Abstract
The mortality rate of alveolar hemorrhage following allogeneic hematopoietic stem cell transplantation is greater than 60% with supportive care and high dose steroids. We performed a retrospective cohort analysis to assess the benefits and risks of rFVIIa as a therapeutic adjunct for alveolar hemorrhage. From 2005 to 2012, 57 episodes of alveolar hemorrhage occurred in 37 patients. Fourteen episodes (in 14 patients) were treated with steroids alone and 43 episodes (in 23 patients) were treated with steroids and rFVIIa. The median (interquartile range) steroid dose was 1.9 mg/kg/d (0.8 – 3.5; methylprednisolone equivalents) and did not differ statistically between the two groups. The median rFVIIa dose was 41 μg/kg (39-62) and a median of 3 doses (2-17) was administered per episode. Concurrent infection was diagnosed in 65% of the episodes. Patients had moderately severe hypoxia (median PaO2/FiO2, 193 [141-262]); 72% required mechanical ventilation and 42% survived to extubation. The addition of rFVIIa did not alter time to resolution of alveolar hemorrhage (p = 0.50), duration of mechanical ventilation (p = 0.89), duration of oxygen supplementation (p = 0.55), or hospital mortality (p = 0.27). Four possible thrombotic events (9% of 43 episodes) occurred with rFVIIa. rFVIIa when used in combination with corticosteroids did not confer clear clinical advantages compared to corticosteroids alone. In patients with AH following hematopoietic stem cell transplant, clinical factors (i.e. worsening infection, multiple organ failure or recrudescence of primary disease) may be more important than the benefit of enhanced hemostasis from rFVIIa.
Keywords: Hematopoietic stem cell transplantation, diffuse alveolar hemorrhage, glucocorticoids, recombinant human factor VIIa
Introduction
Alveolar hemorrhage (AH) is a life-threatening complication of allogeneic hematopoietic stem cell transplantation (HSCT) that occurs in 2-14% of transplant recipients (1). AH may account for up to one-third of cases of acute respiratory failure requiring mechanical ventilation after HSCT and has a mortality rate of more than 60% (2-7). Factors contributing to the development of AH include graft-versus-host disease (GVHD) and pulmonary endothelial and epithelial cell injury due to chemotherapy, conditioning agents or total body irradiation (8-10). In addition to supportive care (i.e., blood product administration, oxygen therapy, mechanical ventilation), high dose corticosteroids have been routinely used to treat AH after HSCT (11-13).
Recent studies have noted similarities in the clinical presentation, management and high mortality of AH after HSCT in the presence or absence of infection (i.e., infection-associated AH (IAH) and diffuse alveolar hemorrhage (DAH), respectively) (5, 6). The difficulty in clinically distinguishing DAH from IAH highlights the concern that high dose corticosteroids may increase infectious risks and cause harm. As a result, three agents have been used as adjunctive therapies to enhance pulmonary hemostasis and to decrease the dose and duration of corticosteroid therapy; aminocaproic acid (14), tranexamic acid (15), and recombinant human factor VIIa (rFVIIa). Aminocaproic acid and tranexamic acid both inhibit plasmin, thereby impeding fibrinolysis. rFVIIa promotes hemostasis via both a tissue factor-dependent pathway at sites of endothelial injury and a tissue factor-independent mechanism, directly activating factors IX and X on the surface of activated platelets (16, 17). While originally approved in the United States for use in patients with hemophilia and inhibitors to factors VIII or IX (18), off-label indications account for the vast majority of its use (19-25). Safety concerns regarding the risk of venous and arterial thromboembolic events and an apparent lack of clinical efficacy in patients without hemophilia have been noted (26-30).
Limited data are available regarding the off-label use of rFVIIa in HSCT recipients (31). A phase II study evaluating rFVIIa for the treatment of hemorrhage after HSCT (including 7 patients with AH) did not demonstrate any overall beneficial effects (32). Case reports of intravenous or intrapulmonary rFVIIa have suggested clinical improvement in HSCT patients with AH (Table S1). However, in a large case series of AH after HSCT (reported as an abstract), rFVIIa did not improve outcomes from respiratory failure or overall survival (33). Thus, the utility of adjunctive rFVIIa therapy for AH post-HSCT remains uncertain.
We describe the use of rFVIIa therapy for AH in a large cohort of HSCT recipients. We evaluated the clinical factors associated with rFVIIa administration including dose, timing, outcome, and thromboembolic events. The characteristics and outcomes of rFVIIa recipients were compared to a contemporaneous cohort of patients with AH treated with corticosteroids alone.
Methods
An expanded Methods section is available in the online supplement.
Patient Identification
Our institutional review board approved this study (11-CC-N228) and granted a waiver of informed consent for its conduct. Three of our hospital databases were queried to identify the patients who developed AH following HSCT from August 2004 to November 2012.
Diagnosis of Alveolar Hemorrhage
AH was defined as the acute onset of signs and/or symptoms of respiratory compromise with evidence of a new or worsening diffuse alveolar pattern on chest radiograph or computed tomography (CT). The diagnosis was established by either a) increasingly bloody return during bronchoalveolar lavage (BAL) and/or the presence of greater than 20% hemosiderin-laden macrophages or b) clinical evidence of respiratory compromise with new or worsening pulmonary infiltrates and either hemoptysis (n=2), blood from an artificial airway (n=5) or histologic evidence of AH at autopsy (n=1). Three of these patients had at least one prior episode of AH documented by bronchoscopy within the previous 14 days. Alternative diagnoses such as fluid overload or congestive heart failure were excluded. The onset of AH was determined retrospectively based on a thorough review of the medical record and defined as the earliest date when either evidence of respiratory compromise was noted or new widespread alveolar infiltrates were found. A recurrent episode of AH was defined as new or worsening hypoxemia requiring increasing supplemental oxygen or the need for mechanical ventilation, evidence of new or worsening alveolar infiltrates on chest radiograph, and clinical deterioration resulting in directed therapy for AH (i.e. starting or increasing corticosteroids and/or resuming rFVIIa treatment). Patients meeting criteria for AH were classified as having either DAH or IAH based on either the absence or presence of a concomitant lower respiratory tract or blood stream infection, respectively.
Patient Characteristics
We reviewed patient demographics including underlying disease, pre-morbid conditions and transplant specific factors including conditioning regimens and GVHD prophylaxis. Laboratory and microbiological data were reviewed. Clinical outcomes including organ failures during the AH episodes were evaluated.
Computed Tomography Analysis
Chest CT images completed 24 hours prior to or within 72 hours from the onset of AH were analyzed with computer aided diagnosis (CAD) techniques to determine the extent and severity of hemorrhage in each patient group. Quantitative metrics of the abnormal lung imaging patterns were analyzed including the volume of pathological lung regions and the ratio of pathological lung volume to total lung volume.
Therapy for Alveolar Hemorrhage
Conventional therapy was defined as corticosteroids and supportive care (e.g., supplemental oxygen and ventilatory support, transfusion of blood products and antimicrobials). Corticosteroid and/or rFVIIa dose and duration of therapy was at the discretion of the treating physicians. Patients were transfused platelets in attempts to achieve levels of more than 50,000/μL, fresh frozen plasma to normalize the PT and aPTT, and cryoprecipitate to achieve a fibrinogen level of greater than 100 mg/dL. The use of any other pro-hemostatic therapy prior to or during an episode of AH was recorded.
In order to examine the effect of rFVIIa on episodes of AH, we compared four separate outcomes with patients treated with corticosteroids alone: (a) ICU length of stay in all patients admitted specifically for respiratory failure due to AH (rFVIIa, n = 23 patients; conventional therapy n = 8 patients), (b) duration of mechanical ventilation for respiratory failure due to AH (rFVIIa n = 25 episodes; conventional therapy n = 7 episodes), (c) time to clinical resolution of AH (rFVIIa n = 30 episodes; conventional therapy n = 10 episodes), and (d) duration of supplemental oxygen use from the onset of the initial episode of AH (all initial episodes included from each group). For the first three comparisons, the onset of the AH episode was defined as either the date of intubation for patients that required mechanical ventilation for AH or in those patients that did not require mechanical ventilation, the date of admission to the ICU for AH. The resolution of an episode was defined as either successful extubation or discharge from the ICU for patients not requiring mechanical ventilation. In some patients, duration of bleeding could not be accurately determined (i.e., a patient intubated for a reason other than AH who then develops AH while receiving mechanical ventilation). In patients who were transferred to the ICU and intubated for AH, time to extubation was used as an indication of bleeding resolution. For supplemental oxygen use, the duration was calculated from the onset of the initial episode of AH until the patient was either off oxygen for 24 hours or death.
For each episode of AH, the total dose, daily mean dose and maximum daily dose (converted to methylprednisolone equivalents per kilogram) was recorded. In 41/57 episodes, the duration of steroid therapy per episode of AH was calculated as the time from the onset of AH to either resolution (i.e., date of extubation or discharge from the ICU as defined above) or death. In the 16 remaining episodes, the duration was determined based on a review of the patient’s medical record. In patients treated with rFVIIa, we assessed the total daily dose per kilogram, the number of doses administered per day of an episode, the number of total doses given per episode and the mean daily dose per kilogram (total dose divided by the number of administrations per day). Adverse events due to the administration of rFVIIa were reviewed and all thrombotic complications within seven days of rFVIIa administration were recorded.
Statistical Analysis
Categorical patient characteristics were compared using either Pearson’s Chi-square test, when appropriate, or Fisher’s exact test. Continuous patient characteristics were compared between two groups using Welch’s t test. When comparing episode-level variables, we used linear mixed models with random subject effects to account for the correlation within each subject. Standard residual diagnostics were used to check model assumptions. Logarithm-transformation was used when necessary. The log-rank test was used to compare survival times from the date of transplant and the onset of AH between the two treatment groups. Survival times from the onset of AH were also compared between the two treatment groups in patients with DAH and those with IAH. We also used propensity scores (generated using logistic regression) to adjust for key baseline differences between the two groups by stratification and regression adjustment. Analyses were conducted using either R statistical package (version 2.15.1; http://www.R-project.org) or SAS® (version 9.4). All p-values are two-tailed and considered significant if p ≤ 0.05.
Results
Patient Characteristics
From 2005 to 2012, 648 patients underwent allogeneic HSCT at the NIH Clinical Center and 37 (5.7%) developed AH. The baseline and transplant characteristics were similar between the rFVIIa and conventional treatment groups (Table 1). Seventy-eight percent of patients received transplants following a reduced-intensity conditioning regimen and 33 patients (89%) received peripheral blood stem cell transplants (Table 1). Time to engraftment, absolute lymphocyte count at day 30 and the incidence of cytomegalovirus reactivation were similar between the groups. The incidence of acute and chronic GVHD prior to the diagnosis of AH was similar between the two groups.
Table 1.
Baseline Patient and Transplant Characteristics
| Characteristics | rFVIIa (N = 23) | Conventional Therapy (N = 14) | P-value |
|---|---|---|---|
| Gender | 13M, 10F | 8M, 6F | 1.00 |
| Age, median (range) | 36 (9-66) | 45 (13-59) | 0.32 |
| Primary disease, n | 0.08 | ||
| Acute Lymphocytic Leukemia | 1 | 3 | |
| Acute Myelogenous Leukemia | 3 | 3 | |
| Chronic Lymphocytic Leukemia | 2 | 1 | |
| Hodgkin’s Disease | 1 | 1 | |
| Myelodysplastic Syndrome | 1 | 1 | |
| Non-Hodgkin Lymphoma | 4 | 5 | |
| Severe Aplastic Anemia | 6 | 0 | |
| Othera | 5 | 0 | |
| Pulmonary function, median (IQR)b | |||
| FVC, liters | 3.30 (2.59-3.96) | 3.72 (2.57-3.90) | 0.44 |
| FVC, % predicted | 90 (78-103) | 82 (73-103) | 0.53 |
| FEV1, liters | 2.60 (1.92-3.05) | 3.02 (2.18-3.17) | 0.79 |
| FEV1, % predicted | 95 (74-104) | 87 (72-98) | 0.39 |
| Diffusion capacity, % predicted | 62 (55-78) | 68 (66-78) | 0.29 |
| Comorbidities, n (%) | 0.71c | ||
| Coronary artery disease | 1 (4) | 1 (7) | |
| Cerebrovascular disease | 2 (9) | 2 (14) | |
| Venous thromboembolism | 3 (13) | 3 (21) | |
| Conditioning regimens | |||
| Reduced-intensity conditioning, n (%) | 19 (83) | 10 (71) | 0.44 |
| Flu/Cy | 8 (35) | 7 (50) | |
| Flu/Cy and equine ATG | 7 (30) | 0 | |
| Other | 4 (18)d | 3 (21)d | |
| Total body irradiation, n (%) | 9 (39) | 6 (43) | 1.00 |
| Myeloablative regimene | 3 (13) | 4 (29) | |
| Reduced-intensity regimenf | 6 (26) | 2 (14) | |
| Stem cell source, n (%) | 0.14 | ||
| Peripheral blood only | 20 (87) | 13 (93) | |
| Bone marrow | 0 | 1 (7) | |
| Peripheral and cord blood | 3 (13) | 0 | |
| Matched transplants, n (%) | 0.69 | ||
| Related | 11(65) | 10 (77) | |
| Unrelated | 6 (35) | 3 (23) | |
| Mismatched transplants, n (%) | 1.00 | ||
| Related | 2 (33) | 1 (100) | |
| Unrelated | 1 (17) | 0 | |
| Related and unrelated | 3 (50)g | 0 | |
| Post-transplant course, n (%) | |||
| Time to engraftment, median days (IQR) | 15 (12-17)h | 12 (10-15) | 0.50 |
| ALC (per μL) at day +30, median (IQR) | 452 (206-789)i | 520 (200-1107)j | 0.94 |
| Cytomegalovirus reactivationk | 14 (61) | 5 (36) | 0.37 |
| Incidence of acute GVHD prior to AH | 11 (48) | 8 (57) | 0.83 |
| Incidence of chronic GVHD prior to AH | 8 (35) | 4 (29) | 1.00 |
| Donor lymphocyte infusionl | 6 (26) | 4 (29) | 0.83 |
| Stem cell boostl | 5 (22) | 4 (29) | 0.70 |
Abbreviations: Interquartile range, (IQR); Forced Vital Capacity, FVC; Forced Expiratory Volume in one second, FEV1; Cyclophosphamide/Fludarabine, Cy/Flu; Absolute Lymphocyte Count, ALC; Graft-versus-host disease, GVHD.
Other = Multiple Myeloma, Sickle Cell Disease, Chronic Granulomatous Disease, GATA2 Deficiency, β-Thalassemia.
All pulmonary function testing was done prior to transplant with the exception of 4 patients (3 in the rFVIIa group and 1 in the conventional group) done post transplant but prior to any episodes of AH. Pulmonary function testing was not available in 2 patients in the conventional group.
Comparison is for the number of patients in each group with at least one comorbidity
rFVIIa group (n): alemtuzumab (2), alemtuzumab and busulfan (1), EPOCH-FR (1); Conventional therapy group (n): EPOCH-F (2), pentostatin (1)
In 6/7 patients, total body irradiation was given twice daily for 4 days as part of a myeloablative conditioning regimen. The total dose was 1200 cGy (150cGy fractions x8) with a 600 cGy mediastinal boost (75 cGy fractions x8). One patient in the rFVIIa group received 400 cGy (50 cGy fractions x8) of total body irradiation as part of a myeloablative regimen.
The dose of total body irradiation therapy given as part of reduced intensity conditioning regimens ranged from 200 cGy to 400 cGy.
Three patients received a haploidentical (related, mismatched) and cord blood (unrelated, mismatched) transplant
Three patients failed to engraft.
Two patients had a total leukocyte count of less than 50 and one patient died before day +30.
One patient died before day +30
Cytomegalovirus reactivation requiring antiviral therapy prior to or within 7 days of an initial episode of AH.
Number of patients who received one or more donor lymphocyte infusions or stem cell boosts ≤ 60 days prior to their initial episode of AH.
Alveolar Hemorrhage
Fifty-seven episodes of AH occurred in 37 patients. Fourteen episodes of AH, occurring in 14 patients, were treated with conventional therapy. The remaining 23 patients developed 43 episodes of AH and were treated with rFVIIa in addition to conventional therapy. Episodes occurred as early as 5 days and as late as almost 7 years post-transplant (Figure S1). AH was confirmed by bronchoscopy in 86% of episodes (35 rFVIIa, 14 conventional therapy). Fifty-six episodes were treated in the ICU (98%). Ten patients (10/23) in the rFVIIa cohort had 2 or more discrete episodes of AH separated by a median (IQR) of 17 days (11 - 41) (Figure S1 and Table 2). None of the patients treated with conventional therapy alone developed recurrent AH.
Table 2.
Episodes of Alveolar Hemorrhage
| Characteristics | rFVIIa (N = 43 episodes, 23 patients) | Conventional Therapy (N = 14 episodes, 14 patients) | P-value |
|---|---|---|---|
| Timing of AH post-transplant, median days (IQR) | 156 (72-514) | 86 (17-183) | 0.52 |
| Patients with ≥ 2 episodes, n (%) | 10 (43) | 0 (0) | 0.006 |
| Episodes per patient, median (IQR) | 1 (1-2) | 1 (1-1) | 0.014 |
| Time interval between episodes, median days (IQR) | 17 (11-41) | --- | --- |
| GVHD prophylaxis, n (%) | |||
| Cyclosporine | 17 (40) | 5 (36) | 1.00 |
| Tacrolimus | 8 (19) | 3 (21) | 1.00 |
| Sirolimus | 10 (23) | 5 (36) | 0.49 |
| Mycophenolate mofetil | 3 (7) | 3 (21) | 0.15 |
| > 1 agent | 4 (9) | 4 (29) | 0.09 |
| None | 9 (21) | 2 (14)a | 0.71 |
| Laboratory variables at onset of AH, median (IQR) | |||
| Platelet count (103/μL) | 43,000 (23,000-61,000) | 33,500 (25,000-63,000) | 0.60 |
| Prothrombin time (sec)b | 15.1 (14.0-17.0)c | 16.5 (14.9-18.3) | 0.58 |
| Activated partial thromboplastin time (sec)b | 39.1 (32.3-44.9)c | 40.4 (35.1-43.2) | 0.85 |
| ANC (per μL) | 1712 (838-4422)d | 3720 (1024-4852)e | 0.39 |
| ALC (per μL) | 268 (95-944)d, f | 255 (115-642)e | 0.42 |
| Organ failures | |||
| SOFA score, median (IQR)g | 13 (11-18) | 12 (11-14) | 0.23 |
| Need for MV, n (%) | 32 (74) | 9 (64) | 0.64 |
| Highest PaO2/FiO2 at the onset of AH, median (IQR)h | 188 (143-243) | 260 (134-347) | 0.30 |
| Vasopressor use, n (%) | 6 (14) | 0 (0) | 0.32 |
| Need for renal replacement therapy, n (%) | 12 (28) | 2 (14) | 0.48 |
| Outcomes | |||
| Hospital mortality in patients requiring MV for AH, n (%) | 13/21 (62) | 6/9 (67) | 1.00 |
| Overall hospital mortality, n (%) | 15 (65) | 6 (43) | 0.27 |
| 30-day survival rate, n (%) | 14 (61) | 11 (79) | 0.31 |
| 60-day survival rate, n (%) | 11 (48) | 6 (43) | 1.00 |
| 180-day survival rate, n (%) | 8 (35) | 4 (29) | 1.00 |
Abbreviations: IQR indicates interquartile range; GVHD, graft-versus-host disease; μL, microliter; sec, seconds; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; SOFA, Sequential Organ Failure Assessment; MV, mechanical ventilation; PaO2, arterial oxygen tension; FiO2, fraction of inspired oxygen.
Two patients were treated with daclizumab and infliximab alone at the onset of AH
Prothrombin time normal range 11.8-14.7 sec; Activated partial thromboplastin time 23.4-34.5 sec.
Data not available for two episodes.
Four patients had a total leukocyte count of less than 30.
Two patients had a total leukocyte count of less than 150.
One patient with chronic lymphocytic leukemia not included.
The PaO2 was not available for calculating the respiratory variable in 10 episodes (rFVIIa group = 7, conventional = 3). In 3 episodes occurring in the rFVIIa treated group, the variable could be imputed based on oxygen saturation. The 7 remaining episodes were excluded in the main comparison (p = 0.23). In a secondary analysis, missing respiratory values were imputed using the lowest possible value in the rFVIIa group and the highest possible value in the conventional group to recalculate the SOFA score, median (IQR) 12 (9-16) and 11 (9-14), respectively (p = 0.19).
Highest PaO2/FiO2 within the first 24 hours of AH onset. A minority of patients in both groups did not have a blood gas done within the first 24 hours of AH onset (rFVIIa group = 5 patients, 8 episodes; conventional group = 3 patients, 3 episodes).
GVHD prophylaxis at the onset of each episode of AH was similar between the two groups and the addition of adjunctive immunosuppression at any time during an episode of AH was also similar (Table 2 and Table S2). The degree of thrombocytopenia during the first 3 days of AH was similar between the two groups (Table 2 and Figure S2). The prothrombin time and activated partial thromboplastin time were prolonged at the onset of AH in both cohorts. The majority of patients had moderately severe hypoxemia with a PaO2/FiO2 ratio (median, IQR) of 193 (141-262) and over two-thirds required mechanical ventilatory support (Table 2) (34).
Hospital mortality, as well as 30d-, 60d- and 180d-survival rates did not significantly differ between the two cohorts (Table 2). Median overall survival following the onset of the initial episode of AH was 80 days in the conventional therapy group and 49 days in the rFVIIa group (p = 0.97) (Figure 1; see Figure S3 for long-term survival curves). In order to adjust for important baseline differences between the two groups, we generated propensity scores using the following variables from the first episode of alveolar hemorrhage: maximum Sequential Organ Failure Assessment (SOFA) score (35), need for vasopressor support, need for renal replacement therapy, lower respiratory tract infection, and bloodstream infection. Despite adjustment based on the propensity score using either stratification (p = 0.24) or regression (p = 0.79) there was no difference in hospital mortality between the two treatment groups.
Figure 1. Survival in Patients Treated with rFVIIa Compared to Conventional Therapy for Alveolar Hemorrhage.
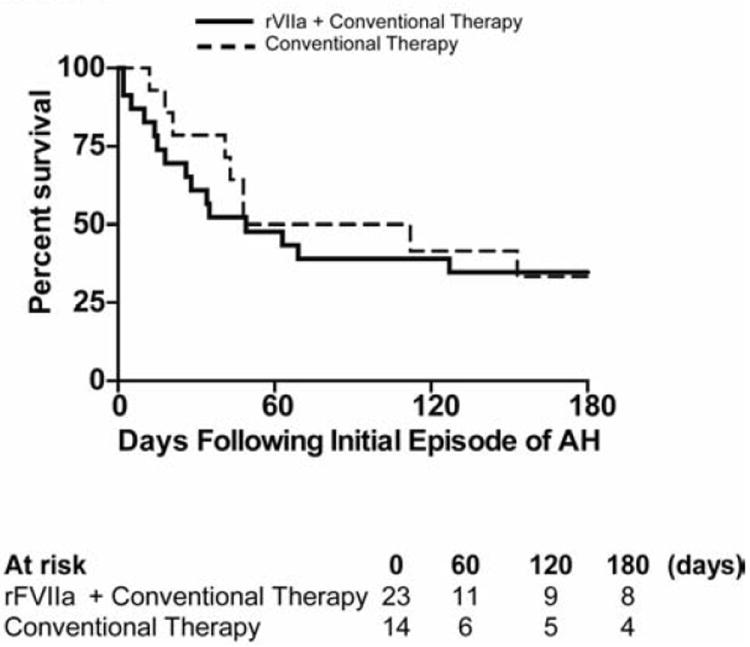
Kaplan-Meier survival curves depicting outcomes in the first 180 day following the onset of the first episode of alveolar hemorrhage. rFVIIa plus conventional therapy (—), conventional therapy (---).
Twenty-seven of the 37 patients with AH had at least one CT scan completed during the study period (40 total scans). The ratio of pathologic lung parenchyma (consolidation and ground-glass opacities) was similar at the onset of AH between the two groups [median (IQR) of 16.4% (12.7-28.9%) in the conventional therapy group and 23.8% (17.9-32.5%) in the rFVIIa group, p = 0.37; Figure S4]. Results were similar when accounting for pleural fluid volume (data not shown). Representative images of increasing amounts of parenchymal involvement are shown in Figure 2A-E. Images demonstrating the distinction between parenchymal infiltrates and pleural effusion, and changes in parenchymal and pleural abnormalities over time in patients with recurrent episodes of AH are found in the online supplement (Figure S5 and video 3). In patients experiencing multiple episodes of AH, parenchymal infiltrates recurred in a similar radiographic distribution relative to their initial episode (video 3).
Figure 2. Extent of Radiographic Pulmonary Pathology at the Onset of Alveolar Hemorrhage.
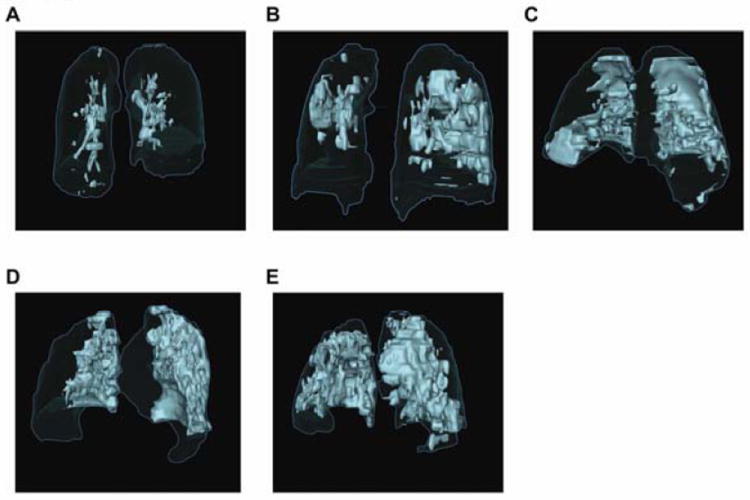
(A-E) Representative surface renderings of pulmonary parenchymal pathology at the onset of alveolar hemorrhage are shown from five different patients treated with rFVIIa and conventional therapy. (A) Patient 8; 8%, (B) Patient 21; 20%, (C) Patient 5; 25%, (D) Patient 13; 37%, and (E) Patient 11; 47%. Total lung volume is outlined in blue and pathological areas are highlighted in light green.
Treatment of Alveolar Hemorrhage
Corticosteroid therapy was used in 95% of the episodes of AH (14/14 episodes in the conventional therapy group and 40/43 episodes in the rFVIIa group; Table 3). The dose of corticosteroids was not increased above the pre-AH dose in 29% (4/14) of episodes in the conventional therapy group (median pre-AH dose, 70 mg/day of methylprednisolone), and 14% (6/43) of episodes in the rFVIIa group (median pre-AH dose 55 mg per day of methylprednisolone). In 3 episodes (2 patients) in the rFVIIa group, corticosteroids were not given. The total dose, daily mean dose and the highest daily dose of therapy were similar between the two groups (Table 3).
Table 3.
Treatment of Alveolar Hemorrhage
| Therapy | rFVIIa (N = 43 episodes, 23 patients) | Conventional Therapy (N = 14 episodes, 14 patients) | P-value |
|---|---|---|---|
| Corticosteroidsa, median (IQR) | |||
| Total dose per episode (mg/kg) | 14.6 (4.8-37.6) | 12.6 (5.0-31.9) | 0.42 |
| Daily mean dose per episode (mg/kg/d) | 1.8 (0.8-3.6) | 2.1 (1.4-2.5) | 0.68 |
| Highest daily dose per episode (mg/kg/d) | 2.8 (1.2-7.9) | 2.2 (2.0-9.6) | 0.97 |
| rFVIIa, median (IQR) | |||
| Dose per administration (μg/kg) | 41 (39-62) | --- | |
| Doses per episode, n, median (IQR; range) | 3 (2-17; 1-57) | --- | |
| Cumulative dose (mg) per episode | 16 (4.8-37.6) | --- | |
| Additional hemostatic agents used, n (%) | 0.43 | ||
| Desmopressin acetate | 8 (18.6) | 1 (7.1) | |
| Aminocaproic acid | 2 (4.7) | 1 (7.1) | |
| Conjugated estrogens | 1 (2.3) | 0 |
Abbreviations: IQR indicates interquartile range; mg, milligrams; kg, kilogram; d, day; μg, microgram.
Expressed in methylprednisolone equivalents.
All patients who received rFVIIa during their initial episode of AH were treated with rFVIIa during each subsequent episode. The first dose of rFVIIa was given within the first 48 hours after the onset of AH in 70% (30/43) of episodes and 53% (23/43) of episodes were treated on day 0, suggesting that the majority of patients were treated early rather than as a rescue therapy. The median rFVIIa dose (IQR) was 41 μg/kg (39-62) and patients received a median of 3 doses (2-17) (Table 3). The mean daily dose of rFVIIa per patient decreased over time (p = 0.007, Figure S6A) and the total daily dose of rFVIIa paralleled this decrease (p = 0.08, Figure S6B). ICU length of stay, duration of mechanical ventilation, time to resolution of bleeding, and the duration of oxygen supplementation in patients who received rFVIIa were similar to patients treated with conventional therapy alone (Figure 3).
Figure 3. Effect of rFVIIa on ICU Length of Stay, Duration of Mechanical Ventilation, Resolution of Alveolar Hemorrhage, and Duration of Supplemental Oxygen Use.
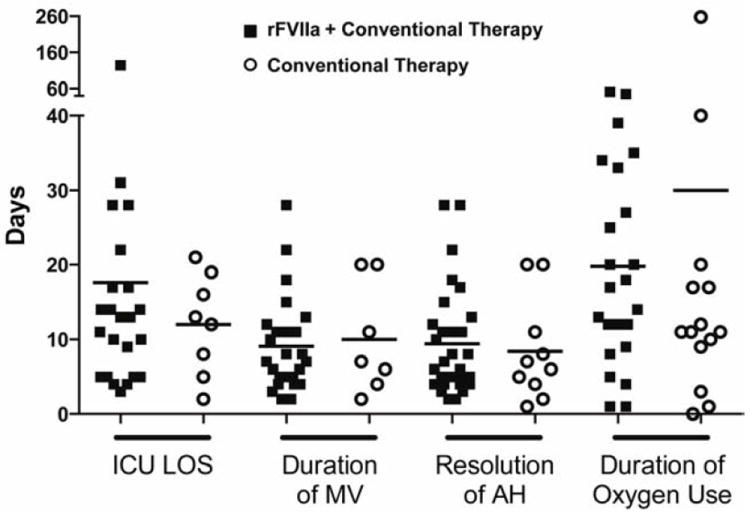
In patients treated with rFVIIa plus conventional therapy (■) compared to patients treated with conventional therapy alone (
 ), there was no difference in ICU length of stay due to alveolar hemorrhage (AH; episodes included in analysis: rFVIIa n = 23/43, conventional therapy n = 8/14; p = 0.63), duration of mechanical ventilation (MV) due to AH (rFVIIa n = 25/43, conventional therapy n = 7/14; p = 0.89), time to resolution of AH (rFVIIa n = 30/43, conventional therapy n = 10/14; p = 0.50); or duration of supplemental oxygen use from the onset of the initial episode of AH (rFVIIa n = 23/23, conventional therapy n = 14/14; p = 0.55).
), there was no difference in ICU length of stay due to alveolar hemorrhage (AH; episodes included in analysis: rFVIIa n = 23/43, conventional therapy n = 8/14; p = 0.63), duration of mechanical ventilation (MV) due to AH (rFVIIa n = 25/43, conventional therapy n = 7/14; p = 0.89), time to resolution of AH (rFVIIa n = 30/43, conventional therapy n = 10/14; p = 0.50); or duration of supplemental oxygen use from the onset of the initial episode of AH (rFVIIa n = 23/23, conventional therapy n = 14/14; p = 0.55).
Incidence and Types of Infection Concurrent With Alveolar Hemorrhage
DAH occurred in 5/14 episodes treated with conventional therapy and 15/43 episodes treated with rFVIIa. In the rFVIIa group, 3 patients accounted for 12 of the 15 episodes of DAH. IAH was present in 64% (9/14) of episodes treated with conventional therapy and 65% (28/43) of episodes treated with rFVIIa (Figure S1). A lower respiratory tract infection (LRTI) was detected in 47% (27/57) of episodes of AH (conventional therapy 6 episodes in 6 patients, rFVIIa 21 episodes in 15 patients) (Table S3). Ten episodes of IAH were associated with concomitant bacteremia (conventional group 3 episodes in 3 patients, rFVIIa 5 episodes in 4 patients) or fungemia (rFVIIa 2 episodes in one patient) in the absence of a LRTI (Table S4). Survival from the onset of the first episode of AH was similar between the two treatment groups in patients with either DAH (p = 0.35; Figure 4A) or IAH (p = 0.56; Figure 4B).
Figure 4. Survival in Patients with Diffuse Alveolar Hemorrhage (DAH) and Infection-Associated Alveolar Hemorrhage (IAH).
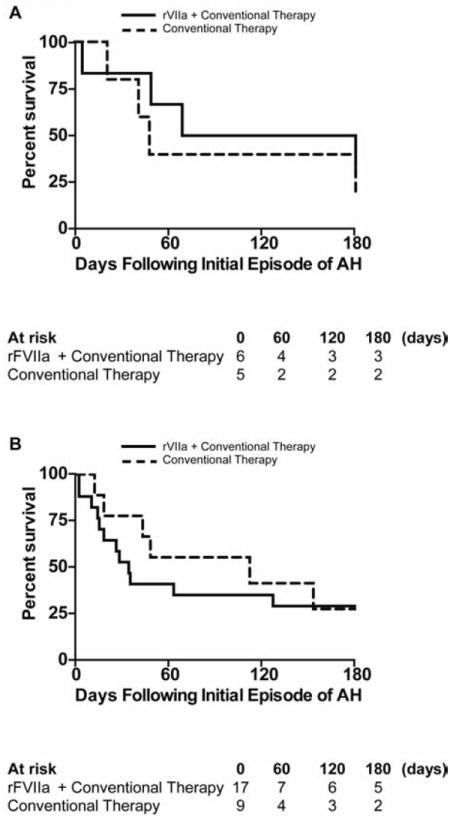
Kaplan-Meier survival curves depicting outcomes in the first 180 day following the onset of the first episode of alveolar hemorrhage in the subgroup of patients with (A) DAH and (B) IAH. rFVIIa plus conventional therapy (—), conventional therapy (---).
Incidence of Thrombotic Events in Patients Treated with rFVIIa
Four thrombotic complications possibly related to rFVIIa therapy were noted in 9% (4/43) of episodes. Complications occurred within one hour and up to four days after rFVIIa administration. These included a blood clot obstructing an endotracheal tube, a basilic vein thrombosis associated with a peripherally inserted central catheter and disseminated intravascular coagulation (DIC) prior to death in two patients; one resulting in the loss of pulses in two extremities (2 days after a dose of rFVIIa) and the second was associated with hemolysis and severe metabolic acidosis (12 hours after a dose of rFVIIa).
Causes of Death
Fifty-seven percent of patients (8/14) treated with conventional therapy survived to hospital discharge. One patient’s death was related to AH and respiratory failure due an angioinvasive mold infection. Three patients died due to progression of their underlying disease. One patient died of respiratory failure due to pneumonia and another patient died of sepsis and multi-organ system failure.
Thirty-five percent of patients (8/23) in the rFVIIa group survived to hospital discharge. Four patients died from respiratory failure due to AH. Three of these patients had concurrent infections (one each with A. baumannii pneumonia, parainfluenza pneumonia and P. aeruginosa bacteremia). One patient’s death in the rFVIIa group was due to progression of their underlying malignancy. The remaining patients’ deaths were attributed to multi-organ system failure in the setting of disseminated fungal infection (n=4), DIC (n=2), carbapenem-resistant K. pneumoniae infection (n=2) and multiple infections (n=2). One patient survived 4 episodes of AH during an initial hospitalization. This patient was re-hospitalized 3 years later and ultimately died of multi-organ failure in the setting of parainfluenza pneumonia and AH.
Post-Mortem Analysis of Lung Parenchyma
Autopsies were performed on 36% (5/14) of patients treated with conventional therapy and 30% (7/23) of patients treated with rFVIIa. Histopathologic findings included intraalveolar hemorrhage with hemosiderin-laden macrophages with elements of diffuse alveolar damage including type II pneumocyte hyperplasia, focal hyaline membranes and fibrin accumulation. In addition, eight autopsies had evidence of organizing pneumonia with inflammation (Table 4, Figure 5A-D). C4d positive staining was noted mainly in larger vessels and within fibrinous deposits and hyaline membranes, but was usually negative in small-caliber vessels and capillaries (Figure 5C and D).
Table 4.
Post Mortem Histopathology Following Alveolar Hemorrhage
| rFVIIa (n = 7) | Conventional Therapy (n = 5) | |
|---|---|---|
| Time (days) of autopsy relative to last dose of therapy for AH (median, IQR) | 3 (2-3) | 20 (7-26) |
| Death due to respiratory failure, n (%) | 2 (29) | 1 (20) |
| Histopathologic Findings (n) | ||
| Dilated airspaces with intraalveolar hemorrhage in different stages of organization with partial clot formation, fibrin accumulation | 7 | 5 |
| Type II pneumocyte hyperplasia | 7 | 5 |
| Hemosiderin laden-macrophages (alveoli and interstitium)a | 6 | 5 |
| Focal hyaline membranes lining alveolar spaces | 6 | 1 |
| Organizing pneumonia with inflammation | 5 | 3 |
| Angioinvasive fungal infection | 2 | 1 |
| Underlying hematologic malignancy | 1b | 2c |
| Thromboemboli | 0 | 0 |
Abbreviations: IQR indicates interquartile range.
One patient without hemosiderin laden macrophages died 1 day after recurrence of alveolar hemorrhage
Gray zone lymphoma
Epstein–Barr virus associated lymphoproliferative disorder and nodular sclerosing Hodgkin’s lymphoma
Figure 5. Autopsy Sections of Representative Lung Histopathology.
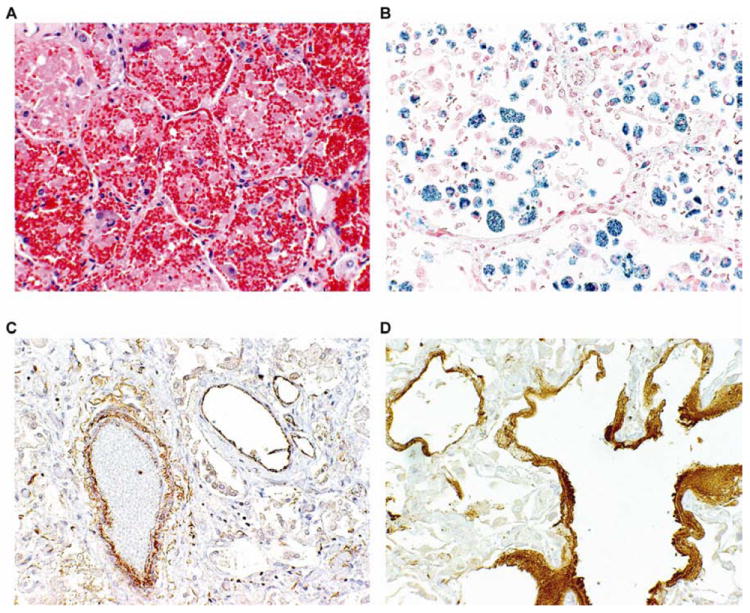
(A) Hematoxylin-eosin stain (magnification, 20X) shows accumulation of red blood cells, fibrin, and hemosiderin-laden macrophages in alveolar spaces. (B) Iron stain (magnification, 20X) highlights hemosiderin-laden macrophages within the alveolar and interstitial space. C4d stain (magnification, 20X) highlights predominantly large-caliber vessels (C) and hyaline membranes (D).
Discussion
We performed a retrospective cohort study to assess the benefits and risks of the off-label use of rFVIIa for patients with AH following HSCT. Alveolar hemorrhage was associated with substantial use of blood products, pharmaceutical and intensive care resources. Over one third of the patients had AH without evidence of lower respiratory tract or blood stream infection while the remaining patients had a concomitant infection that was associated with AH. The majority of episodes were associated with the acute respiratory distress syndrome requiring mechanical ventilatory support. The lung injury was characterized by intraalveolar hemorrhage with diffuse alveolar damage and organizing pneumonia due in part to GVHD-related immunologic mechanisms and the direct and indirect effects of infection (36, 37). As such, anti-inflammatory and antimicrobial therapies were the cornerstones of therapy. We show that rFVIIa can be used as an adjuvant therapy with steroids and supportive care for the treatment of AH. However, the resultant enhanced coagulation from rFVIIa administration did not confer a survival advantage to patients compared to those who received steroids alone. Other factors such as worsening infection, multiple organ failure or recrudescence of primary disease may exceed any survival benefit of enhanced hemostasis in HSCT patients with AH.
Previous reports suggest that AH occurring in the periengraftment period and within the first 30 days following HSCT have better outcomes than AH at later time points post transplant (3, 5). The episodes of AH in our series occurred later after transplantation than previous reports and 27% of patients experienced two or more episodes of AH. The 30-, 60- and 180-day survival rates were comparable or exceeded rates previously described for AH occurring within the first 30 days of transplant (4-6, 38). Improvements in supportive care may have contributed to some of these differences in outcomes. Further, prior chemotherapy and radiation exposure, the presence of GVHD, and concurrent infections contribute to patient heterogeneity and likely impact whether a new therapy will be effective in these patients.
The most frequent cause of death in patients with AH post HSCT in early studies was sepsis and multi-organ system failure (1, 4, 12) while refractory hypoxemic respiratory failure is described in more recent studies (3, 6). The contribution of infection to outcome from AH has yielded conflicting results (5, 6). In the current study, death due to refractory hypoxemia and respiratory failure was uncommon and most of the patients had active infections detected in proximity to an episode of AH. Compared to prior studies (5, 6, 38), our cohort had a higher incidence of lower respiratory tract and bloodstream infections with gram-negative bacilli, molds and fungi. These infections may have contributed directly to pulmonary endothelial and epithelial injury or indirectly by promoting a proinflammatory state and the development of GVHD (39). Furthermore, the preponderance of infection-related deaths in this patient population underscores the difficulty of accurately determining the efficacy of adjunctive rFVIIa for AH since any potential benefit due to enhanced hemostasis is unlikely to impact the outcome of patients who ultimately die of sepsis and multi-organ system failure.
Recurrent episodes of AH occurred in almost half of the patients treated with rFVIIa whereas none of the patients receiving conventional therapy alone experienced recurrence. Recurrent AH alone may reflect a more severely immunocompromised patient population. In this setting, rFVIIa may have had a temporizing effect on the patient’s clinical course pending the effects of more definitive anti-inflammatory and antimicrobial therapy. The high rate of recurrence noted only in patients treated with rFVIIa might suggest a detrimental effect of rFVIIa in the setting of AH. Hyaline membrane formation was more common in our post-mortem histopathology after rFVIIa therapy possibly reflecting more severe lung injury or a consequence of the earlier timing of the autopsy after AH. rFVIIa administration in healthy adults modestly increases plasma interleukin-6 and interleukin-8 levels, an effect that might be potentiated in critically ill patients. The mechanism of rFVIIa-induced pro-inflammatory cytokine production is likely multifactorial, due to thrombin generation (40, 41), activated factor X production (42), or a direct effect of rFVIIa (43). The relevance of these mechanisms to the inflammatory state that exists after HSCT is unknown. However, in a recent meta-analysis of its off-label use for intracranial hemorrhage, cardiac surgery, body trauma, brain trauma or liver transplantation, rFVIIa did not increase in mortality in any of these groups (29). In contrast, rFVIIa was associated with a decreased risk of acute respiratory distress syndrome in body trauma (29).
Recent systematic reviews and meta-analyses have highlighted the increased risk of arterial thromboembolic events with rFVIIa use in patients without hemophilia (27-30). The relative risk of rFVIIa and arterial thrombotic complications was associated with advanced age in cardiac surgery (28) and doses greater than 40 μg/kg in patients with intracranial hemorrhage (29), conditions that typically affect an elderly patient population. In contrast, no increased risk of arterial thromboembolic events was detected in a younger cohort of body trauma patients (29). HSCT patients represented a minority of the patients included in these studies, and thus the applicability of these findings to these patients is uncertain. The dearth of adverse event data from randomized, controlled trials of rFVIIa use in HSCT patients and/or patients with hematological malignancies underscores the fact that risk factors for thrombotic events in this population are unknown and may differ significantly from those in patients included in larger systematic reviews and meta-analyses (28, 31, 44). In the only randomized, placebo-controlled trial of rFVIIa for bleeding following HSCT, the median age of patients was 37 years and there was no evidence of increased thromboembolic events with rFVIIa compared to placebo (32). However, the concurrent use of prophylactic doses of heparin in 22% of patients may have mitigated the risk of thromboembolism (32, 45). Similarly, a retrospective study reported no thrombotic complications in 24 patients treated with rFVIIa for AH post HSCT (33). We describe four thrombotic events possibly related to rFVIIa including a blood clot obstructing an endotracheal tube, an adverse event that has been described previously (46). All four patients were dependent on platelet transfusions at the time of rFVIIa administration, indicating that thrombocytopenia is not protective against thrombosis or DIC. We cannot suggest any screening tool to predict those patients that might develop thrombosis or DIC with the use of rFVIIa in this setting.
The minimum effective dose of rFVIIa for severe bleeding in patients without hemophilia is unknown and doses used in clinical trials vary greatly from as low as 5 μg/kg/dose up to 200 μg/kg/dose given either as a single dose or multiple doses with varying frequencies (30). Decisions regarding dose are particularly relevant to the risk arterial thrombotic complications that appear to be dose dependent (28). In patients with intact mechanisms of thrombin generation, higher rFVIIa doses may augment thrombin production distant to the site of bleeding in a tissue factor-independent manner, resulting in thromboembolic complications without improved hemostatic effects (47). In vitro data suggests that a low dose of rFVIIa (equivalent to 20 μg/kg) has similar efficacy to higher doses (100 – 200 μg/kg) as measured by thrombin generation (48). Similarly, in moderate to severe thrombocytopenia, 50 μg/kg of rFVIIa was as effective as 100 μg/kg at decreasing the Ivy bleeding time (49). Compared to prior reports of rFVIIa therapy for AH following HSCT (Table S1), the median dose per kilogram used in our cohort was lower and decreased over time suggesting increased experience with this therapy with similar clinical effects.
The strengths of our study include the detailed data collection and analysis of each episode of AH, an assessment of concurrent infections, long-term follow up of each patient and inclusion of a contemporaneous comparator cohort treated with corticosteroids and supportive care alone. However, several selection biases for and against the use of rFVIIa likely contributed to our inability to demonstrate any clear advantage or disadvantage of rFVIIa in AH in this cohort. As a single-site retrospective study, selection and ascertainment bias as well as unknown confounders may influence our observations. Our analysis is also likely underpowered to assess some of the clinical variables (mortality, duration of mechanical ventilation, ICU length of stay, and resolution of AH) analyzed between the two cohorts.
In conclusion, rFVIIa, when used in combination with corticosteroids, did not confer a survival advantage compared to corticosteroids alone. Any putative advantage as adjunctive therapy with steroids and supportive care will require a prospective randomized clinical trial. Further understanding of the pathogenic mechanisms contributing to lung injury in an individual patient with AH will likely inform therapeutic options and possibly provide the basis for improved therapies.
Supplementary Material
Acknowledgments
The authors would like to thank Kelly Byrne for her excellent editorial assistance; Judith Welsh for her expertise in information services; Ashley Carpenter her assistance collecting clinical data; and Dr. Hanh Khuu for providing valuable hematopoietic stem cell processing data. The NIH Intramural Research Program of the National Heart Lung and Blood Institute, the National Cancer Institute, the National Institute of Allergy and Infectious Diseases and the NIH Clinical Center supported this work. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
The publisher or recipient acknowledges right of the US government to retain a nonexclusive, royalty-free license in and to any copyright covering the article.
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The online version of the article contains a data supplement.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Afessa B, Tefferi A, Litzow MR, Krowka MJ, Wylam ME, Peters SG. Diffuse alveolar hemorrhage in hematopoietic stem cell transplant recipients. Am J Respir Crit Care Med. 2002;166:641–645. doi: 10.1164/rccm.200112-141cc. [DOI] [PubMed] [Google Scholar]
- 2.Panoskaltsis-Mortari A, Griese M, Madtes DK, et al. An official American Thoracic Society research statement: noninfectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome. Am J Respir Crit Care Med. 2011;183:1262–1279. doi: 10.1164/rccm.2007-413ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afessa B, Tefferi A, Litzow MR, Peters SG. Outcome of diffuse alveolar hemorrhage in hematopoietic stem cell transplant recipients. Am J Respir Crit Care Med. 2002;166:1364–1368. doi: 10.1164/rccm.200208-792OC. [DOI] [PubMed] [Google Scholar]
- 4.Lewis ID, DeFor T, Weisdorf DJ. Increasing incidence of diffuse alveolar hemorrhage following allogeneic bone marrow transplantation: cryptic etiology and uncertain therapy. Bone Marrow Transplant. 2000;26:539–543. doi: 10.1038/sj.bmt.1702546. [DOI] [PubMed] [Google Scholar]
- 5.Majhail NS, Parks K, Defor TE, Weisdorf DJ. Diffuse alveolar hemorrhage and infection-associated alveolar hemorrhage following hematopoietic stem cell transplantation: related and high-risk clinical syndromes. Biol Blood Marrow Transplant. 2006;12:1038–1046. doi: 10.1016/j.bbmt.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Gupta S, Jain A, Warneke CL, et al. Outcome of alveolar hemorrhage in hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2007;40:71–78. doi: 10.1038/sj.bmt.1705695. [DOI] [PubMed] [Google Scholar]
- 7.Huaringa AJ, Leyva FJ, Giralt SA, et al. Outcome of bone marrow transplantation patients requiring mechanical ventilation. Crit Care Med. 2000;28:1014–1017. doi: 10.1097/00003246-200004000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Wojno KJ, Vogelsang GB, Beschorner WE, Santos GW. Pulmonary hemorrhage as a cause of death in allogeneic bone marrow recipients with severe acute graft-versus-host disease. Transplantation. 1994;57:88–92. doi: 10.1097/00007890-199401000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Sisson JH, Thompson AB, Anderson JR, et al. Airway inflammation predicts diffuse alveolar hemorrhage during bone marrow transplantation in patients with Hodgkin disease. Am Rev Respir Dis. 1992;146:439–443. doi: 10.1164/ajrccm/146.2.439. [DOI] [PubMed] [Google Scholar]
- 10.Cooke KR, Kobzik L, Martin TR, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88:3230–3239. [PubMed] [Google Scholar]
- 11.Chao NJ, Duncan SR, Long GD, Horning SJ, Blume KG. Corticosteroid therapy for diffuse alveolar hemorrhage in autologous bone marrow transplant recipients. Ann Intern Med. 1991;114:145–146. doi: 10.7326/0003-4819-114-2-145. [DOI] [PubMed] [Google Scholar]
- 12.Metcalf JP, Rennard SI, Reed EC, et al. Corticosteroids as adjunctive therapy for diffuse alveolar hemorrhage associated with bone marrow transplantation. University of Nebraska Medical Center Bone Marrow Transplant Group. Am J Med. 1994;96:327–334. doi: 10.1016/0002-9343(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 13.Raptis A, Mavroudis D, Suffredini A, et al. High-dose corticosteroid therapy for diffuse alveolar hemorrhage in allogeneic bone marrow stem cell transplant recipients. Bone Marrow Transplant. 1999;24:879–883. doi: 10.1038/sj.bmt.1701995. [DOI] [PubMed] [Google Scholar]
- 14.Wanko SO, Broadwater G, Folz RJ, Chao NJ. Diffuse alveolar hemorrhage: retrospective review of clinical outcome in allogeneic transplant recipients treated with aminocaproic acid. Biol Blood Marrow Transplant. 2006;12:949–953. doi: 10.1016/j.bbmt.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Solomonov A, Fruchter O, Zuckerman T, Brenner B, Yigla M. Pulmonary hemorrhage: A novel mode of therapy. Respir Med. 2009;103:1196–1200. doi: 10.1016/j.rmed.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Gabriel DA, Li X, Monroe DM, 3rd, Roberts HR. Recombinant human factor VIIa (rFVIIa) can activate factor FIX on activated platelets. J Thromb Haemost. 2004;2:1816–1822. doi: 10.1111/j.1538-7836.2004.01015.x. [DOI] [PubMed] [Google Scholar]
- 17.Monroe DM. Further understanding of recombinant activated factor VII mode of action. Semin Hematol. 2008;45:S7–S11. doi: 10.1053/j.seminhematol.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 18.Hedner U, Lee CA. First 20 years with recombinant FVIIa (NovoSeven) Haemophilia. 2011;17:e172–182. doi: 10.1111/j.1365-2516.2010.02352.x. [DOI] [PubMed] [Google Scholar]
- 19.Logan AC, Yank V, Stafford RS. Off-label use of recombinant factor VIIa in U.S. hospitals: analysis of hospital records. Ann Intern Med. 2011;154:516–522. doi: 10.7326/0003-4819-154-8-201104190-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boffard KD, Riou B, Warren B, et al. Recombinant factor VIIa as adjunctive therapy for bleeding control in severely injured trauma patients: two parallel randomized, placebo-controlled, double-blind clinical trials. J Trauma. 2005;59:8–15. doi: 10.1097/01.ta.0000171453.37949.b7. discussion 15-18. [DOI] [PubMed] [Google Scholar]
- 21.Hauser CJ, Boffard K, Dutton R, et al. Results of the CONTROL trial: efficacy and safety of recombinant activated Factor VII in the management of refractory traumatic hemorrhage. J Trauma. 2010;69:489–500. doi: 10.1097/TA.0b013e3181edf36e. [DOI] [PubMed] [Google Scholar]
- 22.Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–2137. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
- 23.Gill R, Herbertson M, Vuylsteke A, et al. Safety and efficacy of recombinant activated factor VII: a randomized placebo-controlled trial in the setting of bleeding after cardiac surgery. Circulation. 2009;120:21–27. doi: 10.1161/CIRCULATIONAHA.108.834275. [DOI] [PubMed] [Google Scholar]
- 24.Bosch J, Thabut D, Bendtsen F, et al. Recombinant factor VIIa for upper gastrointestinal bleeding in patients with cirrhosis: a randomized, double-blind trial. Gastroenterology. 2004;127:1123–1130. doi: 10.1053/j.gastro.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 25.Bosch J, Thabut D, Albillos A, et al. Recombinant factor VIIa for variceal bleeding in patients with advanced cirrhosis: A randomized, controlled trial. Hepatology. 2008;47:1604–1614. doi: 10.1002/hep.22216. [DOI] [PubMed] [Google Scholar]
- 26.O’Connell KA, Wood JJ, Wise RP, Lozier JN, Braun MM. Thromboembolic adverse events after use of recombinant human coagulation factor VIIa. JAMA. 2006;295:293–298. doi: 10.1001/jama.295.3.293. [DOI] [PubMed] [Google Scholar]
- 27.Hsia CC, Chin-Yee IH, McAlister VC. Use of recombinant activated factor VII in patients without hemophilia: a meta-analysis of randomized control trials. Ann Surg. 2008;248:61–68. doi: 10.1097/SLA.0b013e318176c4ec. [DOI] [PubMed] [Google Scholar]
- 28.Levi M, Levy JH, Andersen HF, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med. 2010;363:1791–1800. doi: 10.1056/NEJMoa1006221. [DOI] [PubMed] [Google Scholar]
- 29.Yank V, Tuohy CV, Logan AC, et al. Systematic review: benefits and harms of in-hospital use of recombinant factor VIIa for off-label indications. Ann Intern Med. 2011;154:529–540. doi: 10.7326/0003-4819-154-8-201104190-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simpson E, Lin Y, Stanworth S, Birchall J, Doree C, Hyde C. Recombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophilia. Cochrane Database Syst Rev. 2012;3 doi: 10.1002/14651858.CD005011.pub4. CD005011. [DOI] [PubMed] [Google Scholar]
- 31.Franchini M, Veneri D, Lippi G. The potential role of recombinant activated FVII in the management of critical hemato-oncological bleeding: A systematic review. Bone Marrow Transplantation. 2007;39:729–735. doi: 10.1038/sj.bmt.1705670. [DOI] [PubMed] [Google Scholar]
- 32.Pihusch M, Bacigalupo A, Szer J, et al. Recombinant activated factor VII in treatment of bleeding complications following hematopoietic stem cell transplantation. J Thromb Haemost. 2005;3:1935–1944. doi: 10.1111/j.1538-7836.2005.01523.x. [DOI] [PubMed] [Google Scholar]
- 33.Gupta S, Gupta A, Jain A, Warneke CL, Giralt S, Eapen GA. Outcome of recombinant factor VIIa use for alveolar hemorrhage in hematopoietic stem cell transplant recipients. Blood. 2006;108 doi: 10.1038/sj.bmt.1705695. Abstract 3997. [DOI] [PubMed] [Google Scholar]
- 34.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 35.Vincent JL, de Mendonca A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26:1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Robbins RA, Linder J, Stahl MG, et al. Diffuse alveolar hemorrhage in autologous bone marrow transplant recipients. Am J Med. 1989;87:511–518. doi: 10.1016/s0002-9343(89)80606-0. [DOI] [PubMed] [Google Scholar]
- 37.Agusti C, Ramirez J, Picado C, et al. Diffuse alveolar hemorrhage in allogeneic bone marrow transplantation. A postmortem study. Am J Respir Crit Care Med. 1995;151:1006–1010. doi: 10.1164/ajrccm/151.4.1006. [DOI] [PubMed] [Google Scholar]
- 38.Majhail NS, Parks K, Defor TE, Weisdorf DJ. Alveolar hemorrhage following allogeneic hematopoietic cell transplantation using reduced-intensity conditioning. Bone Marrow Transplant. 2006;38:765–768. doi: 10.1038/sj.bmt.1705521. [DOI] [PubMed] [Google Scholar]
- 39.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones A, Geczy CL. Thrombin and factor Xa enhance the production of interleukin-1. Immunology. 1990;71:236–241. [PMC free article] [PubMed] [Google Scholar]
- 41.Grandaliano G, Valente AJ, Abboud HE. A novel biologic activity of thrombin: stimulation of monocyte chemotactic protein production. J Exp Med. 1994;179:1737–1741. doi: 10.1084/jem.179.5.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cirino G, Cicala C, Bucci M, et al. Factor Xa as an interface between coagulation and inflammation. Molecular mimicry of factor Xa association with effector cell protease receptor-1 induces acute inflammation in vivo. J Clin Invest. 1997;99:2446–2451. doi: 10.1172/JCI119428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cunningham MA, Romas P, Hutchinson P, Holdsworth SR, Tipping PG. Tissue factor and factor VIIa receptor/ligand interactions induce proinflammatory effects in macrophages. Blood. 1999;94:3413–3420. [PubMed] [Google Scholar]
- 44.Mantzios G, Tsirigotis P, Pappa V, et al. Massive pulmonary embolism after treatment with rFVIIa in a thrombocytopenic patient with acute myelogenous leukemia and intractable bleeding. Eur J Haematol. 2007;78:173–174. doi: 10.1111/j.1600-0609.2006.00799.x. [DOI] [PubMed] [Google Scholar]
- 45.Hoots WK. Hemorrhage following hematopoetic stem cell transplantation--which arrows belong in the quiver? J Thromb Haemost. 2005;3:1933–1934. doi: 10.1111/j.1538-7836.2005.01578.x. commentary. [DOI] [PubMed] [Google Scholar]
- 46.Lauer S, Westphal M, Fischer LG, Meissner A, Van Aken H, Freise H. Recombinant factor VIIa and factor VIII treatment for acquired factor VIII deficiency: a case of repeated thrombotic endotracheal occlusion in a mechanically ventilated patient. Crit Care. 2011;15:407. doi: 10.1186/cc10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoots WK. Challenges in the therapeutic use of a “so-called” universal hemostatic agent: recombinant factor VIIa. Hematology Am Soc Hematol Educ Program. 2006:426–431. doi: 10.1182/asheducation-2006.1.426. [DOI] [PubMed] [Google Scholar]
- 48.Altman R, Scazziota A, de Lourdes Herrera M, Gonzalez CD. The hemostatic profile of recombinant activated factor VII. Can low concentrations stop bleeding in off-label indications? Thromb J. 2010;8:8. doi: 10.1186/1477-9560-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kristensen J, Killander A, Hippe E, et al. Clinical experience with recombinant factor VIIa in patients with thrombocytopenia. Haemostasis. 1996;26(Suppl 1):159–164. doi: 10.1159/000217260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


