Abstract
Alcohol is a rich drug affecting both the γ-amino butyric acid (GABA) and glutamatergic neurotransmitter systems. Recent findings from both modeling and pharmacological manipulation have indicated a link between GABAergic activity and oscillations measured in the gamma frequency range (30–80 Hz), but there are no previous reports of alcohol's modulation of gamma-band activity measured by magnetoencephalography (MEG) or electroencephalography (EEG). In this single-blind, placebo-controlled crossover study, 16 participants completed two study days, on one day of which they consumed a dose of 0.8 g/kg alcohol, and on the other day a placebo. MEG recordings of brain activity were taken before and after beverage consumption, using visual grating and finger abduction paradigms known to induce gamma-band activity in the visual and motor cortices respectively. Time–frequency analyses of beamformer source reconstructions in the visual cortex showed that alcohol increased peak gamma amplitude and decreased peak frequency. For the motor task, alcohol increased gamma amplitude in the motor cortex. These data support the notion that gamma oscillations are dependent, in part, on the balance between excitation and inhibition. Disruption of this balance by alcohol, by increasing GABAergic inhibition at GABAA receptors and decreasing glutamatergic excitation at N-methyl-D-aspartic acid receptors, alters both the amplitude and frequency of gamma oscillations. The findings provide further insight into the neuropharmacological action of alcohol.
INTRODUCTION
Alcohol affects many neurotransmitter systems via the disruption of receptor functioning. The two most universal effects are blockade of glutamatergic N-methyl-D-aspartic acid (NMDA) receptors to induce brain-wide decrease in excitation (Grant and Lovinger, 1995; Valenzuela, 1997), and enhancement of γ-amino butyric acid (GABA) type A receptors to increase post-synaptic chloride ion flux and hyperpolarization (see Weiner and Valenzuela, 2006 for a comprehensive review). Thus, in vitro, ethanol is known to increase the amplitude and duration of evoked GABAA inhibitory post-synaptic potentials (IPSPs) and inhibitory post-synaptic current (IPSC) in a slice of the rat central amygdala nucleus (Roberto et al, 2003; Wan et al, 1996). Such alterations are likely to disrupt the fine balance between excitation and inhibition throughout the brain, but the effect of alcohol on dynamic cortical circuits in vivo in humans is not well understood.
Evidence from modeling, multimodal imaging, and pharmacological intervention suggests that changes to synchronous oscillations in the gamma frequency band (30–80 Hz), although they do not directly measure neuronal firing, can be an indicator of disruption to the excitation/inhibition balance, as gamma oscillations are thought to be underpinned by reciprocally connected networks of inhibitory GABAergic interneurons and excitatory glutamatergic pyramidal cells (Buzsáki and Wang, 2012). At a microcircuit level, active GABAergic synapses transiently decrease the probability of pyramidal cell firing, following which synchronized firing of spikes and local field potential oscillations occur (Gonzalez-Burgos and Lewis, 2008). Since alcohol is expected to produce an increase in IPSC decay time, it would lengthen the return time from inhibition and therefore lower the oscillatory frequency. Consistent with this, pharmacological manipulation of GABAergic function in vitro by the barbiturate thiopental reduced the frequency of both fast gamma (>70 Hz) and slow gamma (30–70 Hz) oscillations in rat visual cortex (Oke et al, 2010).
A further prediction is that lower frequencies could facilitate recruitment of pyramidal cells into the network, which would increase oscillatory power (Gonzalez-Burgos and Lewis, 2008). Although this prediction is counter to the intuition that enhancing inhibition should reduce power, it is supported by evidence that benzodiazepines acting at the GABAA receptor increase resting gamma power in occipital and pre-frontal areas (diazepam, Hall et al, 2010), in addition to decreasing alpha power in the visual cortex (lorazepam, Ahveninen et al, 2007) and increasing beta power, and decrease beta frequency in sensori-motor cortex (Jensen et al, 2005).
Very few articles have studied alcohol intoxication using MEG/EEG (eg Kovacevic et al, 2012; Marinkovic et al, 2012; Nikulin et al, 2005), and these have focused on changes to beta-, alpha-, and theta-band oscillations. As such alcohol's effects on gamma activity are incompletely known. Moreover, previous evidence from pharmacological interventions is mixed. Cortical responses to visual stimuli consist of both phase-locked evoked responses and non-phase-locked induced responses. GABA transporter-1 (GAT-1) blockade by tiagabine decreased only evoked responses, whereas no changes in induced gamma power and frequency were detected across placebo and drug conditions (Muthukumaraswamy et al, 2013a). Sedation by propofol (a GABAA agonist) significantly increased induced sustained gamma amplitudes and simultaneously decreased the evoked response (Saxena et al, 2013). Both benzodiazepines and propofol are positive allosteric modulators of the GABAA receptor, which increase chloride ion flux and induce hyperpolarization of the post-synaptic neuron. Tiagabine, on the other hand, acts to increase endogenous GABA levels via GAT-1 blockade. It is possible that these differences in mechanism are responsible for the differences in influence on the gamma response, but exactly how is still unknown.
In motor cortex, simple digit movements induce transient gamma-band frequency oscillations (movement-related gamma synchronization, MRGS; Cheyne et al, 2008), as well as post-movement beta-rebound (PMBR) and beta event-related desynchronization (beta-ERD) in sensori-motor areas (Jurkiewicz et al, 2006). It is likely that each component is generated by anatomically separate cortical circuits (Pfurtscheller and Lopes da Silva, 1999). Benzodiazepines and tiagabine have both been reported to enhance movement induced beta-ERD activity, and tiagabine also reduced PMBR. Surprisingly, neither drug appeared to modulate MRGS (Hall et al, 2010; Jensen et al, 2005; Muthukumaraswamy et al, 2013b).
The current study employed two tasks: a gamma-inducing visual grating paradigm and a simple motor task known to induce gamma- and beta-band activity. These were completed in both pre- and post-drink MEG recording sessions. A simple saccadic eye-movement task was also included to measure sedation.
MATERIALS AND METHODS
Participants and Screening
Sixteen volunteers (eight male, mean age 25.9 years, SD 3.8, mean body weight 75.7 kg, SD 12.7) were recruited after informed consent (procedures approved by Cardiff University School of Psychology Ethics Committee). Participants had no known allergy to alcohol and were taking no medication that was affected by alcohol consumption. All participants abstained from alcohol for 12 h before participation and gave a breath alcohol concentration (BrAC) of 0 μg/100 ml on arrival. Reported mean consumption was moderate, males: 23.9 (9.7), females: 16.5 (5.1) UK units per week (1 unit=8 g ethanol; therefore mean male consumption=191.2 g, females=132 g per week). Participants were screened for alcohol dependence using the Alcohol Use Disorders Identification Test (AUDIT; Babor et al, 2001) and the Severity of Alcohol Dependence Questionnaire (SADQ; Stockwell et al, 1983); scores were reasonably low (AUDIT 8.4 (2.8); SADQ 6.1 (3.9)) and below the alcohol dependence threshold (⩾16). None of the participants reported depression or anxiety symptoms in the Hospital Anxiety and Depression Scale (HADS; Zigmond and Snaith, 1983) at the time of testing (anxiety mean=4.1 (2.2), depression mean=1.7 (3.0)). For saccadic eye movements and the motor task only 14 full data sets were acquired, due to technical difficulties. After screening of data quality by an observer blind to condition (poor data quality defined as low-amplitude gamma response with no clear peak in at least one of the four conditions (pre/post, placebo/alcohol)), four participants were excluded from statistical analyses of visual gamma. For transient visual responses, data from an additional participant were removed using the same criteria. Individual participant fits and excluded participants can be seen in Supplementary Figures S1A–F.
Alcohol Dose and Administration
Participants attended two testing days separated by atleast 24 h. On one testing day, after the initial scanning session, participants were given a dose of alcohol in the form of 40% alcohol by volume vodka; males received 0.8 g/kg of body weight, while females were given 90% of this dose due to differences in body water content (Sutker et al, 1983; Brumback et al, 2007)). This was made up to a 500 ml solution with a carbonated citrus juice drink (Orangina) and divided into 10 equal aliquots of 50 ml each. Participants consumed one aliquot every 3 min and then waited for 15 min to allow absorption of the alcohol. In the placebo condition, participants were given 10 × 50 ml aliquots of Orangina with the rim of the glass sprayed with alcohol and a few drops of alcohol floated on top of the drink (Rose and Duka, 2008). Experimenters were not blind to the experimental intervention.
Procedure
On each testing day participants completed a breathalyzer measurement, were weighed, ate a small sandwich (filling depended upon dietary restrictions, mode calorie content: 427 kcal, range: 359–473 kcal; mode fat content: 23.4 g, range: 22.9–26.6 g) and completed the AUDIT, SADQ, mini-international neuropsychiatric interview (MINI; non-alcohol substance abuse section) and HADS questionnaires. They were then fitted with MEG coils and electrodes, which they kept on for the remainder of the session. Participants then completed a ‘pre-drink' MEG recording. Following this, participants completed the drink challenge as described above. After providing a breathalyzer measurement at 15 min from the last drink, participants completed the ‘post-drink' MEG recording, after which a further breathalyzer measurement was taken at 1 h from the last drink, as well as psychological measures of the Biphasic Alcohol Effects Scale (BAES; Martin et al, 1993) and Subjective High Assessment Scale (SHAS; Schuckit, 1980).
Visual task
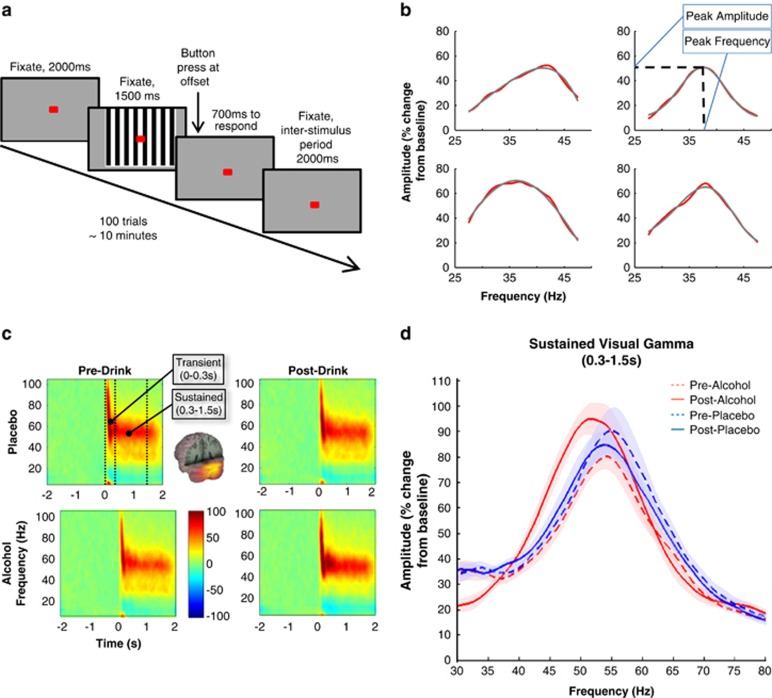
Participants were presented with a vertical, stationary, maximum contrast, three cycles per degree, square-wave grating (8° visual angle) presented on a mean luminance background with a central fixation point (Muthukumaraswamy and Singh, 2013). The screen was positioned centrally at eye level. For 100 trials the stimulus was presented for 1500 ms and a button-press response was given at its offset with right-hand index finger to maintain concentration. Participants were given 750 ms to respond and warned when no or late responses were given. This response period was followed by a 2000-ms inter-stimulus-interval (ISI) (see Figure 1a).
Figure 1.
(a) Paradigm for visual task. (b) An example of skewed Gaussian function fitting to visual data (red) from one participant. Peak amplitude and corresponding frequency are taken from the fitted function (gray). (c) Time–frequency spectrograms of visual task responses. The location of transient responses, thought to be generated from long-ranging bottom-up connections from the thalamus upward to the cortex (Castelo-Branco et al, 1998), and sustained responses, most likely generated by intracortical mechanisms reflecting local cortical circuit activity (Castelo-Branco et al, 1998). Grand-averaged source activity is presented on a 3D-rendered MNI template brain, indicating the stimulus-induced increase in gamma power is located in the primary visual cortex. (d) Grand-averaged amplitude by frequency plots of raw, non-fitted sustained visual gamma responses for each condition. Shaded areas represent ±1 within-subject standard error.
Motor task
Participants performed 100 trials of a cued finger movement task, similar to that described in Muthukumaraswamy (2010) and Muthukumaraswamy et al (2013b). The participants were required to perform ballistic abductions of the right-hand index finger at the onset of an auditory tone pip (same volume for all participants) played through insert headphones (4.5 s ISI) placed by the participant. All participants confirmed that they could hear the tone before the experiment began. The participants' right index finger slightly rested against a small piece of plastic that was attached to an optical displacement system. After the auditory pip (1.5 s), the participants received on-screen feedback with a ‘virtual ruler' for 1 s, indicating how far they had moved relative to a target movement criterion (10 mm).
The visual and motor tasks were presented on a Mitsubishi Diamond Pro 2070 monitor controlled by the Psychophysics Toolbox (Brainard, 1997; Pelli, 1997). The screen size was 1024 by 768 pixels and the monitor frame rate was 100 Hz. The monitor was outside the magnetically shielded room and viewed at 2.15 m through a cut-away portal in the shield.
Saccadic eye movement (SEM)
As an objective measure of sedation, we measured the velocity of 50 saccadic eye movements (Lehtinen et al, 1979), based on a task by Ball et al (1991). Electrooculography (EOG) measurements were used to quantify this velocity. Participants fixated on a red square that alternated from left to right every 1500 ms, prompting 30° saccades along the horizontal mid-point. Stimuli were projected onto a screen at 80 cm viewing distance.
MEG Acquisition
Whole-head MEG recordings were made using a CTF 275-channel radial gradiometer system sampled at 1200 Hz (0–300 Hz bandpass). An additional 29 reference channels were recorded for noise cancellation purposes and the primary sensors were analyzed as synthetic third-order gradiometers (Vrba and Robinson, 2001). Three of the 275 channels were turned off due to excessive sensor noise. Participants were fitted with three electromagnetic head coils (naison and pre-auriculars), which were localized relative to the MEG system immediately before and after the recording session for each task. Participants were also fitted with EOG electrodes, above and below the pupil of the right eye, and 1 cm lateral to the outer canthus of each eye. For the motor task, a bipolar electromyogram was recorded from right dorsal interosseus. EOG and EMG recordings were sampled simultaneously with the MEG recordings. All participants had completed a 1-mm isotropic T1-weighted FSPGR image on the same 3-T full-body GE MRI scanner prior to participation, as part of a different study, to be used for MEG/MRI co-registration. Fiduciary markers were placed on the MR image corresponding to the positions of the electromagnetic head coils as ascertained through photographs of the participants on the day of testing.
Data Analysis
Visual task data were epoched from −2 s before to 2 s after the stimulus onset. For the motor task, data pre-processing was similar to our previous work (Hamandi et al, 2011; Muthukumaraswamy, 2010). In short, EMG onsets were marked using an automated algorithm that marked increases in the rectified EMG signal by 3SDs above the noise floor (Cheyne et al, 2008), subject to the constraint that they occurred within 750 ms of the tone pip. Data were then epoched from 1.5 s before to 3.0 s after the EMG markers. For both tasks each trial was visually inspected and discarded if there were excessive MEG signal artifacts (eg head movements/jaw clenches, blinks); motor trials were further inspected for irregular movement displacements (eg double movements) or irregular EMG activity. Mean number of trials analyzed—visual task: pre-alcohol 82.6 (SD=17.8), post-alcohol 85 (SD=11.2), pre-placebo 82.4 (SD=14.5), post-placebo 77.2 (SD=16.1); motor task: pre-alcohol 83.9 (SD=6.4), post-alcohol 86.3 (SD=10.9), pre-placebo 85.9 (SD=9.1), post-placebo 83.5 (SD=10.3).
Synthetic aperture magnetometry (SAM; Robinson and Vrba, 1999) was used for source localization in the gamma frequency band (visual task: 30–80 Hz, Gaetz et al, 2012; motor task: 60–90 Hz, Muthukumaraswamy et al, 2013b). Additional SAM source localization was conducted in the beta-band (15–30 Hz, Muthukumaraswamy et al, 2013b). Global covariance matrices were generated for each bandpass-filtered data set and beamformer weights were calculated for the whole brain at a 4-mm isotropic voxel resolution using the beamformer algorithm (Robinson and Vrba, 1999).
For the visual data Student t-images of source power changes were calculated using a baseline period of −1.5 to 0 s and an active period of 0 to 1.5 s. The voxel with the largest power increase in the gamma frequency band was located in the occipital lobe for each recording for each participant. In order to generate a time–frequency representation of the stimulus response, the virtual sensor at this voxel was repeatedly band-pass filtered between 1 and 100 Hz at 0.5 Hz frequency step intervals with an 8-Hz bandwidth (third-order Butterworth filter, Van Quyen et al, 2001) and at each frequency step the amplitude envelope was calculated from the analytic signal using the Hilbert transform. A similar analysis was performed on the motor data but using the following times (as used in Muthukumaraswamy et al, 2013b): baseline MRGS=−1.3 to −1 s, active MRGS=0 to 0.3 s, baseline beta-ERD=−1.25 to −0.5 s, active beta-ERD=−0.25 to 0.5 s, baseline PMBR=−1.25 to 0.5 s, active PMBR=1 to 1.75 s. Time–frequency spectra were computed as a percentage change from the pre-stimulus baselines by frequency band. MEG auditory responses to the tone pip were not analyzed as they are known to not contaminate gamma-band responses in the motor cortex (Muthukumaraswamy, 2010).
For the production of grand-average SAM maps, individual SAM images were first spatially normalized onto the MNI (T1) average brain using FMRIB's Linear Affine Registration Tool (Jenkinson and Smith, 2001). This was done by first obtaining a set of warping parameters by registering the participant's anatomical MRI with the average brain and then applying these parameters to the SAM source power maps.
Visual time–frequency spectra were split into two epochs: transient responses (0.0–0.3 s from stimulus onset) and sustained responses (0.3–1.5 s), as typical for this kind of stimulus (Swettenham et al, 2009). The amplitude spectrum for each of these epochs was calculated by averaging the time–frequency maps over these respective time ranges and skewed Gaussian functions were fit to a 20-Hz window centered on the average peak frequency across conditions for each participant, in order to remove noise in the estimation of peak frequencies (Figure 1b and Supplementary Figures S1A–F for individual participant fits). For each visual time–frequency epoch, peak amplitude and corresponding frequency were taken from the fitted functions. For the motor MRGS response, peak frequency and amplitude were taken from the fitted functions.
Source-level evoked responses were calculated from visual virtual sensor data. A low-pass filter of 40 Hz and a baseline period of −0.2 to 0 s were applied. For group-level analysis, to ensure all virtual sensors had the same polarity, data were assigned polarity based on the 80-ms component direction.
For motor beta values, mean power collapsed across 15–30 Hz for the respective active and baseline periods for each participant were calculated.
All statistical analyses were performed using 2 (drug: placebo/alcohol) by 2 (time: pre-drink/post-drink) within-subjects ANOVAs, with the interaction term being of most interest. Within-subject standard errors are used to express variance throughout.
RESULTS
Confirmation of Intoxication
Participants reached a mean peak BrAC of 36.4 μg/100 ml (SD=6.2 μg/100 ml). Saccadic eye movements could be analyzed for 14 participants, and as expected there was an alcohol-induced slowing of eye-movement velocity compared to placebo (significant drug*time interaction: F(1,13)=15.92, p=0.002). In the subjective questionnaires, 13 full data sets were analyzed, and three were incomplete. Significant differences were observed between the placebo and alcohol conditions for both sedative (F(1,12)=35.32, p<0.001) and stimulant feelings (F(1,12)=6.28, p=0.028) measured by the BAES. A significant difference was also observed between placebo and alcohol for the SHAS (F(1,12)=27.66, p<0.001). Reaction time to the offset of visual stimuli was also slower following intoxication, while it was faster following placebo (drug*time interaction F(1,15)=5.70, p=0.031). No significant drug*time interactions were observed from behavioral motor data for both peak movement displacement (F(1,13)=0.114, p=0.741) and the latency at which peak displacement was reached (F(1,13)<0.000, p=0.993). Descriptive statistics for these effects can be found in Supplementary Table S1C.
Visual Gamma
As indicated in Figure 1d, significant drug*time interactions were found for both peak amplitude (F(1,11)=5.317, p=0.042) and frequency (F(1,11)=13.31, p=0.004) of sustained visual gamma responses, such that alcohol increases visual gamma peak amplitude and decreases mean peak frequency. Grand-averaged time–frequency spectrograms for peak gamma-band locations for each condition are presented in Figure 1c. The increase in amplitude can be observed in the spectrogram for the post-alcohol condition as a darker red color.
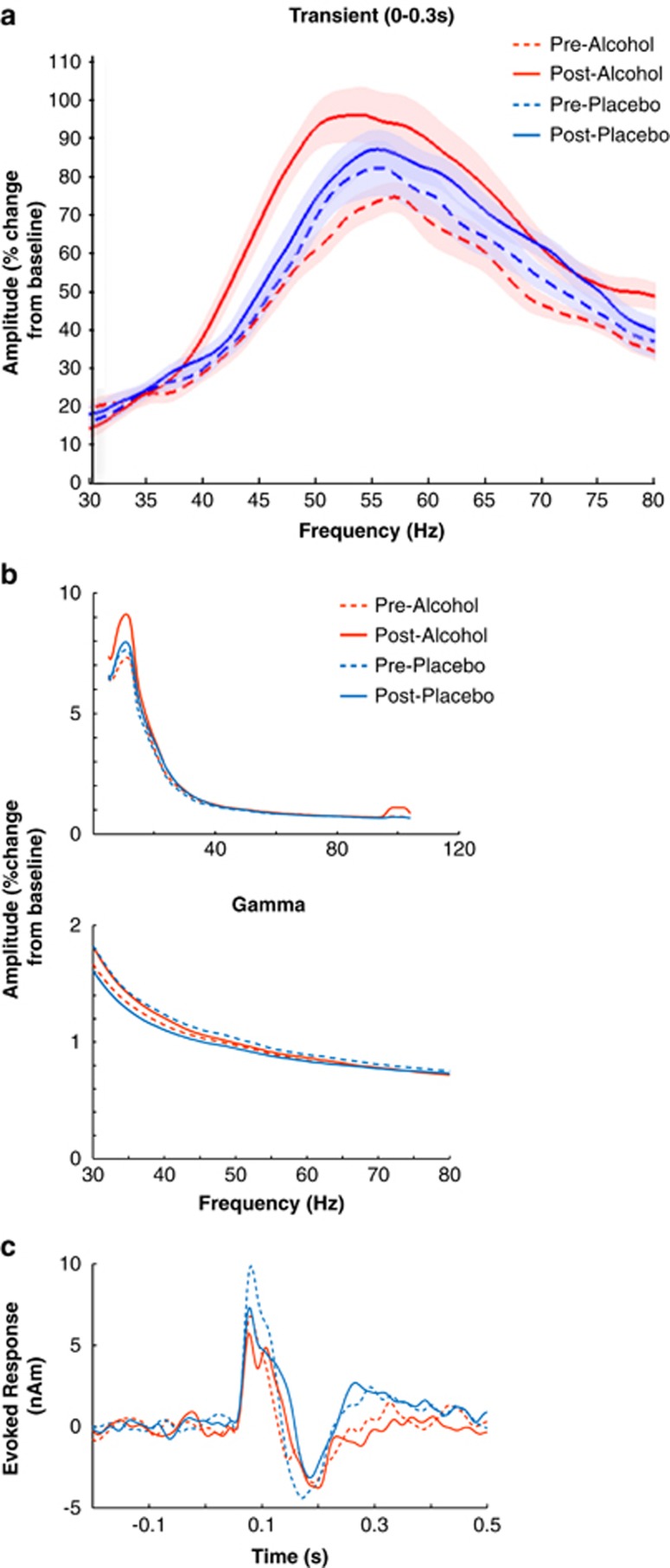
The spectrograms also show that preceding the narrow-band sustained gamma response there is an initial broadband transient gamma response, which is typically present for this type of visual stimulus, though less reliable (Swettenham et al, 2009). As for the sustained data, the mean transient peak frequency decreased with alcohol (F(1,10)=5.50, p=0.041, Figure 2a), but the drug*time interaction for amplitude, though in the same direction, failed to reach significance (F(1,10)=3.99, p=0.074).
Figure 2.
(a) Grand-averaged amplitude by frequency plots of raw, non-fitted transient visual gamma responses for each condition. Shaded areas represent ±1 within-subject standard error. (b) Amplitude by frequency plots of pre-stimulus baseline period activity. The bottom figure indicates only gamma-band activity. (c) Visual evoked responses.
Activity within the pre-stimulus baseline period showed a possible elevation of alpha power in the post-alcohol condition (F(1,11)=3.904, p=0.074) and no differences in the gamma-band (F(1,11)=1.04, p=0.331; see Figure 2b, descriptive statistics in Supplementary Tables S1A and B). Analysis of evoked responses found no differences between pre- and post-drink recordings for both alcohol and placebo, see Figure 2c.
Motor Gamma
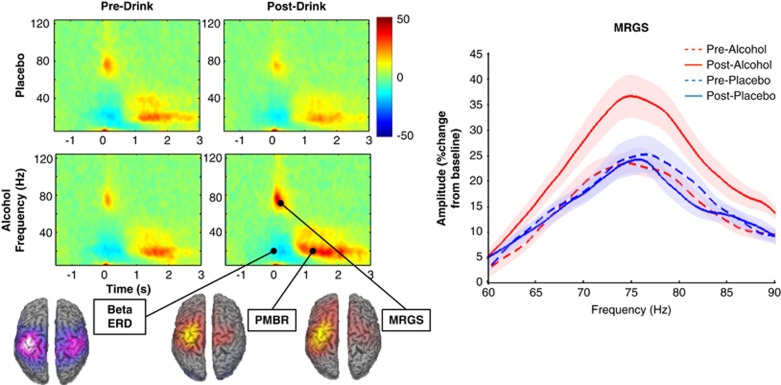
A significant drug*time interaction was found for peak MRGS amplitude (F(1,13)=9.46, p=0.009) but no significant interaction was observed for frequency (F(1,13)=2.02, p=0.179) (Figure 3). Peak gamma amplitude increased under the influence of alcohol.
Figure 3.
Grand-averaged time–frequency spectrograms of motor responses for each condition and a map of grand-averaged source activity across all conditions shown on MNI template brains. For MRGS responses a power by frequency plot of average non-fitted data is presented with shaded within-subject error. There is a clear alcohol-induced increase in amplitude.
Grand-averaged time–frequency spectrograms for the activity recorded during the motor task are shown in Figure 3. The spectrograms display a typical response: a transient gamma response (MRGS) in the 60–90 Hz range at 0–0.3 s, beta-ERD evident at −0.25 to 0.5 s and the sustained PMBR at 1 to 1.75 s, both in the 15–30 Hz range. Grand-averaged SAM maps indicate the pattern of activity of the MRGS, PMBR, and beta-ERD responses (Figure 3).
No significant differences in mean-gamma amplitude during the baseline period were anticipated; therefore, gamma amplitude was calculated as a percentage change from baseline. However, a near-significant drug*time interaction was observed for baseline gamma (F(1,13)=4.41, p=0.056), non-baselined analyses were conducted, which still revealed a significant drug*time interaction for residual, active–baseline period gamma amplitude (F(1,13)=6.42, p=0.025).
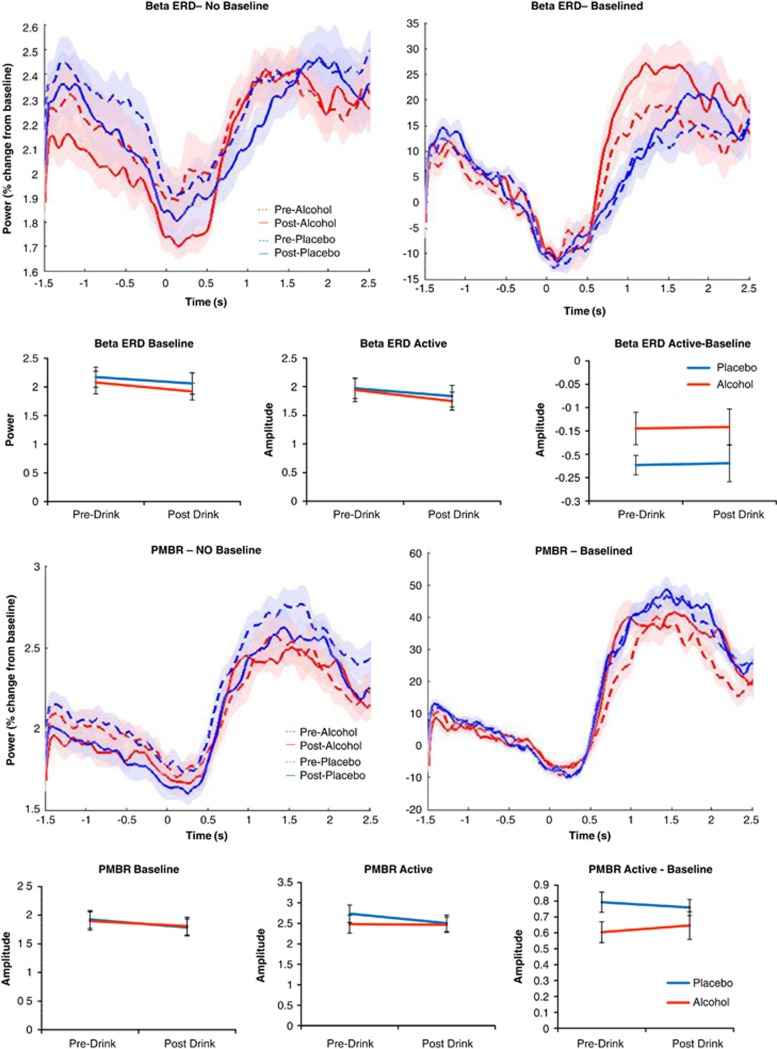
For beta-ERD and PMBR both baselined and non-baselined time–frequency analyses were conducted and revealed that there were differences in baseline period beta power following alcohol ingestion (Figure 4). Using non-baselined data, for both the peak beta-ERD contralateral location and PMBR location there were no significant drug*time interactions for the baseline period (beta-ERD F(1,13)=0.067, p=0.800; PMBR F(1,13)=0.112, p=0.743), the active period (beta-ERD F(1,13)=0.341, p=0.570; PMBR F(1,13)=0.282, p=0.604) or the residual strength, i.e. active—baseline (beta-ERD F(1,13)=0.497, p=0.492; PMBR F(1,13)=0.065, p=0.802).
Figure 4.
Beta event-related desynchronisation (beta-ERD; top) and post-movement beta rebound (PMBR; bottom) data from the motor task. Time by amplitude plots indicate the mean time course of the amplitude of beta activity throughout a trial. Non-baselined plots indicate a discrepancy between conditions in the baseline pre-stimulus period. Plots of mean amplitude across the baseline and active periods and the difference between the two periods indicate no significant interactions between drug and time. Shaded areas and error bars indicate ±1 within-subject standard error.
Exploratory correlational analyses indicated significant correlations between the absolute change in visual gamma frequency from baseline to post-alcohol with BrAC, r=−0.605, n=12, p=0.037. Full correlational matrices can be found in the Supplementary Tables S2A and B.
DISCUSSION
The present experiment examined the effect of a moderate dose of alcohol on temporally organized synchronous neuronal oscillations in human participants. An alcohol-induced increase in peak gamma amplitude was observed for both visual and motor stimulus responses, and a decrease in peak frequency for visual gamma was observed. Since responses were analyzed at posterior sensors, these findings are not likely to be confounded by any alcohol-induced changes in eye movements (Carl et al, 2012). Also, checks for eye-movement-related activity on grand-averaged SAM spatial maps found no evidence of gamma-band activity in areas near extra-ocular muscles. Similar findings to ours are echoed in the in vitro animal literature. For example, under the administration of the barbiturate thiopental, Oke et al (2010) observed an increase in gamma amplitude and a slowing of gamma frequency in rat visual cortex slices.
Positive allosteric modulation of GABAA receptors may be the key driver of changes to synchronous gamma oscillations. Alcohol is known to increase the duration of IPSCs and the amplitude of IPSPs (Roberto et al, 2003; Wan et al, 1996), which in turn is expected to decrease the oscillation frequency of the network (Gonzalez-Burgos and Lewis, 2008), as we observed for visual gamma. The increases in gamma amplitude that we also observed are broadly consistent with the further prediction that there could be greater pyramidal cell recruitment (Gonzalez-Burgos and Lewis, 2008), and also match a previous report that visual gamma amplitude is increased during propofol administration (Saxena et al, 2013). Propofol, like alcohol, is a positive allosteric modulator of the GABAA receptor that similarly alters the IPSCs and IPSPs (Orser et al, 1994). However, with propofol an influence on frequency was not detected, possibly due to two methodological differences. Our experiment used a more optimal visual stimulus, filling a larger proportion of the visual field eliciting a greater amplitude response (Muthukumaraswamy and Singh, 2013). Secondly, we used a fitting procedure, whereas Saxena et al (2013) extracted peak frequency directly from the data, possibly allowing any decrease in frequency to be masked by noise.
Our findings are also in broad concordance with previously observed positive correlations between visual gamma frequency and GABA concentration in the visual cortex measured by magnetic resonance spectroscopy (MRS) (Muthukumaraswamy et al, 2009). During alcohol intoxication MRS has indicated a decrease in GABA concentration (Gomez et al, 2012). Therefore, a decrease in gamma frequency by alcohol fits this trend. However, it is unknown how this pattern should be related to another finding that tiagabine had no detectable effect on amplitude or frequency of visual gamma responses; rather, a reduction of visual evoked responses was detected (Muthukumaraswamy et al, 2013a). Tiagabine acts via the blockade of GAT-1 to increase endogenous GABA levels (Borden et al, 1994). It remains unclear why this should have a different effect on direct enhancement of GABA at GABAA receptors, or on naturally occurring individual differences in GABA levels as measured by MRS. One possibility is that increased concentrations of extracellular GABA may translate to decreased availability for release and/or greater baseline receptor activity could affect network timing or synchrony due to fewer available binding sites for the next release.
For motor gamma responses, pharmaco-MEG investigations using diazepam (Hall et al, 2010) and tiagabine (Muthukumaraswamy et al, 2013b) have found no modulation of the amplitude or frequency of the gamma response. Propofol has not been studied in the context of motor gamma, but, like alcohol, diazepam is a positive allosteric modulator of GABA at GABAA receptors, and thus the apparent lack of any effect contrasts with the clear effect of alcohol on motor gamma amplitude found here. Further, in contrast to the visual-gamma findings, our motor gamma did not show an alcohol-induced alteration to frequency. A possible explanation for this could be the different physiological mechanisms underlying the visual- and motor-gamma responses. Motor-gamma oscillations are thought to be driven subcortically by the subthalamic nucleus (Litvak et al, 2012). This subthalamic drive may make pharmacological manipulations of local circuits in motor area M1 less likely to affect the frequency.
Above we have focussed on the role of GABA, but the action of alcohol on glutamatergic NMDA receptors may also have a critical role. As already mentioned, increases in gamma amplitude may reflect recruitment of additional pyramidal cells in the post-inhibition excitation phase. Alcohol inhibits the excitatory post-synaptic currents (EPSCs) and potentials (EPSPs) induced by NMDA receptors (Lovinger et al, 1989, 1990), further reducing excitation. Counter-intuitively, this could in turn lead to the recruitment of further pyramidal cells, increasing gamma amplitude (Pfurtscheller and Lopes da Silva, 1999; Singer, 1993).
A limitation of our findings is the method for administering alcohol and placebo. Anecdotally, a number of participants reported knowledge of the drink condition they had been assigned on each day, which is impossible to avoid given that participants are familiar with the symptoms of mild alcohol doses. This may have altered their attention during experimental tasks affecting their gamma-band response (Kahlbrock et al, 2012). However, unlike broadband visual gamma, the narrow-band response studied here has previously been shown to be insensitive to attentional manipulation (Koelewijn et al, 2013), suggesting it is purely a bottom-up driven stimulus response. The method of administration was selected because it has been successfully used by a number of studies administering alcohol (Nutt et al, 2007; Rose and Duka, 2008).
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
Special thanks to all who assisted with the running of the study, especially Alex Shaw, Jenny Brealy, and Kacper Wieczorek for assisting with data collection, Anne Lingford-Hughes and Angela Attwood for advice on alcohol administration and breathalyzers, and Geoffrey Mégardon and Brice Dassy for their contribution to developing the Gaussian function fits. Anne Campbell receives a studentship funded by Alcohol Research UK and Cardiff University School of Psychology. This study was supported by CUBRIC and the School of Psychology at Cardiff University, together with the MRC/EPSRC funded UK MEG Partnership Grant (MR/K005464/1).
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Ahveninen J, Lin F, Kivisaari R, Autti T, Hämäläinen M, Stufflebeam S, et al. MRI-constrained spectral imaging of benzodiazepine modulation of spontaneous neuromagnetic activity in human cortex. NeuroImage. 2007;35:577–582. doi: 10.1016/j.neuroimage.2006.12.033. [DOI] [PubMed] [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG.2001The Alcohol Use Disorders Identification Test. Guidelines for Use in Primary Health Care2nd edn, World Health Organisation: Geneva, Switzerland [Google Scholar]
- Ball DM, Glue P, Wilson S, Nutt DJ, Unit CP. Pharmacology of saccadic eye movements in man 1. Psychopharmacology (Berl) 1991;105:361–367. doi: 10.1007/BF02244431. [DOI] [PubMed] [Google Scholar]
- Borden LA, Murali Dhar TG, Smith KE, Weinshank RL, Branchek TA, Gluchowski C. Tiagabine, SK&F 89976-A, CI-966, and NNC-711 are selective for the cloned GABA transporter GAT-1. Eur J Pharmacol. 1994;269:219–224. doi: 10.1016/0922-4106(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- Brumback T, Cao D, King A. Effects of alcohol on psychomotor performance and perceived impairment in heavy binge social drinkers. Drug Alcohol Depend. 2007;91:10–17. doi: 10.1016/j.drugalcdep.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Wang X-J. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203–225. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carl C, Açık A, König P, Engel AK, Hipp JF. The saccadic spike artefact in MEG. NeuroImage. 2012;59:1657–1667. doi: 10.1016/j.neuroimage.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Castelo-Branco M, Neuenschwander S, Singer W. Synchronization of visual responses between the cortex, lateral geniculate nucleus, and retine in the anesthetized cat. J Neucosci. 1998;18:6395–6410. doi: 10.1523/JNEUROSCI.18-16-06395.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyne D, Bells S, Ferrari P, Gaetz W, Bostan AC. Self-paced movements induce high-frequency gamma oscillations in primary motor cortex. Neuroimage. 2008;42:332–342. doi: 10.1016/j.neuroimage.2008.04.178. [DOI] [PubMed] [Google Scholar]
- Gaetz W, Roberts TPL, Singh KD, Muthukumaraswamy SD. Functional and structural correlates of the aging brain: relating visual cortex (V1) gamma band responses to age-related structural change. Hum Brain Mapp. 2012;33:2035–2046. doi: 10.1002/hbm.21339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez R, Behar KL, Watzl J, Weinzimer SA, Gulanski B, Sanacora G, et al. Intravenous ethanol infusion decreases human cortical γ-aminobutyric acid and N-acetylaspartate as measured with proton magnetic resonance spectroscopy at 4 tesla. Biol Psychiatry. 2012;71:239–246. doi: 10.1016/j.biopsych.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis D. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Lovinger DM. Cellular and behavioural neurobiology of alcohol: receptor-mediated neuronal processes. Clin Neurosci. 1995;3:155–164. [PubMed] [Google Scholar]
- Hall SD, Barnes GR, Furlong PL, Seri S, Hillebrand A. Neuronal network pharmacodynamics of GABAergic modulation in the human cortex determined using pharmaco-magnetoencephalography. Hum Brain Mapp. 2010;31:581–594. doi: 10.1002/hbm.20889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamandi K, Singh KD, Muthukumaraswamy S. Reduced movement-related β desynchronisation in juvenile myoclonic epilepsy: a MEG study of task specific cortical modulation. Clin Neurophysiol. 2011;122:2128–2138. doi: 10.1016/j.clinph.2011.04.017. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jensen O, Goel P, Kopell N, Pohja M, Hari R, Ermentrout B. On the human sensorimotor-cortex beta rhythm: sources and modeling. Neuroimage. 2005;26:347–355. doi: 10.1016/j.neuroimage.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Jurkiewicz MT, Gaetz WC, Bostan AC, Cheyne D. Post-movement beta rebound is generated in motor cortex: evidence from neuromagnetic recordings. Neuroimage. 2006;32:1281–1289. doi: 10.1016/j.neuroimage.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Kahlbrock N, Butz M, May ES, Schnitzler A. Sustained gamma band synchronization in early visual areas reflects the level of selective attention. Neuroimage. 2012;59:673–681. doi: 10.1016/j.neuroimage.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Koelewijn L, Rich AN, Muthukumaraswamy SD, Singh KD. Spatial attention increases high-frequency gamma synchronisation in human medial visual cortex. Neuroimage. 2013;79:295–303. doi: 10.1016/j.neuroimage.2013.04.108. [DOI] [PubMed] [Google Scholar]
- Kovacevic S, Azma S, Irimia A, Sherfey J, Halgren E, Marinkovic K. Theta oscillations are sensitive to both early and late conflict processing stages: effects of alcohol intoxication. PLoS One. 2012;7:e43957. doi: 10.1371/journal.pone.0043957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtinen I, Lang AH, Jäntti V, Keskinen E. Acute effects of alcohol on saccadic eye movements. Psychopharmacolgy. 1979;63:17–23. doi: 10.1007/BF00426915. [DOI] [PubMed] [Google Scholar]
- Litvak V, Eusebio A, Jha A, Oostenveld R, Barnes G, Foltynie T, et al. Movement-related changes in local and long-range synchronization in Parkinson's disease revealed by simulatneous megnetoencephalography and intracranial recordings. J Neurosci. 2012;32:10541–10553. doi: 10.1523/JNEUROSCI.0767-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. NMDA receptor-mediated synaptic excitation selectively inhibited by ethanol in hippocampal slice from adult rat. J Neurosci. 1990;10:1372–1379. doi: 10.1523/JNEUROSCI.10-04-01372.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Rosen BQ, Cox B, Kovacevic S. Event-related theta power during lexical-semantic retrieval and decision conflict is modulated by alcohol intoxication: anatomically constrained MEG. Front Psychol. 2012;3:121. doi: 10.3389/fpsyg.2012.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD. Functional properties of human primary motor cortex gamma oscillations. J Neurophysiol. 2010;104:2873–2885. doi: 10.1152/jn.00607.2010. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Edden RAE, Jones DK, Swettenham JB, Singh KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci USA. 2009;106:8356–8361. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Myers JFM, Wilson SJ, Nutt DJ, Hamandi K, Lingford-Hughes A, et al. Elevating endogenous GABA levels with GAT-1 blockade modulates evoked but not induced responses in human visual cortex. Neuropsychopharmacology. 2013;1:1–8. doi: 10.1038/npp.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Myers JFM, Wilson SJ, Nutt DJ, Lingford-Hughes A, Singh KD, et al. The effects of elevated endogenous GABA levels on movement-related network oscillations. Neuroimage. 2013;66:36–41. doi: 10.1016/j.neuroimage.2012.10.054. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Singh KD. Visual gamma oscillations: the effects of stimulus type, visual field coverage and stimulus motion on MEG and EEG recordings. Neuroimage. 2013;69:223–230. doi: 10.1016/j.neuroimage.2012.12.038. [DOI] [PubMed] [Google Scholar]
- Nikulin VV, Nikulina AV, Yamashita H, Rossi EM, Kähkönen S. Effects of alcohol on spontaneous neuronal oscillations: a combined magnetoencephalography and electroencephalography study. Prog Neuro-Psychopharmacol Biol Psychiatry. 2005;29:687–693. doi: 10.1016/j.pnpbp.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Besson M, Wilson SJ, Dawson GR, Lingford-Hughes AR. Blockade of alcohol's amnestic activity in humans by an alpha5 subtype benzodiazepine receptor inverse agonist. Neuropharmacology. 2007;53:810–820. doi: 10.1016/j.neuropharm.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Oke OO, Magony A, Anver H, Ward PD, Jiruska P, Jefferys JGR, et al. High-frequency gamma oscillations coexist with low-frequency gamma oscillations in the rat visual cortex in vitro. Eur J Neurosci. 2010;31:1435–1445. doi: 10.1111/j.1460-9568.2010.07171.x. [DOI] [PubMed] [Google Scholar]
- Orser A, Pennefather PS, Macdonald JF. Propofol modulates activation and desensitization of GABA, receptors in cultured murine hippocampal neurons. J Neurosci. 1994;74:7747–7760. doi: 10.1523/JNEUROSCI.14-12-07747.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10:437–442. [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and post-synaptic sites in rat central amygdala neurons. Proc Natl Acad Sci USA. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SE, Vrba J.1999Functional neuroimaging by synthetic aperture manetometry (SAM)In: Yoshimoto T, Kotani M, Kuriki S, Karibe H, Nakasato N (Eds.)Recent Advances in Biomagnetism, Tohoku University Press: Sendai; 302–305. [Google Scholar]
- Rose AK, Duka T. Effects of alcohol on inhibitory processes. Behav Pharmacol. 2008;19:284–291. doi: 10.1097/FBP.0b013e328308f1b2. [DOI] [PubMed] [Google Scholar]
- Saxena N, Muthukumaraswamy SD, Diukova A, Singh K, Hall J, Wise R. Enhanced stimulus-induced gamma activity in humans during propofol-induced sedation. PLoS One. 2013;8:e57685. doi: 10.1371/journal.pone.0057685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Self-rating of alcohol intoxication by young men with and without family histories of alcoholism. J Stud Alcohol. 1980;41:242–249. doi: 10.15288/jsa.1980.41.242. [DOI] [PubMed] [Google Scholar]
- Singer W. Synchronization of cortical activity and its putative role in information processing and learning. Annu Rev Physiol. 1993;55:349–374. doi: 10.1146/annurev.ph.55.030193.002025. [DOI] [PubMed] [Google Scholar]
- Stockwell T, Murphy D, Hodgson R. Severity of alcohol dependence questionnaire (SADQ) Br J Addict. 1983;78:45–156. doi: 10.1111/j.1360-0443.1983.tb05502.x. [DOI] [PubMed] [Google Scholar]
- Sutker PB, Tabakoff B, Goist KC, Randall CL. Acute alcohol intoxication, mood states and alcohol metabolism in women and men. Pharmacol Biochem Behav. 1983;18 (Suppl 1:349–354. doi: 10.1016/0091-3057(83)90198-3. [DOI] [PubMed] [Google Scholar]
- Swettenham JB, Muthukumaraswamy SD, Singh KD. Spectral properties of induced and evoked gamma oscillations in human early visual cortex to moving and stationary stimuli. J Neurophysiol. 2009;102:1241–1253. doi: 10.1152/jn.91044.2008. [DOI] [PubMed] [Google Scholar]
- Valenzuela CF. Alcohol and neurotransmitter interactions. Alcohol Health Res World. 1997;21:144–148. [PMC free article] [PubMed] [Google Scholar]
- Van Quyen M, Le, Foucher J, Lachaux J, Rodriguez E, Lutz A, Martinerie J, et al. Comparison of Hilbert transform and wavelet methods for the analysis of neuronal synchrony. J Neurosci Methods. 2001;111:83–98. doi: 10.1016/s0165-0270(01)00372-7. [DOI] [PubMed] [Google Scholar]
- Vrba J, Robinson SE. Signal processing in magnetoencephalography. Methods. 2001;25:249–271. doi: 10.1006/meth.2001.1238. [DOI] [PubMed] [Google Scholar]
- Wan FJ, Berton F, Madamba SG, Francesconi W, Siggins GR. Low ethanol concentrations enhance GABAergic inhibitory postsynaptic potentials in hippocampal pyramidal neurons only after block of GABAB receptors. Proc Natl Acad Sci USA. 1996;93:5049–5054. doi: 10.1073/pnas.93.10.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther. 2006;111:533–554. doi: 10.1016/j.pharmthera.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;37:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.