonary hypertension. J Am Soc of Echocardiogr. 2011;24(10):1101–8. doi: 10.1016/j.echo.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Pirat B, McCulloch ML, Zoghbi WA. Evaluation of global and regional right ventricular systolic function in patients with pulmonary hypertension using a novel speckle tracking method. Am J Cardiol. 2006;98(5):699–704. doi: 10.1016/j.amjcard.2006.03.056. [DOI] [PubMed] [Google Scholar]
- 30.Teske AJ, De Boeck BWL, Melman PG, Sieswerda GT, Doevendans PA, Cramer MJM. Echocardiographic quantification of myocardial function using tissue deformation imaging, a guide to image acquisition and analysis using tissue Doppler and speckle tracking. Cardiovasc Ultrasound. 2007;5:27. doi: 10.1186/1476-7120-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tong C, Li C, Song J, Liu H, Deng Y. Assessment of right ventricular free wall longitudinal myocardial deformation using speckle tracking imaging in normal subjects. J Huazhong Univ Sci Technolog Med Sci. 2008;28(2):194–6. doi: 10.1007/s11596-008-0220-8. [DOI] [PubMed] [Google Scholar]
- 32.Schattke S, Knebel F, Grohmann A, Dreger H, Kmezik F, Riemekasten G, et al. Early right ventricular systolic dysfunction in patients with systemic sclerosis without pulmonary hypertension: A Doppler Tissue and Speckle Tracking echocardiography study. Cardiovasc Ultrasound. 2010;8:3. doi: 10.1186/1476-7120-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meris A, Faletra F, Conca C, Klersy C, Regoli F, Klimusina J, et al. Timing and magnitude of regional right ventricular function: A speckle tracking-derived strain study of normal subjects and patients with right ventricular dysfunction. J Am Soc Echocardiogr. 2010;23(8):823–31. doi: 10.1016/j.echo.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 34.López-Candales A, Rajagopalan N, Saxena N, Gulyasy B, Edelman K, Bazaz R. Right ventricular systolic function is not the sole determinant of tricuspid annular motion. Am J Cardiol. 2006;98(7):973–7. doi: 10.1016/j.amjcard.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 35.Sitbon O, Lascoux-Combe C, Delfraissy JF, Yeni PG, Raffi F, Zuttere DD, et al. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med. 2008;177:108–113. doi: 10.1164/rccm.200704-541OC. [DOI] [PubMed] [Google Scholar]
- 36.Quezada M, Martin-Carbonero L, Soriano V, Vispo E, Valencia E, Moreno V, et al. Prevalence and risk factors associated with PH in HIV-infected patients on regular follow-up. AIDS. 2012;26(11):1387–92. doi: 10.1097/QAD.0b013e328354f5a1. [DOI] [PubMed] [Google Scholar]
- 37.Mondy K, Gottdiener J, Overton T, Henry K, Bush T, Conley L, et al. High prevalence of echocardiographic abnormalities among HIV- infected persons in the era of highly active antiretroviral therapy. Clin Infect Dis. 2011;52:378–386. doi: 10.1093/cid/ciq066. [DOI] [PubMed] [Google Scholar]
- 38.Reinsch N, Buhr C, Krings P, Kaelsch H, Kahlert P, Konorza T, et al. Effect of gender and highly active antiretroviral therapy on HIV-related pulmonary arterial hypertension: Results of the HIV-HEART Study. HIV Med. 2008;9(7):550–6. doi: 10.1111/j.1468-1293.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 39.Sani MU. Myocardial disease in human immunodeficiency virus (HIV) infection: a review. Wien Klin Wochenschr. 2008;120(3-4):77–87. doi: 10.1007/s00508-008-0935-3. [DOI] [PubMed] [Google Scholar]
- 40.Santamore WP, Dell’Italia LJ. Ventricular interdependence: Significant left ventricular contributions to right ventricular systolic function. Prog Cardiovasc Dis. 1998;40(4):289–308. doi: 10.1016/s0033-0620(98)80049-2. [DOI] [PubMed] [Google Scholar]
- 41.Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008;117(11):1436–48. doi: 10.1161/CIRCULATIONAHA.107.653576. [DOI] [PubMed] [Google Scholar]
- 42.Gan CTJ, Lankhaar JW, Marcus JT, Westerhof N, Marques KM, Bronzwaer JGF, et al. Impaired left ventricular filling due to right-to-left ventricular interaction in patients with pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol. 2006;290:H1528–33. doi: 10.1152/ajpheart.01031.2005. [DOI] [PubMed] [Google Scholar]